Antiamoebic Activities of Indolocarbazole Metabolites Isolated from Streptomyces sanyensis Cultures
Abstract
:1. Introduction
2. Results and Discussion
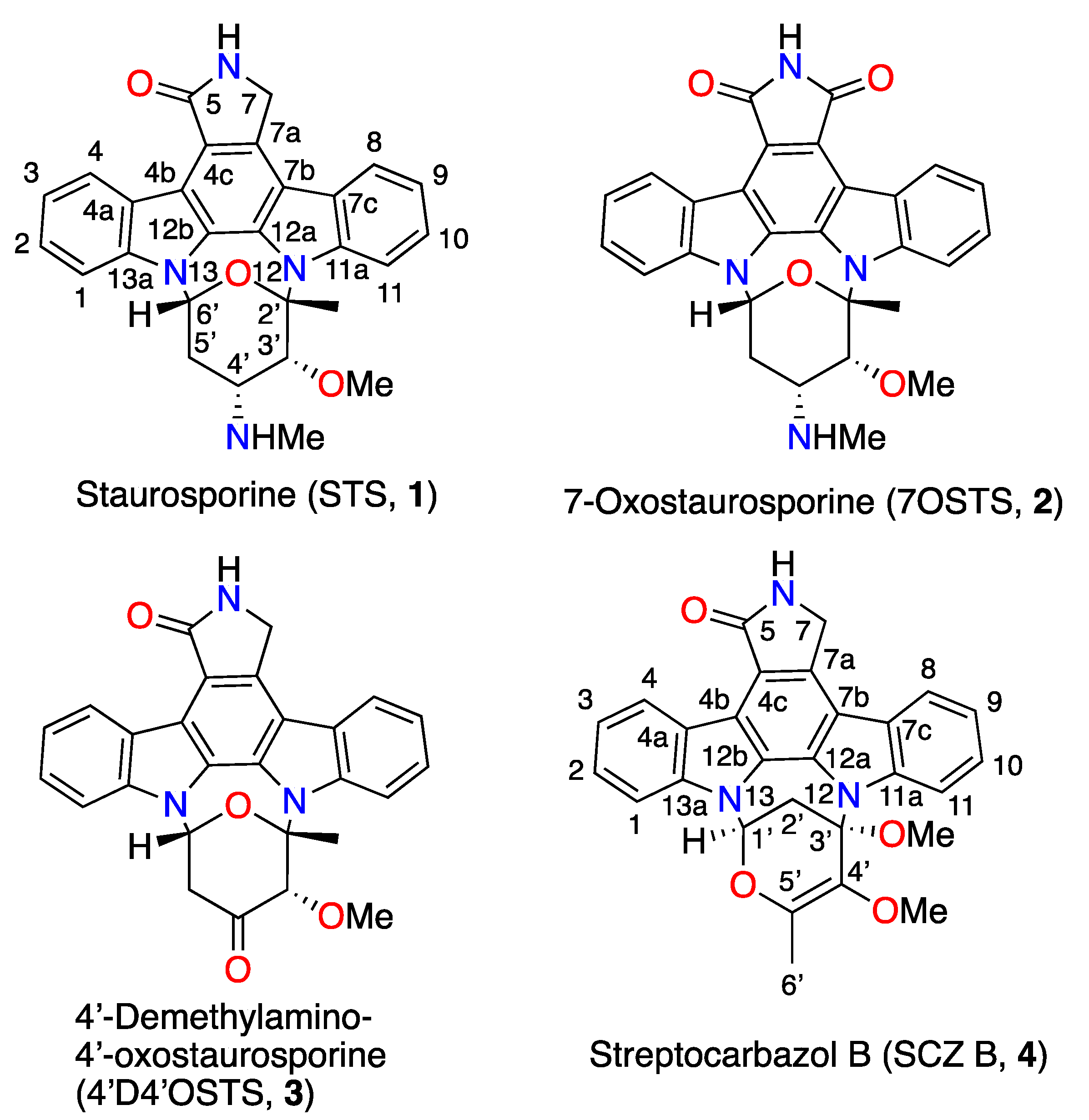
2.1. Strain Isolation, Culture, and Identification of Indolocarbazol Metabolites
2.2. Anti-Acanthamoeba Activity on Parasite Trophozoites
2.3. Cytotoxicity Assays in Murine Macrophages
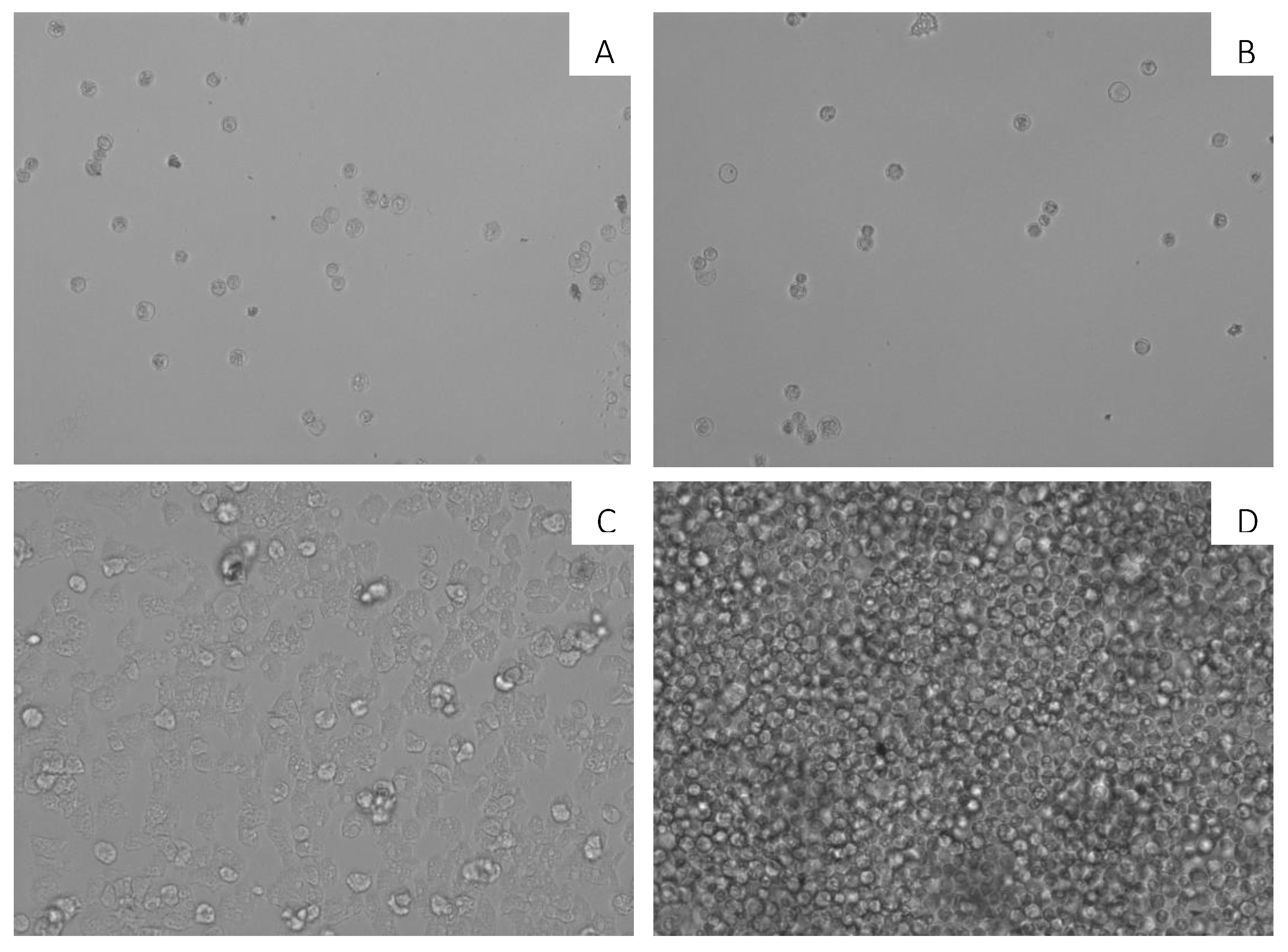
2.4. In Vitro Activity against Acanthamoeba castellanii Neff Cysts
2.5. Evaluation of the Mechanism of Action of 7OSTS
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Cultures and Preparation of Crude Extracts
3.4. Isolation of Indolocarbazole Metabolites
3.4.1. Staurosporine, (STS), (1)
3.4.2. 7-Oxostaurosporine, (7OSTS), (2)
3.4.3. 4’-Demethylamino-4′-oxostaurosporine, (4′D4′OSTS), (3)
3.4.4. Streptocarbazole B, (SCZ B), (4)
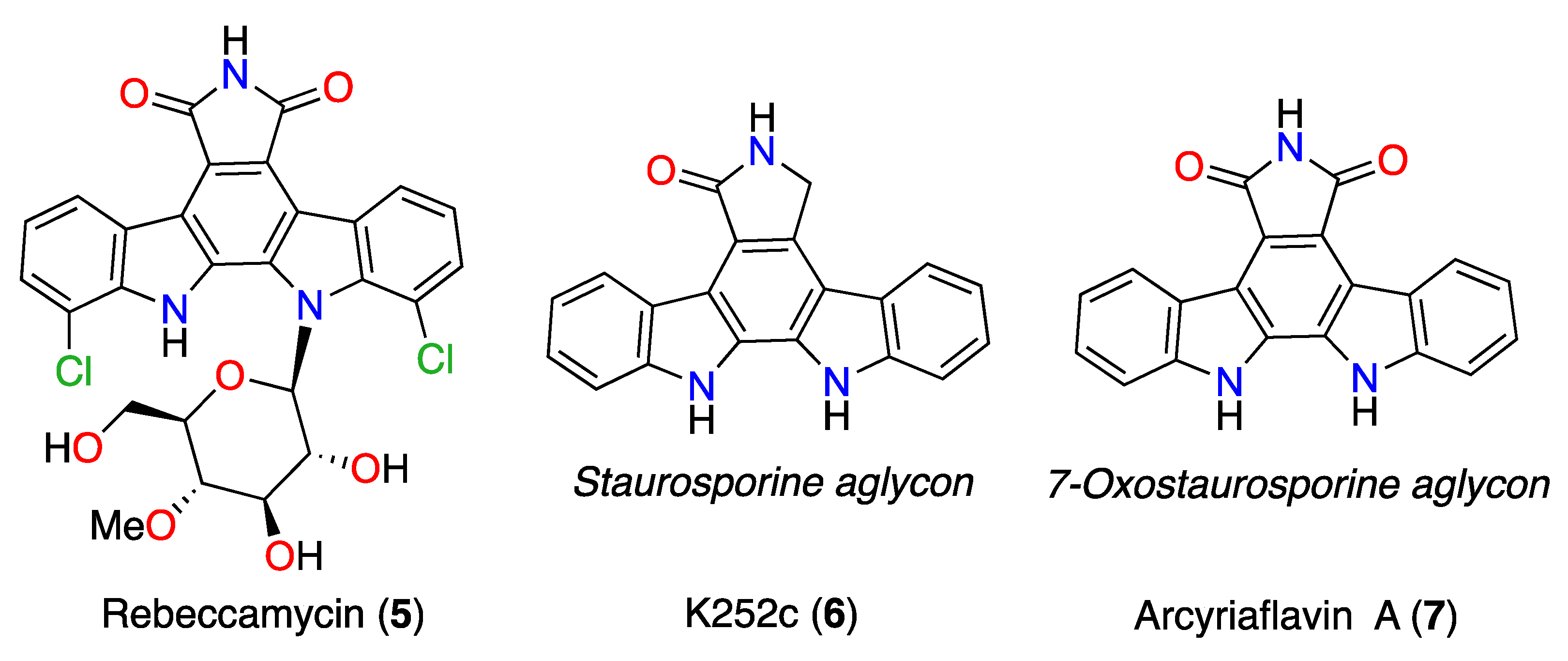
3.5. Commercial ICZ Analogs 5–7
3.6. Cell Culture
3.7. In Vitro Activity Against Acanthamoeba spp. Trophozoites
3.8. In Vitro Activity against Acanthamoeba Castellanii Neff Cysts
3.9. Cytotoxicity Test
3.10. Statistical Analysis
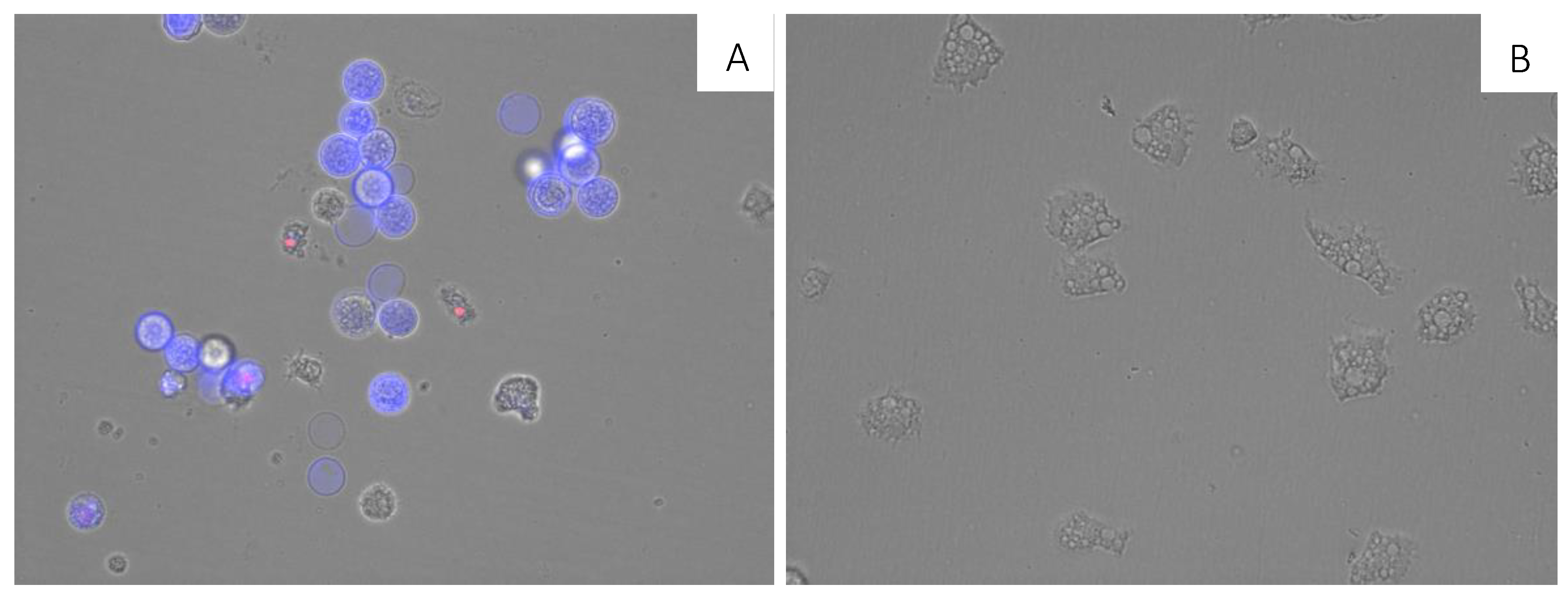
3.11. Double-Stain Assay for Programmed Cell Death Determination
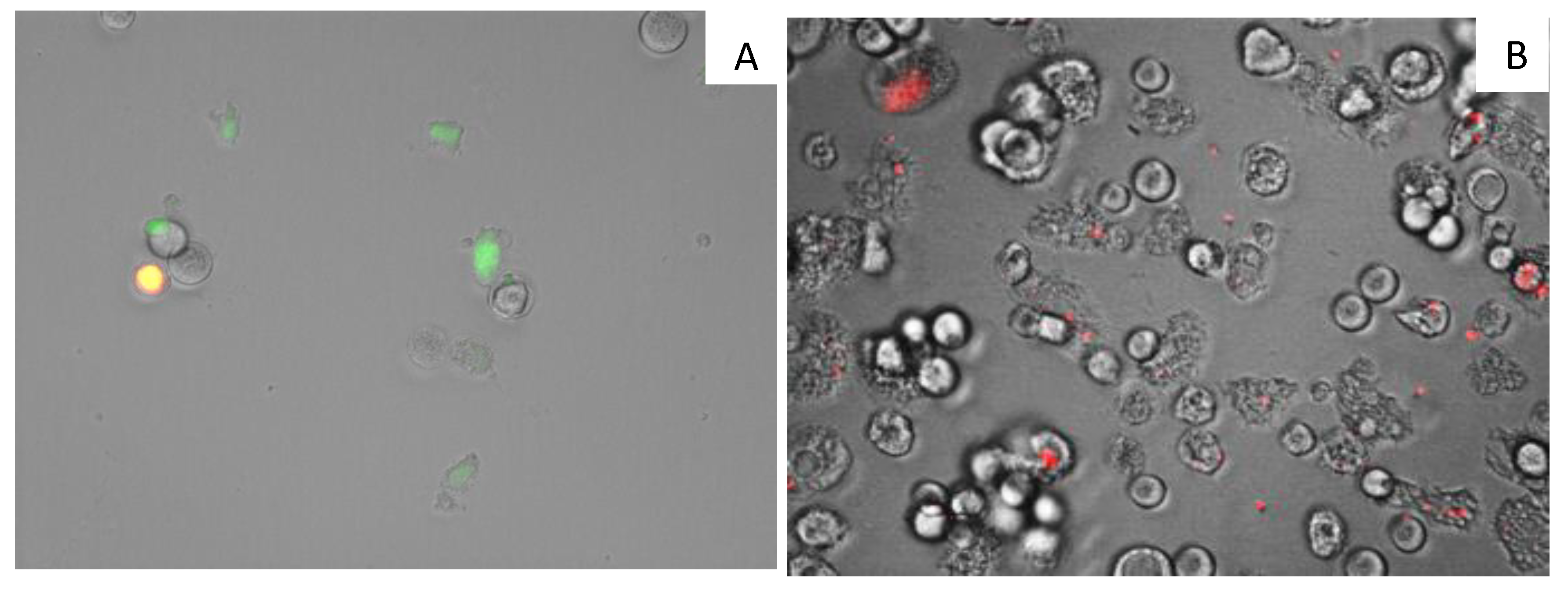
3.12. Analysis of Mitochondrial Membrane Potential
3.13. Measurement of ATP Levels
3.14. Plasma Membrane Permeability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sánchez, C.; Mendez, C.M.; Salas, J.A. Indolocarbazole natural products: Occurrence, biosynthesis, and biological activity. Nat. Prod. Rep. 2006, 23, 1007–1045. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Omura, S. Chemical biology of natural indolocarbazole products: 30 years since the discovery of staurosporine. J. Antibiot. 2009, 62, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanosik, T.; Rannug, A.; Rannug, U.; Whalström, N.; Slätt, J. Chemistry and properties of Indolocarbazoles. Chem. Rev. 2018, 118, 9058–9128. [Google Scholar] [CrossRef] [PubMed]
- Omura, S.; Iwai, Y.; Hirano, A.; Nakagawa, A.; Awaya, J.; Tsuchiya, H.; Takahashi, Y.; Masuma, R. A new alkaloid AM-2282 of Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J. Antibiot. 1977, 30, 275–282. [Google Scholar] [CrossRef]
- Tamaoki, T.; Nomoto, H.; Takahashi, I.; Kato, Y.; Morimoto, M.; Tomita, F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem. Biophys. Res. Commun. 1986, 135, 397–402. [Google Scholar] [CrossRef]
- Lawrie, A.M.; Noble, M.E.M.; Tunnah, P.; Brown, N.R.; Johnson, L.N.; Endicott, J.A. Protein kinase inhibition by staurosporine revealed in details of the molecular interaction with CDK2. Nat. Struct. Biol. 1997, 4, 796–801. [Google Scholar] [CrossRef]
- Kase, H.; Iwahashi, K.; Nakanishi, S.; Matsuda, Y.; Yamada, K.; Takahashi, M.; Murakata, C.; Sato, A.; Kaneko, M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Commun. 1987, 30, 436–440. [Google Scholar] [CrossRef]
- Tapley, P.; Lamballe, F.; Barbacid, M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene 1992, 7, 371–381. [Google Scholar]
- Yamashita, Y.; Fujii, N.; Murakata, C.; Ashizawa, T.; Okabe, M.; Nakano, H. Induction of Mammalian DNA Topoisomerase I Mediated DNA Cleavage by Antitumor Indolocarbazole Derivatives. Biochemistry 1992, 31, 12069–12075. [Google Scholar] [CrossRef]
- Welburn, S.; Macleod, E.; Figarella, K.; Duzensko, M. Programmed cell death in African trypanosomes. Parasitology 2006, 132 (Suppl. 1), S7–S18. [Google Scholar] [CrossRef]
- Yin, J.; Howe, J.; Tan, K. Staurosporine-induced programmed cell death in Blastocystis occurs independently of caspases and cathepsins and is augmented by calpain inhibition. Microbiology 2010, 156, 1284–1293. [Google Scholar] [CrossRef] [Green Version]
- Bruges, G.; Betancourt, M.; March, M.; Sanchez, E.; Mijares, A. Apoptotic-like activity of staurosporine in axenic cultures of Trypanosoma evansi. Rev. Inst. Med. Trop. 2012, 54, 103–118. [Google Scholar] [CrossRef]
- Naula, C.; Parsons, M.; Mottram, J. Protein kinases as drug targets in trypanosomes and Leishmania. Biochim. Biophys. Acta 2005, 1754, 151–159. [Google Scholar] [CrossRef]
- Barth, T.; Bruges, G.; Meiwes, A.; Mogk, S.; Mudogo, C.; Duszenko, M. Staurosporine-Induced Cell Death in Trypanosoma brucei and the Role of Endonuclease G during Apoptosis. Open J. Apoptosis 2014, 3, 16–31. [Google Scholar] [CrossRef]
- Osada, H.; Koshino, H.; Kudo, T.; Onose, R.; Isono, K. A new inhibitor of protein kinase c, rk- 1409 (7-oxostaurosporine) I. Taxonomy and biological activity. J. Antibiot. 1992, 45, 189–194. [Google Scholar] [CrossRef]
- Cai, Y.; Fredenhagen, A.; Hug, P.; Meyer, T. Further minor metabolites of Staurosporine produced by a Streptomyces longisporoflavus strain. J. Antibiot. 1996, 49, 519–526. [Google Scholar] [CrossRef]
- Fu, P.; Yang, C.; Wang, Y.; Liu, P.; Ma, Y.; Xu, L.; Su, M.; Hong, K.; Zhu, W. Streptocarbazoles A and B, Two Novel Indolocarbazoles from the Marine-Derived Actinomycete Strain Streptomyces sp. FMA. Org. Lett. 2012, 14, 2422–2425. [Google Scholar] [CrossRef]
- Cartuche, L.; Sifaoui, I.; Cruz, D.; Reyes-Batlle, M.; Lopez-Arencibia, A.; Fernández, J.J.; Diaz-Marrero, A.R.; Piñero, J.E.; Lorenzo-Morales, J. Staurosporine from Streptomyces sanyensis activates Programmed Cell Death in Acanthamoeba via the mitochondrial pathway and presents low cytotoxicity levels to vertebrate cells. Sci. Rep. 2019, 9, 11651. [Google Scholar] [CrossRef]
- Taravaud, A.; Loiseau, P.M.; Pomel, S. In vitro evaluation of antimicrobial agents on Acanthamoeba sp. and evidence of a natural resilience to amphotericin B. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 328–336. [Google Scholar] [CrossRef]
- García-Davis, S.; Sifaoui, I.; Reyes-Batlle, M.; Viveros-Valdez, E.; Piñero, J.E.; Lorenzo-Morales, J.; Fernández, J.J.; Díaz-Marrero, A.R. Anti-Acanthamoeba Activity of Brominated Sesquiterpenes from Laurencia johnstonii. Mar. Drugs 2018, 16, 443. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Díaz-Marrero, A.R.; Cen-Pacheco, F.; Sifaoui, I.; Reyes-Batlle, M.; Souto, M.L.; Hernández Daranas, A.; Piñero, J.E.; Fernández, J.J. Evaluation of Oxasqualenoids from the Red Alga Laurencia viridis against Acanthamoeba. Mar. Drugs 2019, 17, 420. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowski, S.; Sajid, M.; Reece, S.E. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasites Vectors 2011, 4, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Gani, O.A.S.M.; Engh, R.A. Protein kinase inhibition of clinically important staurosporine analogues. Nat. Prod. Rep. 2010, 27, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Tanramluk, D. On the Origins of Enzyme Inhibitor Selectivity and Promiscuity: A Case of Study of Protein Kinase Binding to Staurosporine. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2009. [Google Scholar]
- Long, B.H.; Rose, W.C.; Vyas, D.M.; Matson, J.A.; Forenza, S. Discovery of antitumor indolocarbazoles: Rebeccamycin, NSC 655649, and fluoroindolocarbazoles. Curr. Med. Chem. Anticancer Agents 2002, 2, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.R.; Belin, L.; Sancelme, M.; Prudhomme, M.; Ollier, M.; Rapp, M.; Sevère, D.; Riou, F.-F.; Fabbro, D.; Meyer, T. Structure-activity relationships in a series of substituted indolocarbazoles: Topoisomerase I and protein kinase C inhibition and antitumoral and antimicrobial properties. J. Med. Chem. 1996, 39, 4471–4477. [Google Scholar] [CrossRef]
- Cinelli, M.A. Topoisomerase 1B poisons: Over a half-century of drug leads, clinical candidates and serendipitous discoveries. Med. Res. Rev. 2019, 39, 1294–1337. [Google Scholar] [CrossRef]
- Bhaduri, S.; Ranjan, N.; Arya, D.P. An overview of recent advances in duplex DNA recognition by small molecules. Beilstein J. Org. Chem. 2018, 14, 1051–1086. [Google Scholar] [CrossRef]
- Sánchez, C.; Zhu, L.; Braña, A.F.; Salas, A.P.; Rohr, J.; Méndez, C.; Salas, J.A. Combinatorial Biosynthesis of Antitumor Indolocarbazole Compounds. Proc. Natl. Acad. Sci. USA 2005, 102, 46–466. [Google Scholar] [CrossRef]
- Bush, J.A.; Long, B.H.; Catino, J.J.; Bradner, W.T.; Tomita, K. Production and biological activity of rebeccamycin, a novel antitumor agent. J. Antibiot. (Tokyo) 1987, 40, 668–678. [Google Scholar] [CrossRef]
- Sanchez-Martinez, C.; Shih, C.; Faul, M.M.; Zhu, G.; Paal, M.; Somoza, C.; Li, T.; Kumrich, C.A.; Winneroski, L.L.; Xun, Z.; et al. Aryl[a]pyrrolo[3,4-c]carbazoles as selective cyclin D1-CDK4 inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 3835–3839. [Google Scholar] [CrossRef]
- González-Robles, A.; Salazar-Villatoro, L.; Omaña-Molina, M.; Reyes-Batlle, M.; Martín-Navarro, C.M.; Lorenzo-Morales, J. Morphological Features and In Vitro Cytopathic Effect of Acanthamoeba griffini Trophozoites Isolated from a Clinical Case. J. Parasitol. Res. 2014, 2014, 256310. [Google Scholar] [CrossRef]
- McBride, J.; Ingram, P.R.; Henriquez, F.L.; Roberts, C.W. Development of colorimetric microtiter plate assay for assessment of antimicrobials against Acanthamoeba. J. Clin. Microbiol. 2005, 43, 629–634. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Kliescikova, J.; Martinez-Carretero, E.; De Pablos, L.M.; Profotova, B.; Nohynkova, E.; Osuna, A.; Valladares, B. Glycogen phosphorylase in Acanthamoeba spp.: Determining the role of the enzyme during the encystment process using RNA interference. Eukaryot. Cell 2008, 7, 509–517. [Google Scholar] [CrossRef]
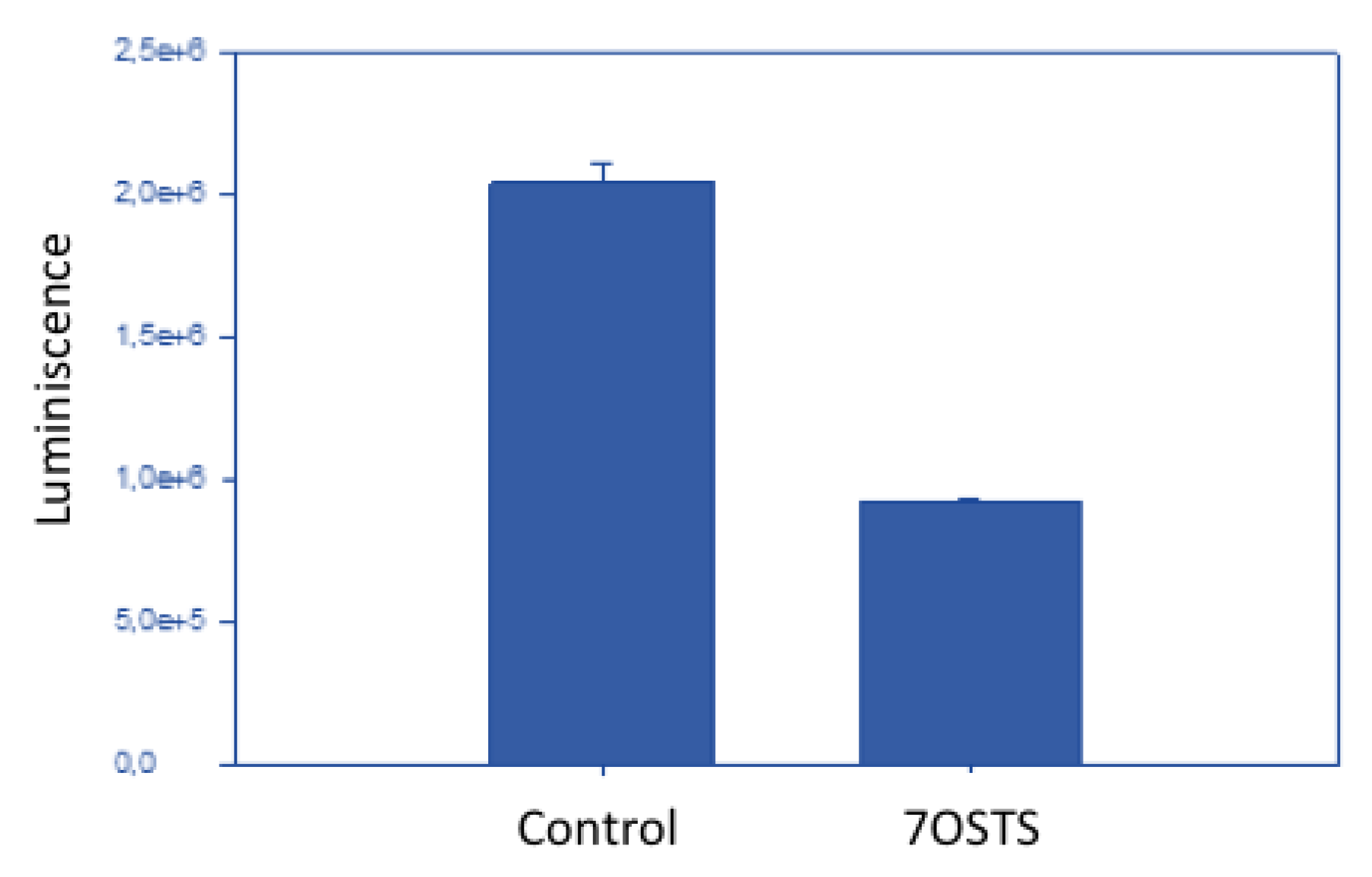


| Compounds | A. castellanii Neff | A. griffini | A. polyphaga |
|---|---|---|---|
| STS (1) [18] | 0.57 ± 0.12 | 0.97± 0.12 | 2.25 ± 0.42 |
| 7OSTS (2) | 0.83 ± 0.09 | 0.96 ± 0.19 | 5.51 ± 0.09 |
| 4’D4’OSTS (3) | 7.53 ± 0.73 | 23.86 ± 0.55 | >40 |
| ICZ B (4) | 6.83 ± 2.60 | 14.51 ±0.38 | >40 |
| Rebeccamycin (5) | 5.37 ± 0.23 | 5.02 ± 0.18 | 7.47 ± 0.61 |
| K252c (6) | 6.17 ± 0.73 | 2.25 ± 0.32 | 5.59 ± 0.34 |
| Arcyriaflavin A (7) | 7.32 ± 0.45 | 7.53 ± 1.11 | 7.59 ± 0.73 |
| Chlorhexidine * | 3.02 ± 0.89 | 3.73 ± 0.98 | 4.76 ± 0.08 |
| Voriconazole * | 0.94 ± 0.29 | 0.10 ± 0.02 | 10.10 ± 2.21 |
| Compounds | Macrophage J774A.1 CC50 (µM) |
|---|---|
| STS (1) | 8.74 ± 0.72 |
| 7OSTS (2) | 5.20 ± 1.75 |
| 4’D4’OSTS (3) | >40 |
| ICZ B (4) | >40 |
| Chlorhexidine * | 7.40 ± 0.39 |
| Voriconazole * | 7.56 ± 0.77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartuche, L.; Reyes-Batlle, M.; Sifaoui, I.; Arberas-Jiménez, I.; Piñero, J.E.; Fernández, J.J.; Lorenzo-Morales, J.; Díaz-Marrero, A.R. Antiamoebic Activities of Indolocarbazole Metabolites Isolated from Streptomyces sanyensis Cultures. Mar. Drugs 2019, 17, 588. https://doi.org/10.3390/md17100588
Cartuche L, Reyes-Batlle M, Sifaoui I, Arberas-Jiménez I, Piñero JE, Fernández JJ, Lorenzo-Morales J, Díaz-Marrero AR. Antiamoebic Activities of Indolocarbazole Metabolites Isolated from Streptomyces sanyensis Cultures. Marine Drugs. 2019; 17(10):588. https://doi.org/10.3390/md17100588
Chicago/Turabian StyleCartuche, Luis, María Reyes-Batlle, Ines Sifaoui, Iñigo Arberas-Jiménez, José E. Piñero, José J. Fernández, Jacob Lorenzo-Morales, and Ana R. Díaz-Marrero. 2019. "Antiamoebic Activities of Indolocarbazole Metabolites Isolated from Streptomyces sanyensis Cultures" Marine Drugs 17, no. 10: 588. https://doi.org/10.3390/md17100588
APA StyleCartuche, L., Reyes-Batlle, M., Sifaoui, I., Arberas-Jiménez, I., Piñero, J. E., Fernández, J. J., Lorenzo-Morales, J., & Díaz-Marrero, A. R. (2019). Antiamoebic Activities of Indolocarbazole Metabolites Isolated from Streptomyces sanyensis Cultures. Marine Drugs, 17(10), 588. https://doi.org/10.3390/md17100588











