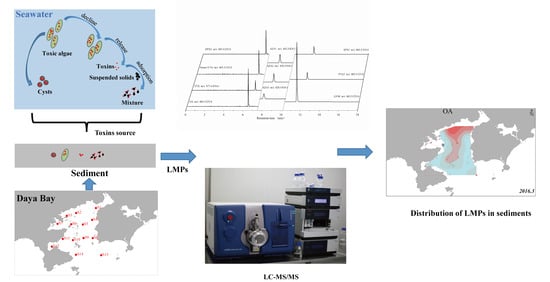
Sediment as a Potential Pool for Lipophilic Marine Phycotoxins with the Case Study of Daya Bay of China
Abstract
1. Introduction
2. Results and Discussion
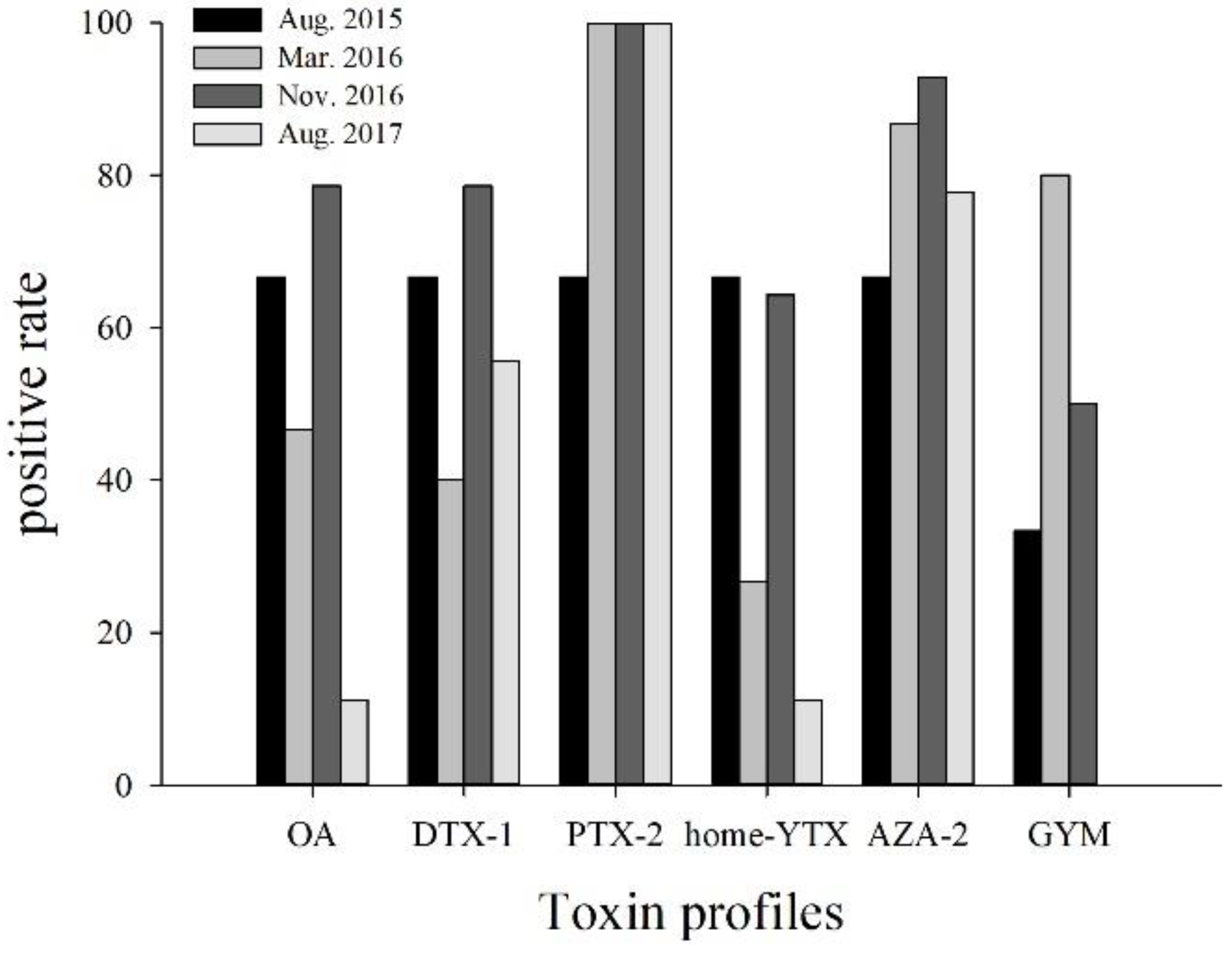
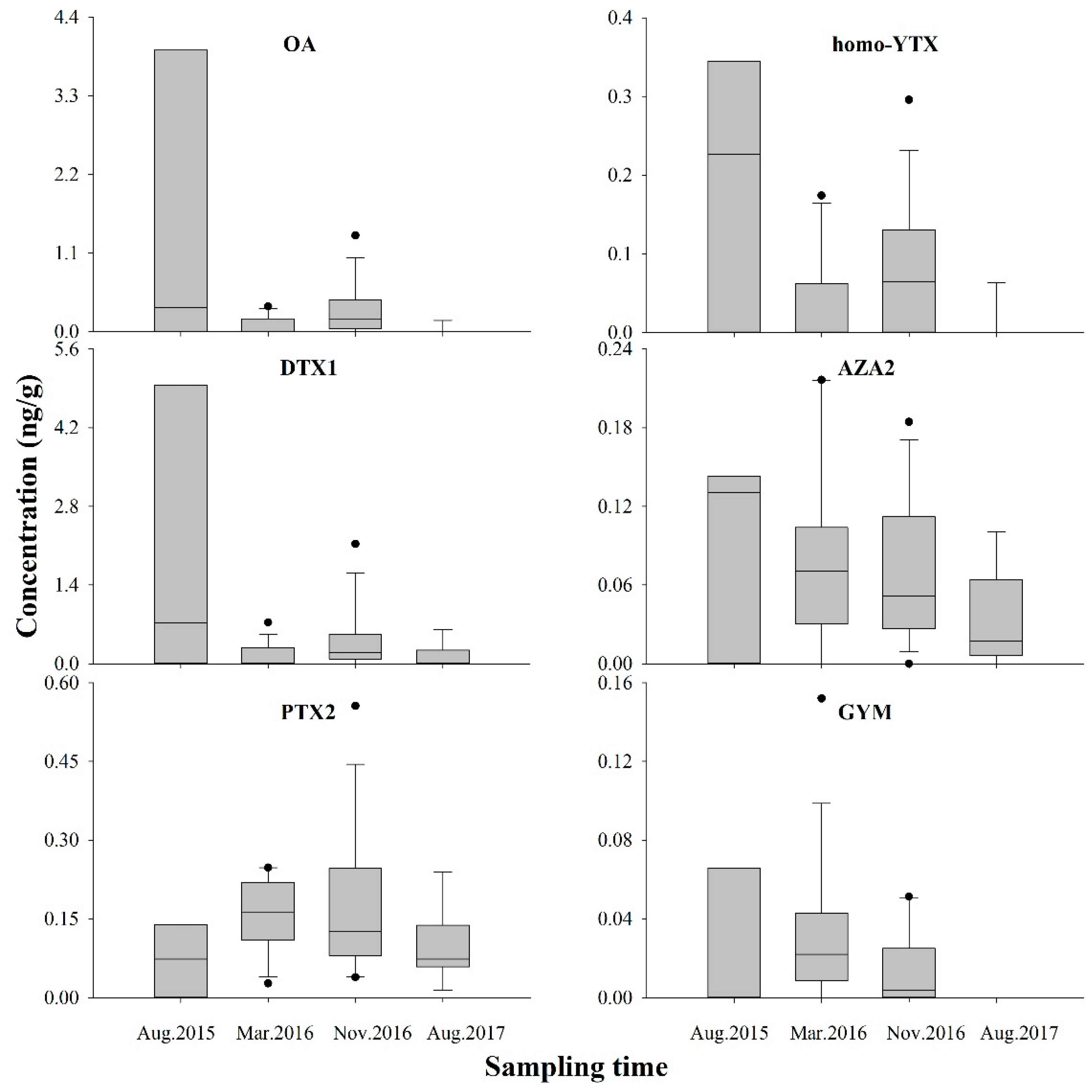
2.1. Occurrence of LMPs in the Sediment of DYB
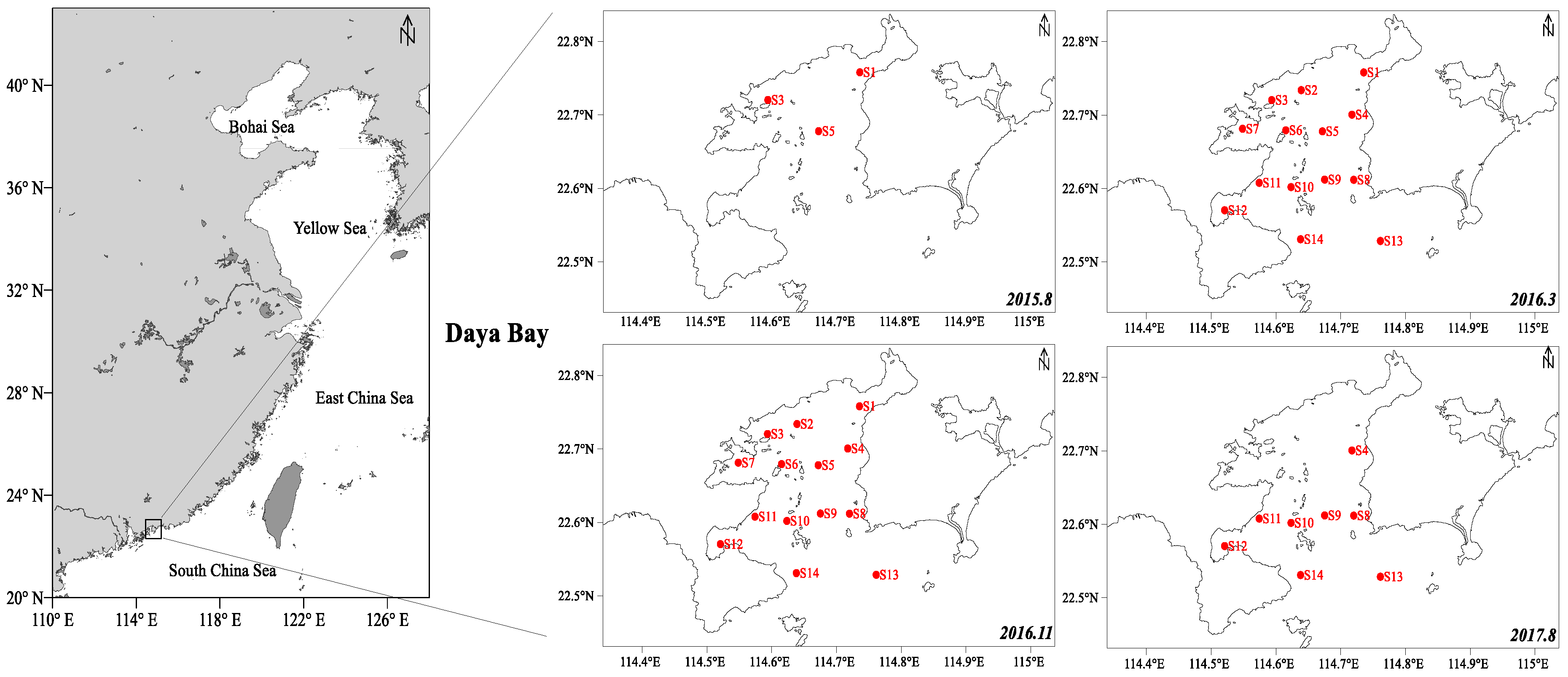
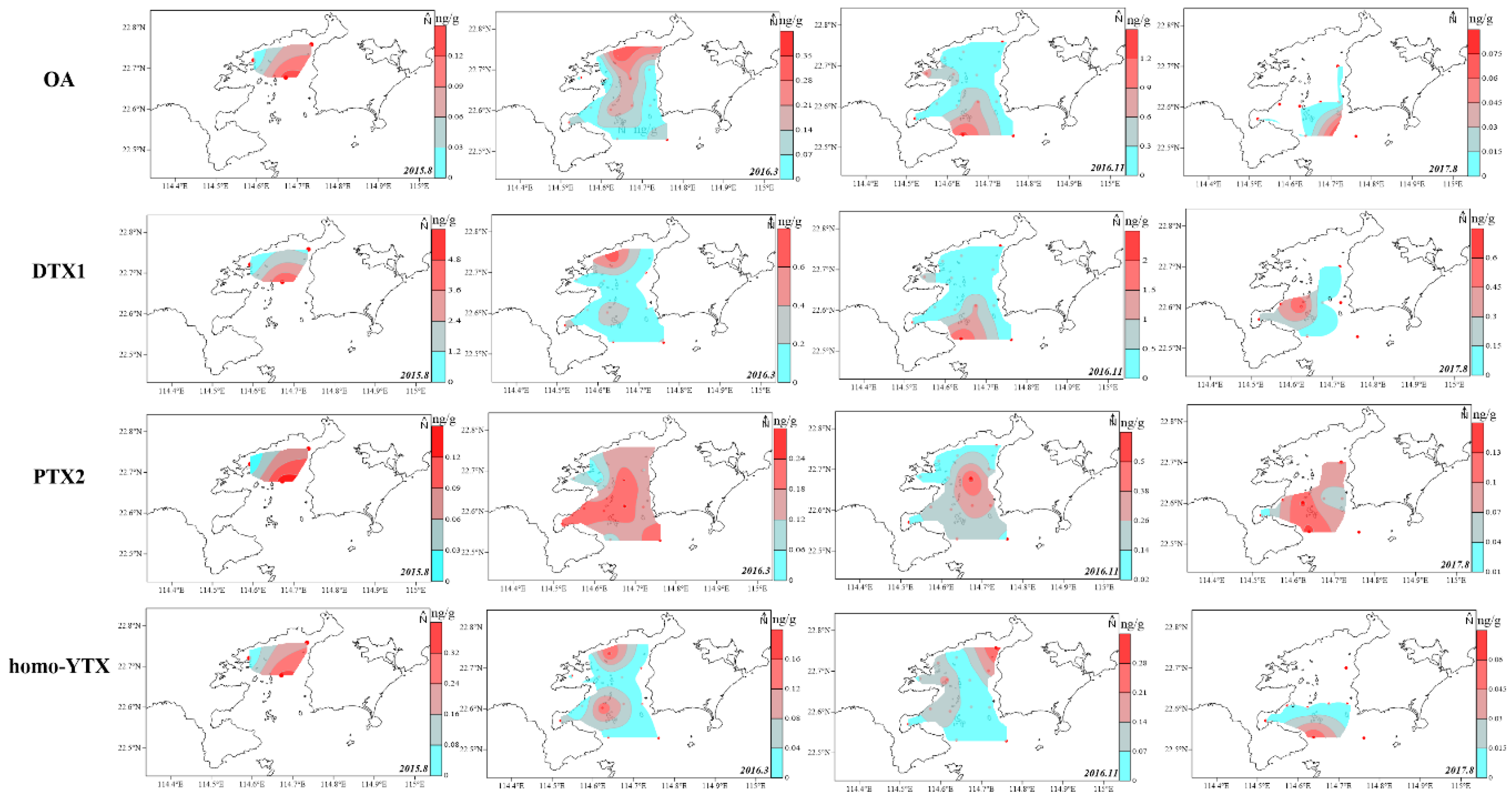
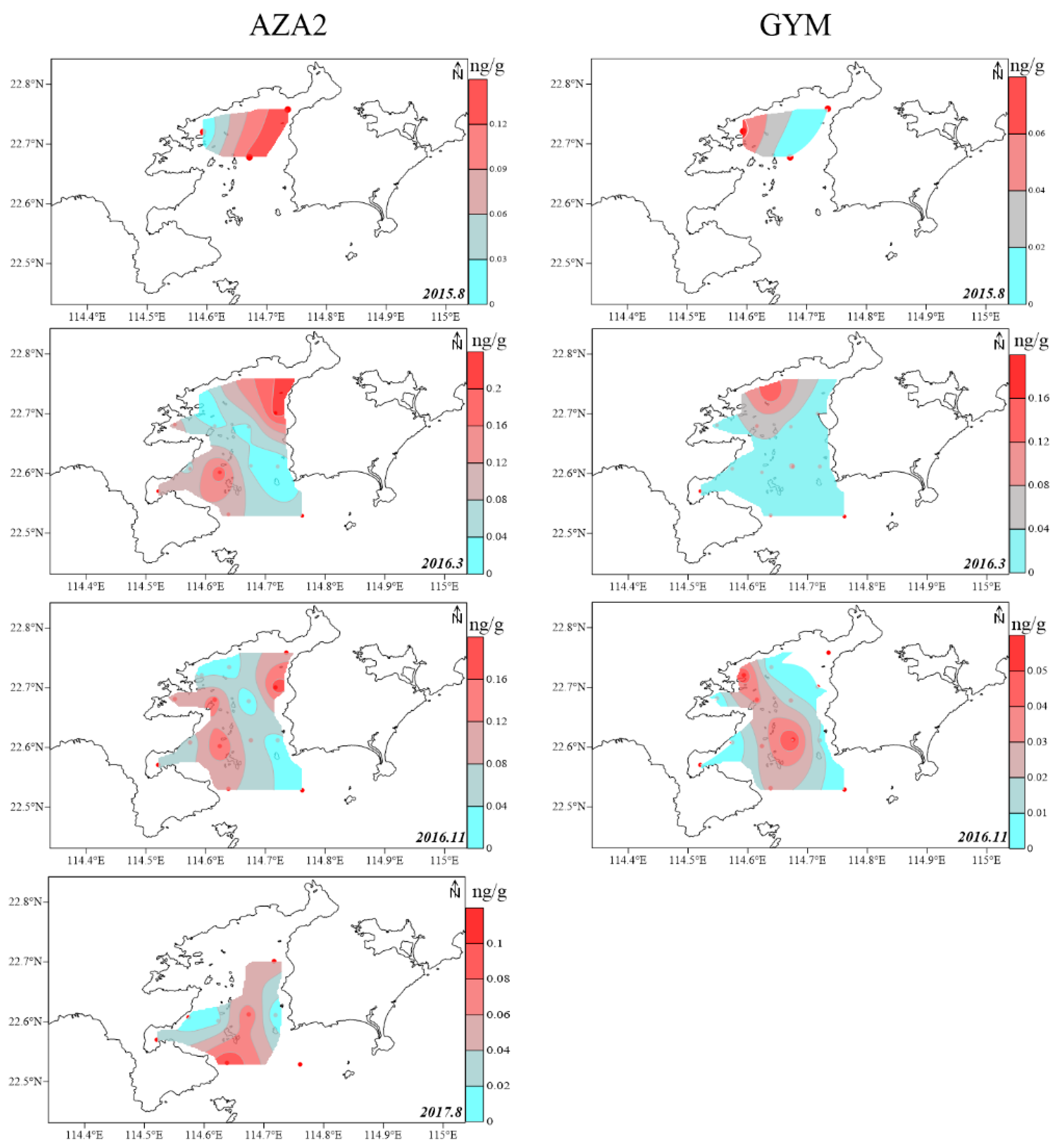
2.2. Spatial Distribution of LMPs in the Sediment of DYB
2.3. Potential Source of LMPs in Sediment of DYB
2.4. Relationship Between Physicochemical Property of Sediment and Distribution of LMPs in the Sediment of DYB
3. Experimental Section
3.1. Chemical and Reagents
3.2. Investigated Area and Sampling Collection
3.3. Sample Pre-Treatment and Toxin Extraction
3.4. Analysis Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- These, A.; Scholz, J.; Preiss, W.A. Sensitive method for the determination of lipophilic marine biotoxins in extracts of mussels and processed shellfish by high-performance liquid chromatography-tandem mass spectrometry based on enrichment by solid-phase extraction. J. Chromatogr. A 2009, 1216, 4529–4538. [Google Scholar] [CrossRef] [PubMed]
- Landrum, P.F.; Fisher, S.W. Influence of Lipids on the Bioaccumulation and Trophic Transfer of Organic Contaminants in Aquatic Organisms. In Lipids in Freshwater Ecosystems; Springer: New York, NY, USA, 1999; pp. 203–234. [Google Scholar]
- Li, A.; Sun, G.; Qiu, J.; Fan, L. Lipophilic shellfish toxins in Dinophysis caudate picked cells and in shellfish from the East China Sea. Environ. Sci. Pollut. Res. 2015, 22, 3116–3126. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, R.C.; Kong, F.Z.; Li, C.; Dai, L.; Chen, Z.F.; Zhou, M.J. Lipophilic marine toxins discovered in the Bohai Sea using high performance liquid chromatography coupled with tandem mass spectrometry. Chemosphere 2017, 183, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, R.C.; Kong, F.Z.; Li, C.; Chen, Z.F.; Geng, H.X.; Zhou, M.J. Contamination status of lipophilic marine toxins in shellfish samples from the Bohai Sea, China. Environ. Pollut. 2019, 249, 171–180. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Chen, J.; Shi, Q.; Zhang, R.; Wang, S.; Wang, X. Detection, occurrence and monthly variations of typical lipophilic marine toxins associated with diarrhetic shellfish poisoning in the coastal seawater of Qingdao City, China. Chemosphere 2014, 111, 560–567. [Google Scholar] [CrossRef]
- Chen, J.H.; Li, X.; Wang, S.; Chen, F.R.; Cao, W.; Sun, C.J.; Zheng, L.; Wang, X.R. Screening of lipophilic marine toxins in marine aquaculture environment using liquid chromatography-mass spectrometry. Chemosphere 2017, 168, 32–40. [Google Scholar] [CrossRef]
- Kuuppo, P.; Uronen, P.; Petermann, A.; Tamminen, T.; Granéli, E. Pectenotoxin-2 and dinophysistoxin-1 in suspended and sedimenting organic matter in the Baltic Sea. Limnol. Oceanogr. 2006, 51, 2300–2307. [Google Scholar] [CrossRef]
- Voorspoels, S.; Covaci, A.; Maervoet, J.; De Meester, I.; Schepens, P. Distribution of PCBs/OCPs in benthic organisms and fish from the North Sea Continental Shelf and Scheldt estuary. Mar. Pollut. Bull. 2004, 49, 393–404. [Google Scholar] [CrossRef]
- Mouradian, M.; Panetta, R.J.; Vernal, A.; Gelinas, Y. Dinosterols or dynocycts to estimate dinoflagellate contributions to marine sedimentary organic matter. Limnol. Oceanogr. 2007, 52, 2569–2581. [Google Scholar] [CrossRef]
- Hitchcock, G.L.; Fourqurean, J.W.; Drake, J.L.; Mead, R.N.; Heil, C.A. Brevetoxin persistence in sediments and seagrass epiphytes of east Florida coastal waters. Harmful Algae 2012, 13, 89–94. [Google Scholar] [CrossRef]
- Wang, Y.L.; Chen, J.H.; Li, Z.Y.; Wang, S.; Shi, Q.; Cao, W.; Zheng, X.L.; Sun, C.J.; Wang, X.R.; Zheng, L. Determination of typical lipophilic marine toxins in marine sediments from three coastal bays of China using liquid chromatography-tandem mass spectrometry after accelerated solvent extraction. Mar. Pollut. Bull. 2015, 101, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Han, T.Z.; Li, X.T.; He, X.P.; Wang, Y.L.; Chen, F.R.; Song, X.C.; Zhou, D.S.; Wang, X.R. Occurrence and distribution of marine natural organic pollutants: Lipophilic marine algal toxins in the Yellow Sea and the Bohai Sea, China. Sci. Total Environ. 2018, 612, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Huang, L.M.; Zhang, J.L.; Huang, H.H.; Li, T.; Su, Q. Harmful algal blooms (HABs) in Daya Bay, China: An in situ study of primary production and environmental impacts. Mar. Pollut. Bull. 2009, 58, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Yan, W.; Chi, J.S.; Zhang, G. Spatial and vertical distribution of organochlorine pesticides in sediments from Daya Bay, South China. Mar. Pollut. Bull. 2008, 56, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Mu, D.H.; Li, Y.F.; Cao, Y.; Zhang, Y.J. Recent eutrophication and human disturbance in Daya Bay, the South China Sea: Dinoflagellate cyst and geochemical evidence. Estuar. Coast. Shelf Sci. 2011, 92, 403–414. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, L.; Li, Y.; Zhang, J.; Tan, Z.J.; Wu, H.Y.; Jiang, T.J.; Lu, S.H. Occurrence of marine algal toxins in oyster and phytoplankton samples in Daya Bay, South China Sea. Chemosphere 2017, 183, 80–88. [Google Scholar] [CrossRef]
- Fisher, T.T.; Law, R.J.; Rumney, H.S.; Kirby, M.F.; Kelly, C. Towards a scheme of toxic equivalency factors (TEFs) for the acute toxicity of PAHs in sediment. Ecotoxicol. Environ. Saf. 2011, 74, 2245–2251. [Google Scholar] [CrossRef]
- Li, A.F.; Li, M.H.; Qiu, J.B.; Song, J.L.; Ji, Y.; Hu, Y.; Wang, S.Q.; Che, Y.J. Effect of suspended particulate matter on the accumulation of dissolved diarrhetic shellfish toxins by mussels (Mytilus galloprovincialis) under laboratory conditions. Toxins 2018, 10, 273. [Google Scholar] [CrossRef]
- Suzuki, T.; Yasumoto, T. Liquid chromatography-electrospray ionization mass spectrometry of the diarrhetic shellfish-poisoning toxins okadaic acid, dinophysistoxin-1 and pectenotoxin-6 in bivalves. J. Chromatogr. A 2000, 874, 199–206. [Google Scholar] [CrossRef]
- Klöpper, S.; Scharek, R.; Gerdts, G. Diarrhetic shellfish toxicity in relation to the abundance of Dinophysis spp. in the German Bight near Helgoland. Mar. Ecol. Prog. Ser. 2003, 259, 93–102. [Google Scholar] [CrossRef]
- Gao, X.L.; Chen, S.Y.; Long, A.M. Composition and sources of organic matter and its solvent extractable components in surface sediments of a bay under serious anthropogenic influences: Daya Bay, China. Mar. Pollut. Bull. 2008, 56, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Paz, B.; Daranas, A.H.; Norte, M.; Riobo, P.; Franco, J.M.; Fernandez, J.J. Yessotoxins, a group of marine polyether toxins: An overview. Mar. Drugs 2008, 6, 73–102. [Google Scholar] [CrossRef] [PubMed]
- Paz, B.; Riobó, P.; Ramilo, I.; Franco, J.M. Yessotoxins profile in strains of Protoceratium reticulatum from Spain and USA. Toxicon 2007, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Luo, Z.; Krock, B.; Witt, M.; Tillmann, U. Morphology, phylogeny and azaspiracid profile of Azadinium poporum (Dinophyceae) from the China sea. Harmful Algae 2013, 21–22, 64–75. [Google Scholar] [CrossRef]
- Krock, B.; Tillmann, U.; Witt, M.; Gu, H.F. Azaspiracid variability of Azadinium poporum (Dinophyceae) from the China Sea. Harmful Algae 2014, 36, 22–28. [Google Scholar] [CrossRef]
- Kharrat, R.; Servent, D.; Girard, E.; Ouanounou, G.; Amar, M.; Marrouchi, R.; Benoit, E.; Molgo, J. The marine phycotoxin gymnodimine targets muscular and neuronal nicotinic acetylcholine receptor subtypes with high affinity. J. Neurochem. 2008, 107, 952–963. [Google Scholar] [CrossRef]
- Gu, H.F. Morphology, phylogenetic position, and ecophysiology of Alexandrium ostenfeldii (Dinophyceae) from the Bohai Sea, China. J. Syst. Evol. 2011, 49, 606–616. [Google Scholar] [CrossRef]
- Wang, Z.H.; Chen, J.F.; Xu, N.; Qi, Y.Z. Dynamics on cell densities of diatom, dinoflagellate and relationship with environmental factors in Aotou area, Daya Bay, South China Sea. Oceanol. Limnol. Sin. 2015, 36, 186–192. (In Chinese) [Google Scholar]
- Zhang, H.; Li, Y.; Cen, J.Y.; Wang, H.L.; Cui, L.; Dong, Y.L.; Lu, S.H. Morphotypes of Prorocentrum lima (Dinophyceae) from Hainan Island, South China Sea: Morphological and molecular characterization. Phycologia 2015, 54, 503–516. [Google Scholar] [CrossRef]
- Wang, J.H.; Wu, J.X. Occurrence and potential risks of harmful algal blooms in the East China Sea. Sci. Total Environ. 2009, 107, 4012–4021. [Google Scholar] [CrossRef]
- Niu, L.X.; Cai, H.Y.; Van Gelder, P.H.A.J.M.; Luo, P.Z.; Liu, F.; Yang, Q.S. Dynamics of polycyclic aromatic hydrocarbons (PAHs) in water column of Pearl River estuary (China): Seasonal pattern, environmental fate and source implication. Appl. Geochem. 2018, 90, 39–49. [Google Scholar] [CrossRef]
- Wu, H.Y.; Luan, Q.S.; Guo, M.M.; Gu, H.F.; Zhai, Y.X.; Tan, Z.J. Phycotoxins in scallops (Patinopecten yessoensis) in relation to source, composition and temporal variation of phytoplankton and cysts in North Yellow Sea, China. Mar. Pollut. Bull. 2018, 135, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.H.; Henry, M.S.; Higham, C.J.; Patricia, B.; Sengco, M.R.; Anderson, D.M. Removal of harmful algal cells (Karenia brevis) and toxins from seawater culture by clay flocculation. Harmful Algae 2004, 3, 141–148. [Google Scholar] [CrossRef]
- Lin, S.; Hsieh, I.J.; Huang, K.M.; Wang, C.H. Influence of the Yangtze River and grain size on the spatial variations of heavy metals and organic carbon in the East China Sea continental shelf sediments. Chem. Geol. 2002, 182, 377–394. [Google Scholar] [CrossRef]
- Yan, W.C.; Chi, J.S.; Wang, Z.Y.; Huang, W.X.; Zhang, G. Spatial and temporal distribution of polycyclic aromatic hydrocarbons (PAHs) in sediments from Daya Bay, South China. Environ. Pollut. 2009, 157, 1823–1830. [Google Scholar] [CrossRef]
- O’Meara, T.; Gibbs, E.; Thrush, S.F. Rapid organic matter assay of organic matter degradation across depth gradients within marine sediments. Methods Ecol. Evol. 2018, 9, 245–253. [Google Scholar] [CrossRef]
| Species | Toxin Profiles | Category | Producing Toxins | Location | Reference |
|---|---|---|---|---|---|
| D. caudata D. acuminata complex | okadaic acid/pectenotoxin | vegetative cell | OA, DTX1, PTX2, and PTX2sa | DYB | [17] |
| P. lima | okadaic acid | vegetative cell | OA and DTX1 | DYB, Hainan Island | [29,30] |
| P. reticulatum L. polyedrum G. spinifera | yessotoxin | cyst | - | the coast of the SCS | [22,31] |
| K. selliformis A. ostenfeldii | gymnodimine | vegetative cell | GYM | Hongkong sea area | [17,28] |
| A. poporum | azaspiracids | cyst | AZA2 and AZA40 | Guangxi sea area, SCS | [25,26] |
| A. ostenfeldii | spirolide | cyst | - | - | [28] |
| Toxin | ESI Polarity | Precursor Ion | Q1 m/z | Q3 m/z | DP | CE | LOD (ng g−1) | LOQ (ng g−1) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | ||||||||
| OA a | ESI − | [M − H]− | 803.5 | 255.0 | −150 | −66 | 0.007 | 0.02 | |
| 112.9 | −150 | −92 | |||||||
| YTX a | ESI − | [M − 2H]2− | 570.5 | 467.3 | −130 | −43 | 0.090 | 0.03 | |
| 396.2 | −130 | −48 | |||||||
| DTX1 a | ESI − | [M − H]− | 817.5 | 255.1 | −180 | −64 | 0.010 | 0.03 | |
| 113.0 | −180 | −100 | |||||||
| homo-YTX a | ESI − | [M − 2H]2− | 577.4 | 474.4 | −130 | −50 | 0.090 | 0.03 | |
| 403.4 | −130 | −50 | |||||||
| DTX2 b | ESI − | [M − H]− | 803.5 | 255.2 | −180 | −64 | 0.010 | 0.03 | |
| 113.1 | −180 | −100 | |||||||
| AZA1 a | ESI + | [M + H]+ | 842.5 | 824.5 | 150 | 47 | 0.035 | 0.11 | |
| 806.3 | 150 | 54 | |||||||
| AZA2 a | ESI + | [M + H]+ | 856.5 | 838.5 | 150 | 47 | 0.080 | 0.03 | |
| 672.4 | 150 | 78 | |||||||
| AZA3 a | ESI + | [M + H]+ | 828.5 | 810.5 | 150 | 47 | 0.080 | 0.03 | |
| 658.4 | 150 | 78 | |||||||
| SPX1 a | ESI + | [M + H]+ | 692.5 | 444.3 | 153 | 51 | 0.020 | 0.06 | |
| 164.2 | 153 | 55 | |||||||
| GYM a | ESI + | [M + H]+ | 508.4 | 490.3 | 135 | 33 | 0.010 | 0.03 | |
| 162.3 | 135 | 49 | |||||||
| PTX2 a | ESI+ | [M + NH4]+ | 876.5 | 823.4 | 150 | 36 | 0.060 | 0.02 | |
| 213.1 | 150 | 44 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, P.; Du, S.; Lin, Z.; Zhou, Y.; Chen, L.; Yu, R.; Zhang, L. Sediment as a Potential Pool for Lipophilic Marine Phycotoxins with the Case Study of Daya Bay of China. Mar. Drugs 2019, 17, 623. https://doi.org/10.3390/md17110623
Liu Y, Zhang P, Du S, Lin Z, Zhou Y, Chen L, Yu R, Zhang L. Sediment as a Potential Pool for Lipophilic Marine Phycotoxins with the Case Study of Daya Bay of China. Marine Drugs. 2019; 17(11):623. https://doi.org/10.3390/md17110623
Chicago/Turabian StyleLiu, Yang, Peng Zhang, Sen Du, Zhuoru Lin, Yanyan Zhou, Lizhao Chen, Rencheng Yu, and Li Zhang. 2019. "Sediment as a Potential Pool for Lipophilic Marine Phycotoxins with the Case Study of Daya Bay of China" Marine Drugs 17, no. 11: 623. https://doi.org/10.3390/md17110623
APA StyleLiu, Y., Zhang, P., Du, S., Lin, Z., Zhou, Y., Chen, L., Yu, R., & Zhang, L. (2019). Sediment as a Potential Pool for Lipophilic Marine Phycotoxins with the Case Study of Daya Bay of China. Marine Drugs, 17(11), 623. https://doi.org/10.3390/md17110623