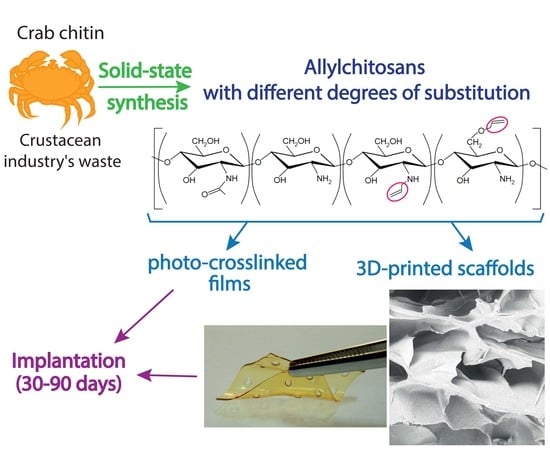
From Aggregates to Porous Three-Dimensional Scaffolds through a Mechanochemical Approach to Design Photosensitive Chitosan Derivatives
Abstract
1. Introduction
2. Results and Discussion
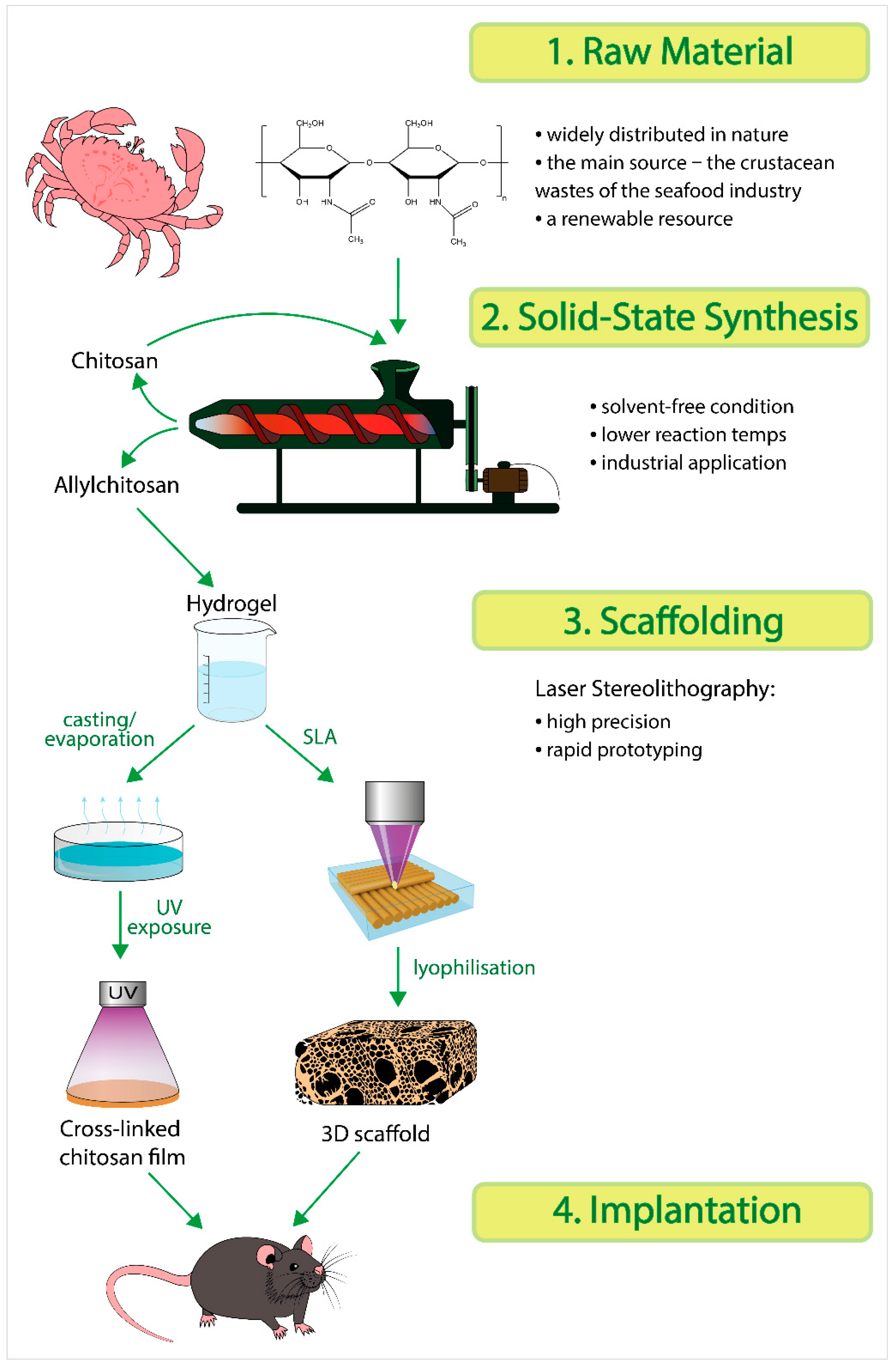
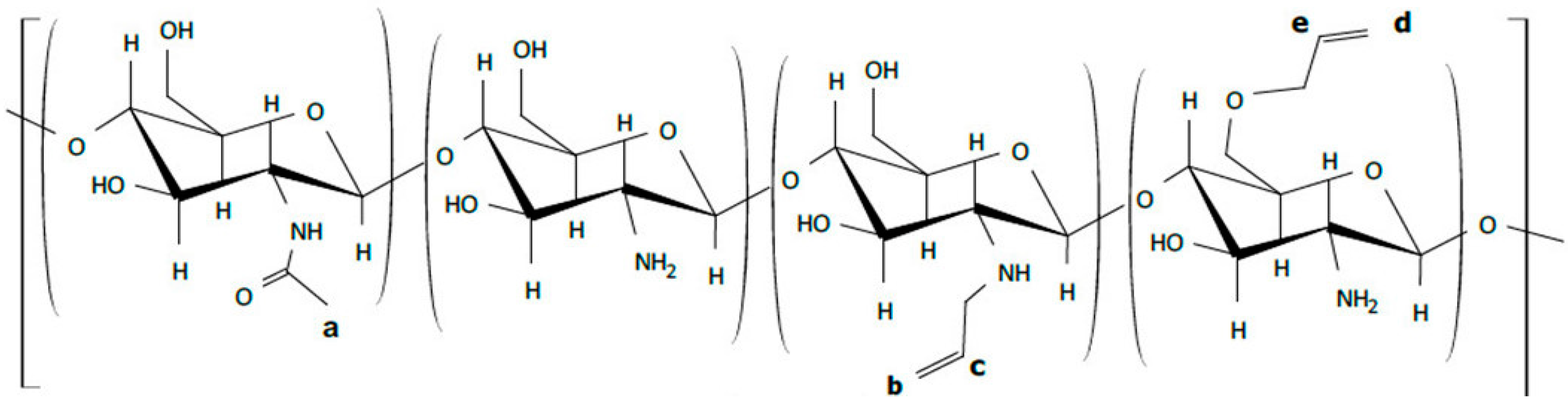
2.1. Synthesis of Allylchitosans (AC) and Their Properties
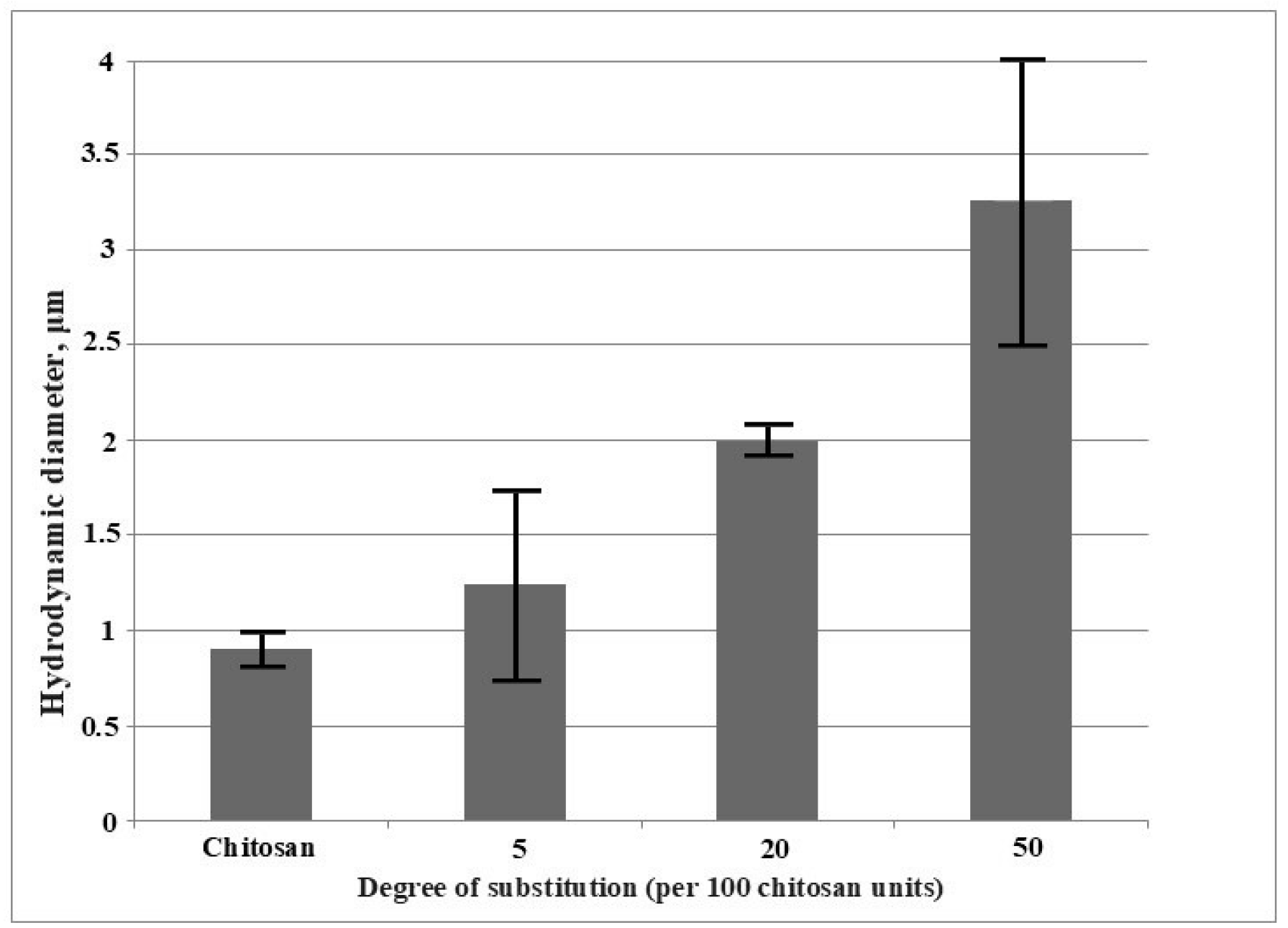
2.1.1. Hydrodynamic Diameter of Aggregates in the Samples in Aqueous Solutions
2.1.2. Mechanical Properties of the Film Samples
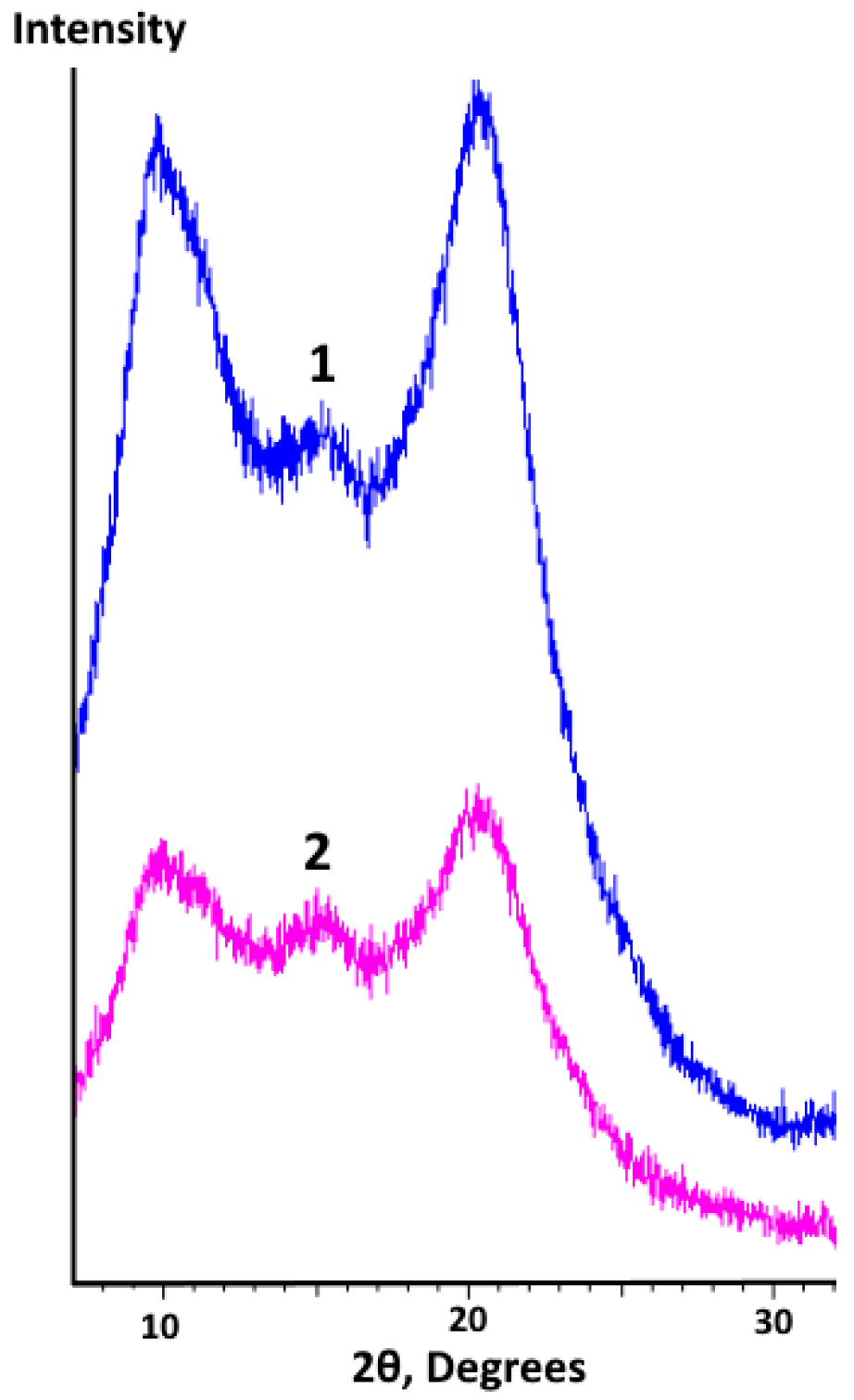
2.1.3. X-Ray Diffraction (XRD) Analysis of Film Samples
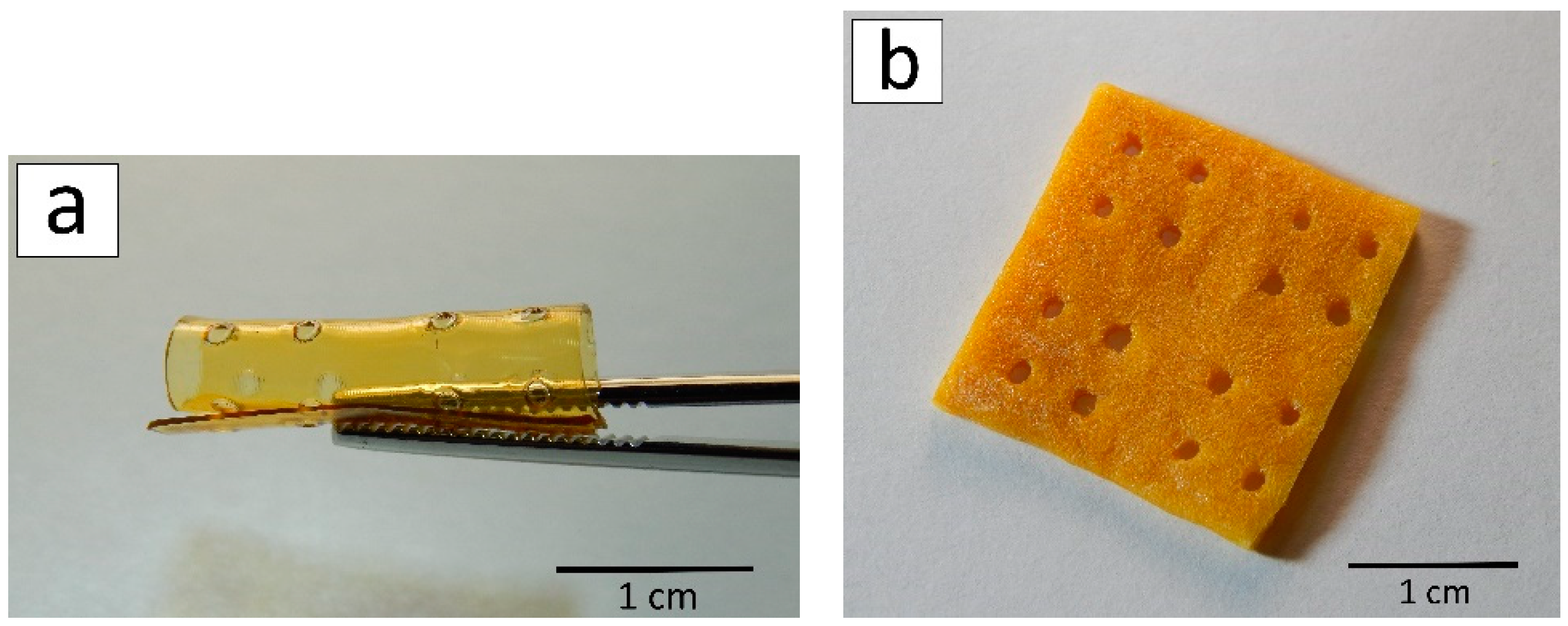
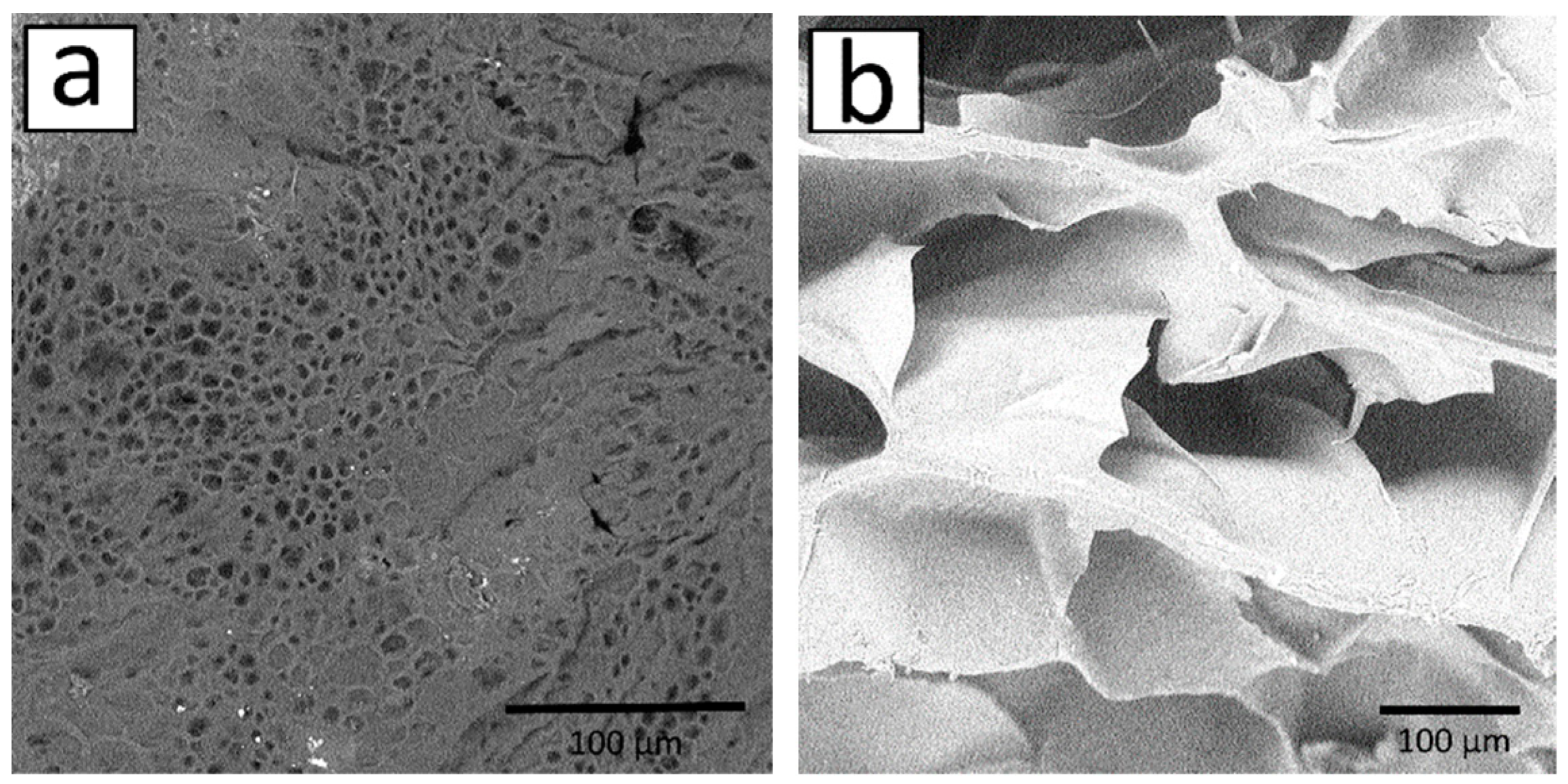
2.2. Three-Dimensional Scaffolds
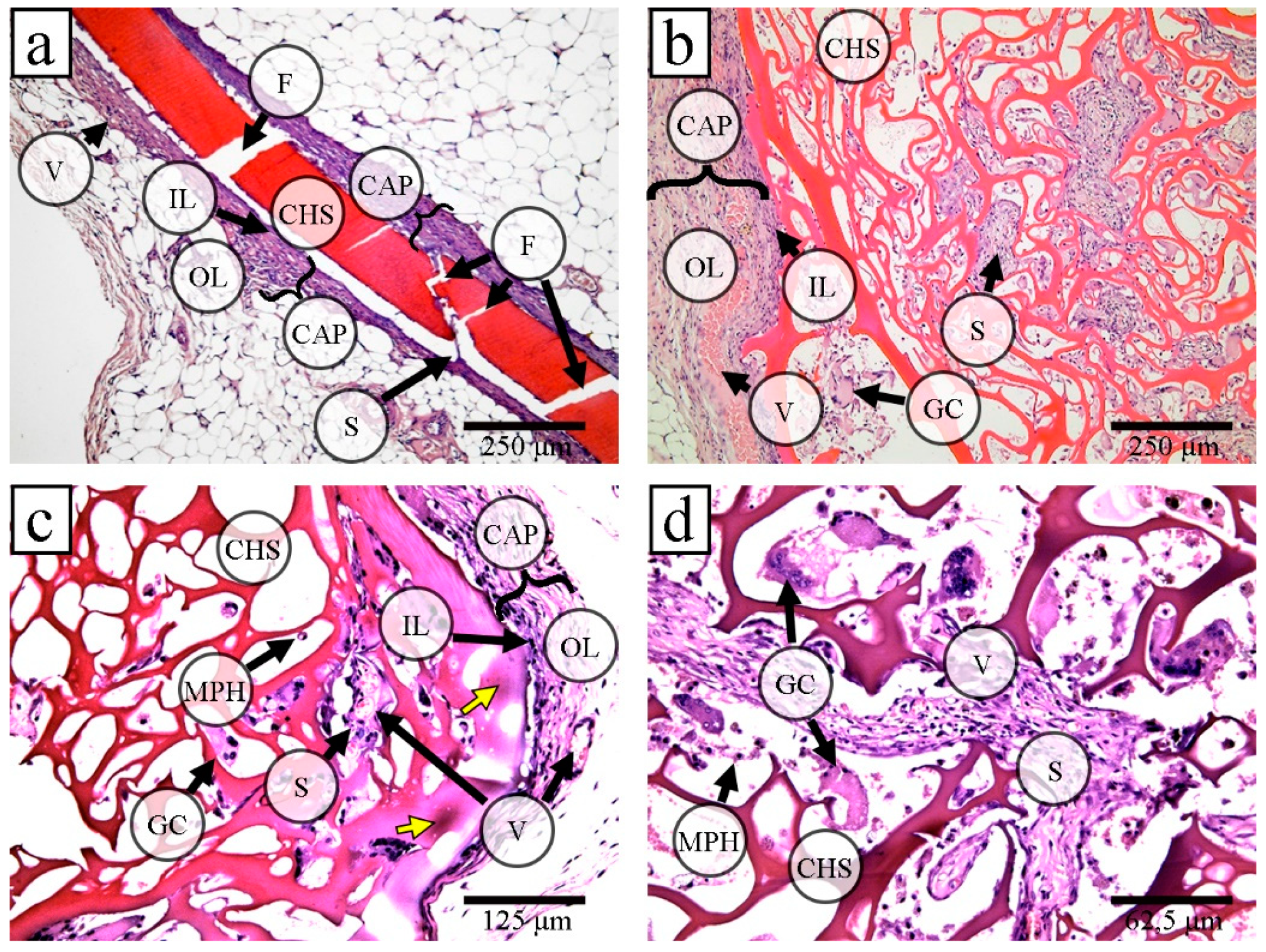
2.3. Implantation of Films and Porous 3D Scaffolds based on Allylchitosans
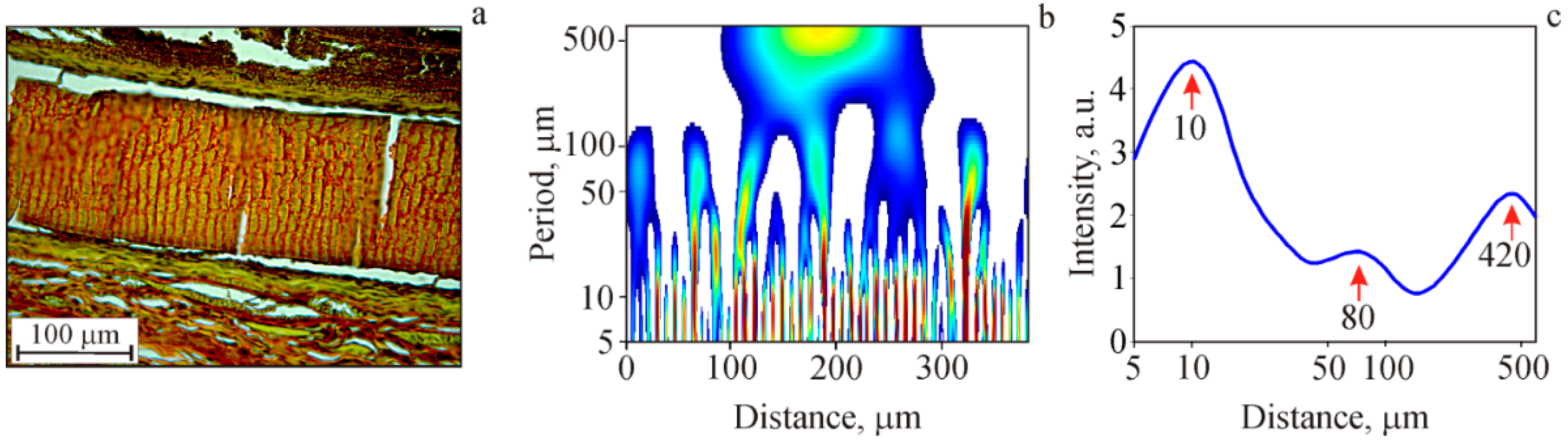
Internal Structure of Film Samples
3. Materials and Methods
3.1. Synthesis of Allylchitosans
3.2. Preparation of Films and 3D Scaffolds
3.3. Characterization of Film Samples and 3D Scaffolds
3.3.1. Hydrodynamic Diameter of Samples in Aqueous Solutions
3.3.2. Mechanical Properties of Film Samples
3.3.3. XRD Analysis
3.3.4. SEM and the Pore Surface Area Estimation
3.4. Implantation
3.5. Histological Analysis
Identification of the Internal Film Structure (Wavelet Analysis)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sayari, N.; Sila, A.; Abdelmalek, B.E.; Abdallah, R.B.; Ellouz-Chaabouni, S.; Bougatef, A.; Balti, R. Chitin and chitosan from the Norway lobster by-products: Antimicrobial and anti-proliferative activities. Int. J. Biol. Macromol. 2016, 87, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Thapa, B.; Narain, R. Mechanism, current challenges and new approaches for non viral gene delivery. Polym. Nanomater. Gene Ther. 2016, 1–27. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed. Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Peña, A.; Sánchez, N.S.; Calahorra, M. Effects of chitosan on Candida albicans: Conditions for its antifungal activity. BioMed Res. Int. 2013, 2013, 527549. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of chitosan-a challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Leithner, K.; Bernkop-Schnürch, A. Chitosan and Derivatives for Biopharmaceutical Use: Mucoadhesive Properties. Chitosan-Based Syst. Biopharm. Deliv. Target. Polym. Ther. 2012, 159–180. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Muzzarelli, C. Chitosan Chemistry: Relevance to the Biomedical Sciences. In Polysaccharides I; Springer: Berlin/Heidelberg, Germany, 2005; pp. 151–209. [Google Scholar] [CrossRef]
- Prabaharan, M. Review Paper: Chitosan Derivatives as Promising Materials for Controlled Drug Delivery. J. Biomater. Appl. 2008, 23, 5–36. [Google Scholar] [CrossRef]
- Yadav, H.K.S.; Joshi, G.B.; Singh, M.N.; Shivakumar, H.G. Naturally Occurring Chitosan and Chitosan Derivatives: A Review. Curr. Drug Ther. 2011, 6, 2–11. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Ahmed, J.; Roos, Y.H.; Rahman, S.; Ray, S.S. Glass Transition and Phase Transitions in Food and Biological Materials. n.d. Available online: https://www.wiley.com/en-us/Glass+Transition+and+Phase+Transitions+in+Food+and+Biological+Materials-p-9781118935729 (accessed on 6 July 2018).
- Ball, R.; Bajaj, P.; Whitehead, K. Achieving long-term stability of lipid nanoparticles: Examining the effect of pH, temperature, and lyophilization. Int. J. Nanomed. 2016, 12, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Aljaeid, B. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef]
- Jana, S.; Jana, S. Particulate Technology for Delivery of Therapeutics; Springer: New York, NY, USA, 2017; Available online: https://books.google.ru/books?id=xng5DwAAQBAJ&dq=Sougata,+and+Subrata+Jana,+eds.+Particulate+Technology+for+Delivery+of+Therapeutics.+Springer,+2017&hl=ru&source=gbs_navlinks_s (accessed on 6 July 2018).
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Lyakhov, N.Z.; Grigorieva, T.F.; Barinova, A.P.; Vorsina, I.A. Mechanochemical synthesis of organic compounds and composites with their participation. Russ. Chem. Rev. 2010, 79, 189–203. [Google Scholar] [CrossRef]
- Prut, E.V.; Zelenetskii, A.N. Chemical modification and blending of polymers in an extruder reactor. Russ. Chem. Rev. 2001, 70, 65–79. [Google Scholar] [CrossRef]
- Akopova, T.A.; Demina, T.S.; Zelenetskii, A.N. Amphiphilic systems based on polysaccharides produced by solid-phase synthesis—A review. Fibre Chem. 2012, 44, 217–220. [Google Scholar] [CrossRef]
- Ferguson, A.N.; O’Neill, A.G. Focus on Chitosan Research; Nova Science Publishers: Hauppauge, NY, USA, 2011; Available online: https://www.novapublishers.com/catalog/product_info.php?products_id=33937 (accessed on 19 July 2018).
- Crawford, D.E.; Casaban, J. Recent Developments in Mechanochemical Materials Synthesis by Extrusion. Adv. Mater. 2016, 28, 5747–5754. [Google Scholar] [CrossRef]
- Timashev, P.S.; Bardakova, K.N.; Minaev, N.V.; Demina, T.S.; Mishchenko, T.A.; Mitroshina, E.V.; Akovantseva, A.A.; Koroleva, A.V.; Asyutin, D.S.; Pimenova, L.F.; et al. Compatibility of cells of the nervous system with structured biodegradable chitosan-based hydrogel matrices. Appl. Biochem. Microbiol. 2016, 52, 508–514. [Google Scholar] [CrossRef]
- Demina, T.S.; Bardakova, K.N.; Minaev, N.V.; Svidchenko, E.A.; Istomin, A.V.; Goncharuk, G.P.; Vladimirov, L.V.; Grachev, A.V.; Zelenetskii, A.N.; Timashev, P.S.; et al. Two-photon-induced microstereolithography of chitosan-g-oligolactides as a function of their stereochemical composition. Polymers 2017, 9, 302. [Google Scholar] [CrossRef]
- Timashev, P.S.; Bardakova, K.N.; Demina, T.S.; Pudovkina, G.I.; Novikov, M.M.; Markov, M.A.; Asyutin, D.S.; Pimenova, L.F.; Svidchenko, E.M.; Ermakov, A.M.; et al. Novel biocompatible material based on solid-state modified chitosan for laser stereolithography. Sovrem. Tehnol. Med. 2015, 7. [Google Scholar] [CrossRef]
- Akopova, T.A.; Demina, T.S.; Bagratashvili, V.N.; Bardakova, K.N.; Novikov, M.M.; Selezneva, I.I.; Istomin, A.V.; Svidchenko, E.A.; Cherkaev, G.V.; Surin, N.M.; et al. Solid state synthesis of chitosan and its unsaturated derivatives for laser microfabrication of 3D scaffolds. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012079. [Google Scholar] [CrossRef]
- Nud’ga, L.A.; Petrova, V.A.; Lebedeva, M.F. Effect of Allyl Substitution in Chitosan on the Structure of Graft Copolymers. Russ. J. Appl. Chem. 2003, 76, 1978–1982. [Google Scholar] [CrossRef]
- VPhilippova, I.; Andreeva, O.E.; Khokhlov, A.S.; Islamov, A.R.; Kuklin, A.K.; Gordeliy, A.I. Charge-Induced Microphase Separation in Polyelectrolyte Hydrogels with Associating Hydrophobic Side Chains: Small-Angle Neutron Scattering Study. Langmuir 2003, 19, 7240–7248. [Google Scholar] [CrossRef]
- Kurisawa, M.; Yokoyama, M.; Okano, T. Transfection efficiency increases by incorporating hydrophobic monomer units into polymeric gene carriers. J. Control. Release 2000, 68, 1–8. [Google Scholar] [CrossRef]
- Popa-Nita, S.; Alcouffe, P.; Rochas, C.; David, L.; Domard, A. Continuum of structural organization from chitosan solutions to derived physical forms. Biomacromolecules 2010, 11, 6–12. [Google Scholar] [CrossRef]
- Ottøy, M.H.; Vårum, K.M.; Christensen, B.E.; Anthonsen, M.W.; Smidsrød, O. Preparative and analytical size-exclusion chromatography of chitosans. Carbohydr. Polym. 1996, 31, 253–261. [Google Scholar] [CrossRef]
- Schatz, C.; Pichot, C.; Delair, T.; Viton, C.; Domard, A. Static Light Scattering Studies on Chitosan Solutions: From Macromolecular Chains to Colloidal Dispersions. Langmuir 2003, 19, 9896–9903. [Google Scholar] [CrossRef]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the Factors Affecting the Solubility of Chitosan in Water. Macromol. Chem. Phys. 2010, 211, 426–433. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Souza, B.W.S.; Cerqueira, M.A.; Martins, J.T.; Casariego, A.; Teixeira, J.A.; Vicente, A.A. Influence of electric fields on the structure of chitosan edible coatings. Food Hydrocoll. 2010, 24, 330–335. [Google Scholar] [CrossRef]
- Khan, M.A.; Ferdous, S.; Mustafa, A.I. Improvement of physico-mechanical properties of chitosan films by photocuring with acrylic monomers. J. Polym. Environ. 2005, 13, 193–201. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef]
- Kim, K.M.; Son, J.H.; Kim, S.-K.; Weller, C.L.; Hanna, M.A. Properties of Chitosan Films as a Function of pH and Solvent Type. J. Food Sci. 2006, 71, E119–E124. [Google Scholar] [CrossRef]
- Dong, Y.; Sakurai, K.; Wu, Y.; Kondo, Y. Multiple crystalline morphologies of N-Alkyl chitosan solution cast films. Polym. Bull. 2002, 49, 189–195. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.A.; Pinotti, A. Correlations between structural, barrier, thermal and mechanical properties of plasticized gelatin films. Innov. Food Sci. Emerg. Technol. 2010, 11, 369–375. [Google Scholar] [CrossRef]
- Bangyekan, C.; Aht-Ong, D.; Srikulkit, K. Preparation and properties evaluation of chitosan-coated cassava starch films. Carbohydr. Polym. 2006, 63, 61–71. [Google Scholar] [CrossRef]
- Rotta, J.; Minatti, E.; Barreto, P.L.M. Determination of structural and mechanical properties, diffractometry, and thermal analysis of chitosan and hydroxypropylmethylcellulose (HPMC) films plasticized with sorbitol. Food Sci. Technol. 2011, 31, 450–455. [Google Scholar] [CrossRef]
- Modrzejewska, Z.; Maniukiewicz, W.; Wojtasz-Pająk, A. Determination of hydrogel chitosan membrane structure. Pol. Chitin Soc. Monogr. XI 2006, 113–121. [Google Scholar]
- Wan, D.; Wu, Y.; Yu, H.; Wen, A. Biodegradable Polylactide/Chitosan Blend Membranes. Biomacromolecules 2006, 7, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Yahya, M.Z.; Harun, M.K.; Ali, A.M.; Mohammat, M.F.; Hanafiah, M.A.; Ibrahim, S.C.; Mustaffa, M.; Darus, Z.M.; Latif, F. XRD and Surface Morphology Studies on Chitosan-Based Film Electrolytes. J. Appl. Sci. 2006, 6, 3150–3154. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Wallace, J.; Kim, S.Y.; Kalyanasundaram, S.; Andalman, A.S.; Davidson, T.J.; Mirzabekov, J.J.; Zalocusky, K.A.; Mattis, J.; Denisin, A.K.; et al. Structural and molecular interrogation of intact biological systems. Nature 2013, 497, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Do, A.; Khorsand, B.; Geary, S.M.; Salem, A.K.; Therapeutics, E.; City, I. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Sands, R.W.; Bhatta, D.; Arany, P.; Verbeke, C.S.; Edwards, D.A. Injectable preformed scaffolds with shape-memory properties. Proc. Natl. Acad. Sci. USA 2012, 109, 19590–19595. [Google Scholar] [CrossRef]
- Zhang, H.; Hussain, I.; Brust, M.; Butler, M.F.; Rannard, S.P.; Cooper, A.I. Aligned two- and three-dimensional structures by directional freezing of polymers and nanoparticles. Nat. Mater. 2005, 4, 787–793. [Google Scholar] [CrossRef]
- Farrugia, B.L.; Whitelock, J.M.; Jung, M.; McGrath, B.; O’Grady, R.L.; McCarthy, S.J.; Lord, M.S. The localisation of inflammatory cells and expression of associated proteoglycans in response to implanted chitosan. Biomaterials 2014, 35, 1462–1477. [Google Scholar] [CrossRef]
- Kwak, B.K.; Shim, H.J.; Han, S.-M.; Park, E.S. Chitin-based Embolic Materials in the Renal Artery of Rabbits: Pathologic Evaluation of an Absorbable Particulate Agent. Radiology 2005, 236, 151–158. [Google Scholar] [CrossRef]
- VandeVord, P.J.; Matthew, H.W.T.; DeSilva, S.P.; Mayton, L.; Wu, B.; Wooley, P.H. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 2002, 59, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Tator, C.H.; Shoichet, M.S. Chitosan implants in the rat spinal cord: Biocompatibility and biodegradation. J. Biomed. Mater. Res. Part A 2011, 97, 395–404. [Google Scholar] [CrossRef]
- Peluso, G.; Petillo, O.; Ranieri, M.; Santin, M.; Ambrosic, L.; Calabró, D.; Avallone, B.; Balsamo, G. Chitosan-mediated stimulation of macrophage function. Biomaterials 1994, 15, 1215–1220. [Google Scholar] [CrossRef]
- Tomihata, K.; Ikada, Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 1997, 18, 567–575. [Google Scholar] [CrossRef]
- Barbosa, J.N.; Amaral, I.F.; Águas, A.P.; Barbosa, M.A. Evaluation of the effect of the degree of acetylation on the inflammatory response to 3D porous chitosan scaffold. J. Biomed. Mater. Res. Part A 2009, 93, 20–28. [Google Scholar] [CrossRef]
- Hartl, L.; Zach, S.; Seidl-Seiboth, V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2012, 93, 533–543. [Google Scholar] [CrossRef]
- Park, C.J.; Gabrielson, N.P.; Pack, D.W.; Jamison, R.D.; Johnson, A.J.W. The effect of chitosan on the migration of neutrophil-like HL60 cells, mediated by IL-8. Biomaterials 2009, 30, 436–444. [Google Scholar] [CrossRef]
- Simard, P.; Galarneau, H.; Marois, S.; Rusu, D.; Hoemann, C.D.; Poubelle, P.E.; El-Gabalawy, H.; Fernandes, M.J. Neutrophils exhibit distinct phenotypes toward chitosans with different degrees of deacetylation: Implications for cartilage repair. Arthritis Res. Ther. 2009, 11, R74. [Google Scholar] [CrossRef]
- Nishimura, K.; Nishimura, S.; Nishi, N.; Saiki, I.; Tokura, S.; Azuma, I. Immunological activity of chitin and its derivatives. Vaccine 1984, 2, 93–99. [Google Scholar] [CrossRef]
- Hwang, H.-G.; Choi, H.-J.; Lee, B.-I.; Yoon, H.-K.; Nam, S.-H.; Choi, H.-K. Multi-resolution wavelet-transformed image analysis of histological sections of breast carcinomas. Anal. Cell. Oncol. 2005, 27, 237–244. [Google Scholar]
- Astaf’eva, N.M. Wavelet analysis: Basic theory and some applications. Uspekhi Fizicheskikh Nauk 1996, 166, 1145–1170. [Google Scholar] [CrossRef]
- Park, H.; Choi, B.; Nguyen, J.; Fan, J.; Shafi, S.; Klokkevold, P.; Lee, M. Anionic carbohydrate-containing chitosan scaffolds for bone regeneration. Carbohydr. Polym. 2013, 97, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Rogovina, S.Z.; Akopova, T.A.; Vikhoreva, G.A. Investigation of properties of chitosan obtained by solid-phase and suspension methods. J. Appl. Polym. Sci. 1998, 70, 927–933. [Google Scholar] [CrossRef]
- Aleksandrov, A.I.; Akopova, T.A.; Shevchenko, V.G.; Cherkaev, G.V.; Degtyarev, E.N. A Biocompatible Nanocomposite Based on Allyl Chitosan and Vinyltriethoxysilane for Tissue Engineering. Popym. Sci. Ser. B 2017, 59, 97–108. [Google Scholar] [CrossRef]
- Serra, J.P. Image Analysis and Mathematical Morphology; Academic Press: Cambridge, MA, USA, 1982; Available online: https://dl.acm.org/citation.cfm?id=1098652 (accessed on 15 July 2018).
- Schneider, O.D.; Mohn, D.; Fuhrer, R.; Klein, K.; Kämpf, K.; Nuss, K.M.; Sidler, M.; Zlinszky, K.; von Rechenberg, B.; Stark, W.J. Biocompatibility and Bone Formation of Flexible, Cotton Wool-like PLGA/Calcium Phosphate Nanocomposites in Sheep. Open Orthop. J. 2011, 5, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Melman, L.; Jenkins, E.D.; Hamilton, N.A.; Bender, L.C.; Brodt, M.D.; Deeken, C.R.; Greco, S.C.; Frisella, M.M.; Matthews, B.D. Histologic and biomechanical evaluation of a novel macroporous polytetrafluoroethylene knit mesh compared to lightweight and heavyweight polypropylene mesh in a porcine model of ventral incisional hernia repair. Hernia 2011, 15, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Bagratashvili, V.N.; Rybaltovsky, A.O.; Minaev, N.V.; Timashev, P.S.; Firsov, V.V.; Yusupov, V.I. Laser-induced atomic assembling of periodic layered nanostructures of silver nanoparticles in fluoro-polymer film matrix. Laser Phys. Lett. 2010, 7, 401–404. [Google Scholar] [CrossRef]
| Sample | DS (mol%) | Before UV Exposure | After UV Exposure | ||||
|---|---|---|---|---|---|---|---|
| σ (MPa) | E (MPa) | ε (%) | σ (MPa) | E (MPa) | ε (%) | ||
| Chitosan | 0 | 37 ± 2 | 1800 ± 200 | 18 ± 3 | 39 ± 3 | 1900 ± 200 | 18 ± 3 |
| AC2 | 5 | 37 ± 2 | 1800 ± 200 | 26 ± 3 | 41 ± 3 | 1900 ± 100 | 21 ± 3 |
| AC4 | 20 | 38 ± 2 | 2100 ± 200 | 25 ± 3 | 38 ± 2 | 1800 ± 200 | 19 ± 3 |
| AC5 | 50 | 33 ± 2 | 1900 ± 200 | 23 ± 3 | 33 ± 2 | 1400 ± 200 | 17 ± 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardakova, K.N.; Akopova, T.A.; Kurkov, A.V.; Goncharuk, G.P.; Butnaru, D.V.; Burdukovskii, V.F.; Antoshin, A.A.; Farion, I.A.; Zharikova, T.M.; Shekhter, A.B.; et al. From Aggregates to Porous Three-Dimensional Scaffolds through a Mechanochemical Approach to Design Photosensitive Chitosan Derivatives. Mar. Drugs 2019, 17, 48. https://doi.org/10.3390/md17010048
Bardakova KN, Akopova TA, Kurkov AV, Goncharuk GP, Butnaru DV, Burdukovskii VF, Antoshin AA, Farion IA, Zharikova TM, Shekhter AB, et al. From Aggregates to Porous Three-Dimensional Scaffolds through a Mechanochemical Approach to Design Photosensitive Chitosan Derivatives. Marine Drugs. 2019; 17(1):48. https://doi.org/10.3390/md17010048
Chicago/Turabian StyleBardakova, Kseniia N., Tatiana A. Akopova, Alexander V. Kurkov, Galina P. Goncharuk, Denis V. Butnaru, Vitaliy F. Burdukovskii, Artem A. Antoshin, Ivan A. Farion, Tatiana M. Zharikova, Anatoliy B. Shekhter, and et al. 2019. "From Aggregates to Porous Three-Dimensional Scaffolds through a Mechanochemical Approach to Design Photosensitive Chitosan Derivatives" Marine Drugs 17, no. 1: 48. https://doi.org/10.3390/md17010048
APA StyleBardakova, K. N., Akopova, T. A., Kurkov, A. V., Goncharuk, G. P., Butnaru, D. V., Burdukovskii, V. F., Antoshin, A. A., Farion, I. A., Zharikova, T. M., Shekhter, A. B., Yusupov, V. I., Timashev, P. S., & Rochev, Y. A. (2019). From Aggregates to Porous Three-Dimensional Scaffolds through a Mechanochemical Approach to Design Photosensitive Chitosan Derivatives. Marine Drugs, 17(1), 48. https://doi.org/10.3390/md17010048