Differences between Motile and Nonmotile Cells of Haematococcus pluvialis in the Production of Astaxanthin at Different Light Intensities
Abstract
:1. Introduction
2. Results
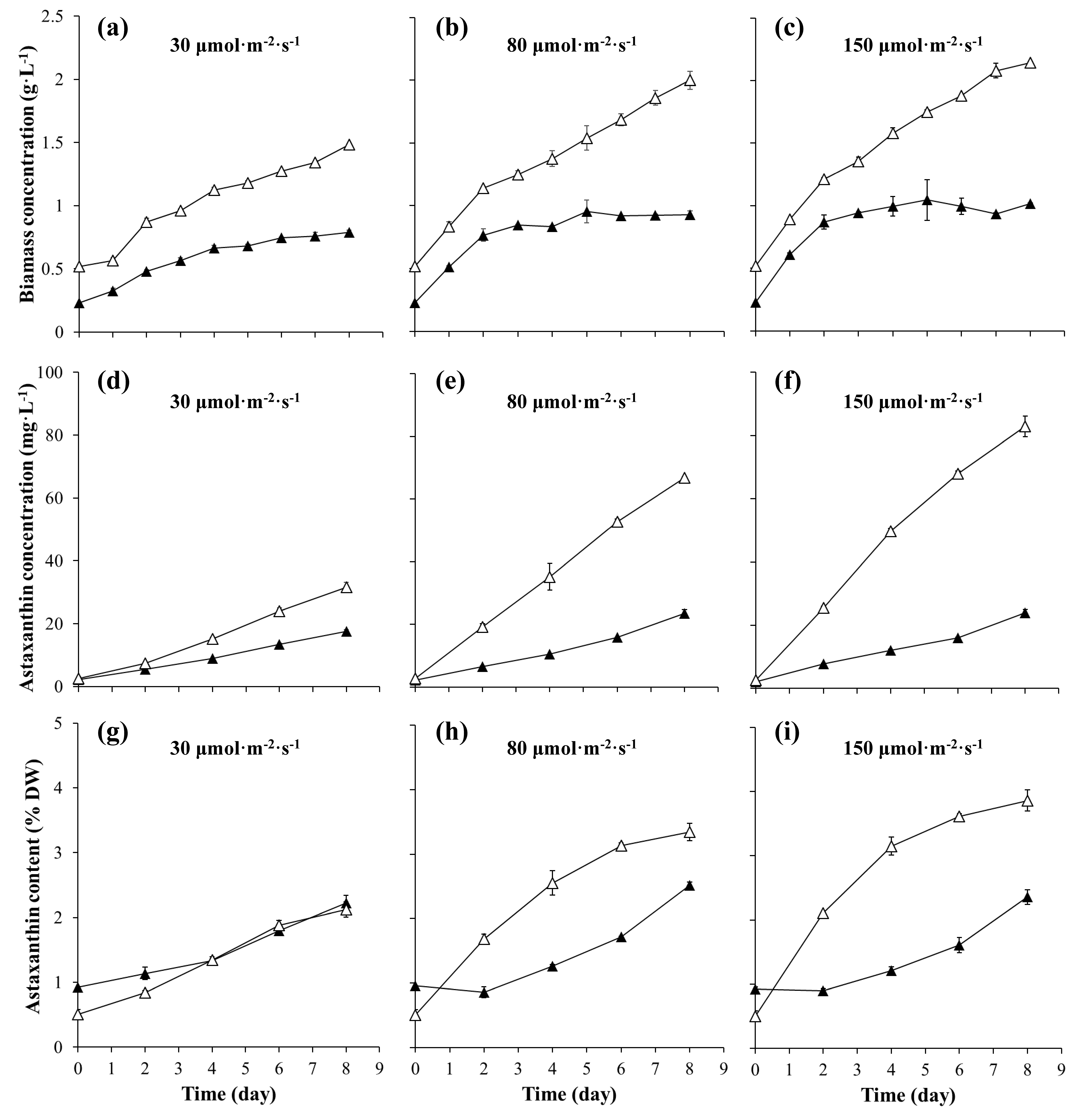
2.1. The Accumulation of Biomass and Astaxanthin
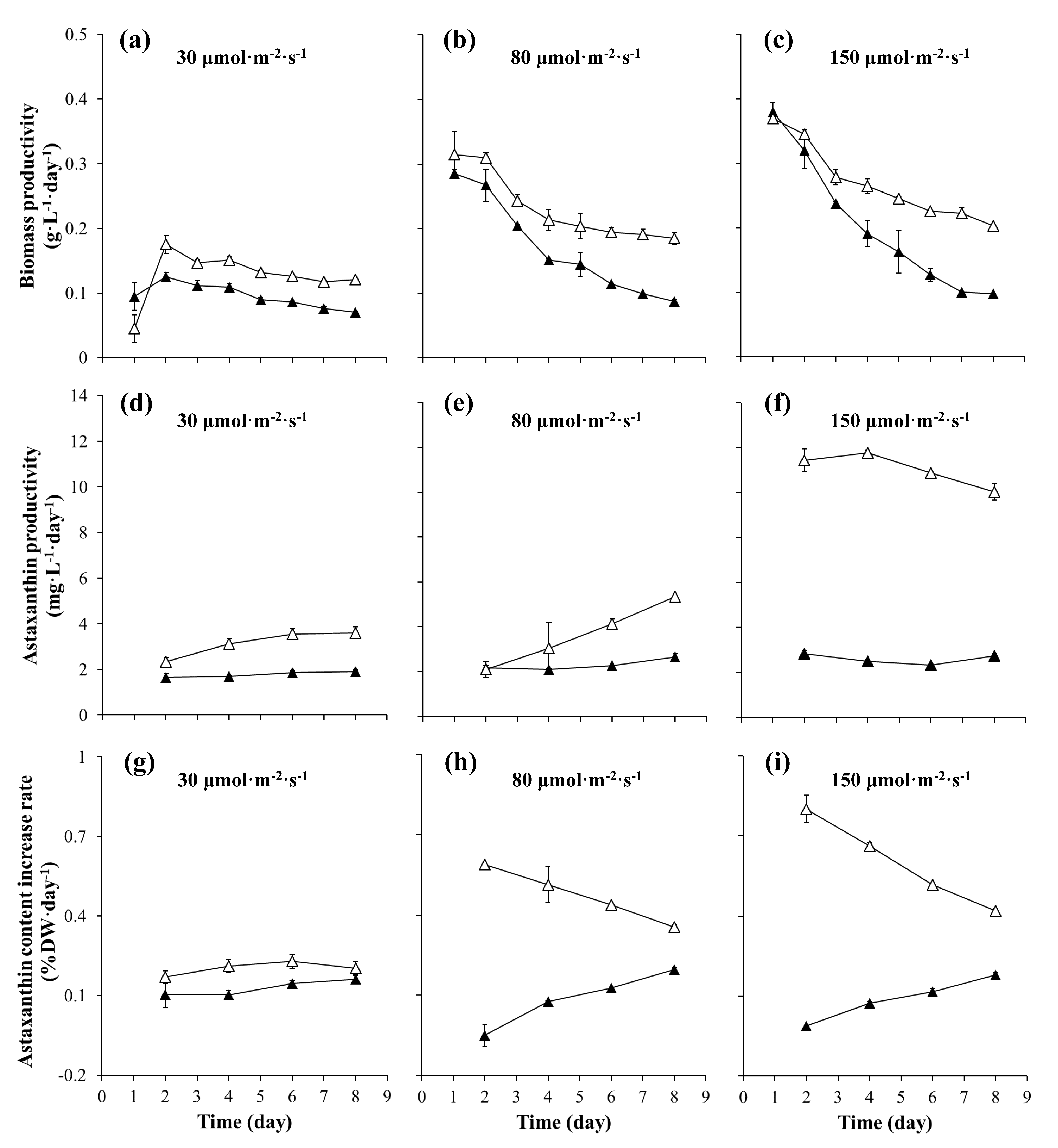
2.2. The Productivity of Biomass and Astaxanthin
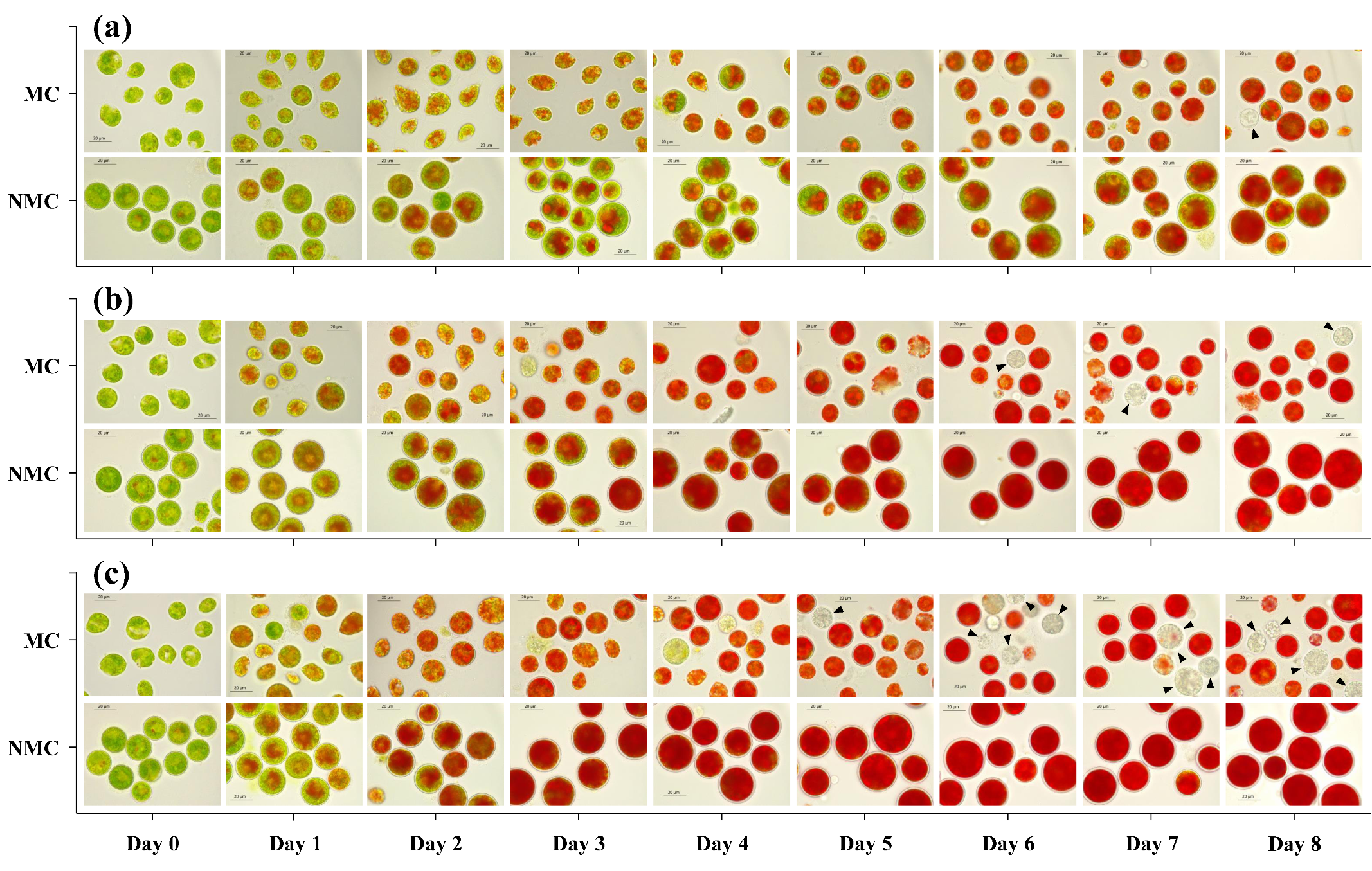
2.3. The Cell Morphology and Viability
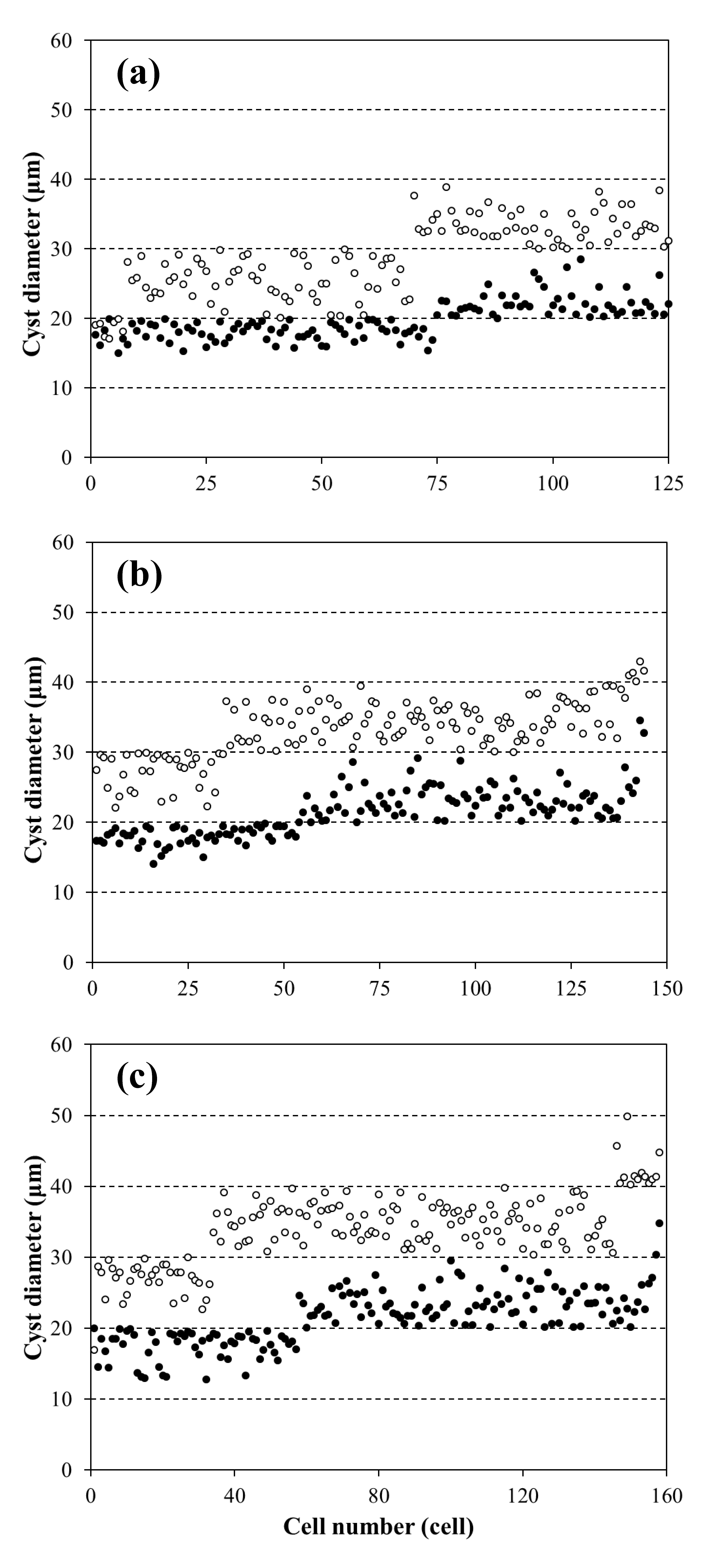
2.4. The Diameter of Cysts
3. Discussion
4. Materials and Methods
4.1. H. pluvialis Strain and Growth Conditions
4.2. Stress Conditions
4.3. Analytical Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Goswami, G.; Chaudhuri, S.; Dutta, D. The present perspective of astaxanthin with reference to biosynthesis and pharmacological importance. World J. Microb. Biot. 2010, 26, 1925–1939. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential anti-atherosclerotic properties of astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H. Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Mar. Drugs 2015, 13, 4310–4330. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.A.; An, G.H. Astaxanthin from microbial sources. Crit. Rev. Biotechnol. 1991, 11, 297–326. [Google Scholar] [CrossRef]
- Wu, H.; Niu, H.; Shao, A.; Wu, C.; Dixon, B.J.; Zhang, J.; Yang, S.; Wang, Y. Astaxanthin as a potential neuroprotective agent for neurological diseases. Mar. Drugs 2015, 13, 5750–5766. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green alga Haematococcus pluvialis. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plantarum. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Droop, M.R. Haematococcus pluvialis and its allies; III: Organic nutrition. Rev. Algologique 1961, 4, 247–259. [Google Scholar]
- Chekanov, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Sidorov, R.; Solovchenko, A. Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the White Sea coastal rocks (Russia). Mar. Drugs 2014, 12, 4504–4520. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.T. A technical review of Haematococcus algae. NatuRoseTM Tech. Bull. 1999, 60, 1–12. [Google Scholar]
- Elliot, A.M. Morphology and life history of Haematococcus pluvialis. Arch. Protistenk. 1934, 82, 250–272. [Google Scholar]
- Shah, M.; Mahfuzur, R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, J.; Sommerfeld, M.; Hu, Q. Susceptibility and protective mechanisms of motile and nonmotile cells of Haematococcus pluvialis (Chlorophyceae) to photooxidative stress. J. Appl. Phycol. 2012, 48, 693–705. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhang, L. Cell cycles and proliferation patterns in Haematococcus pluvialis. Chin. J. Oceanol. Limnol. 2017, 35, 1205–1211. [Google Scholar] [CrossRef]
- Fan, L.; Vonshak, A.; Boussiba, S. Effect of temperature and irradiance on growth of Haematococcus pluvialis (chlorophyceae). J Phycol. 1994, 30, 829–833. [Google Scholar] [CrossRef]
- Hagen, C.H.; Braune, W.; Greulich, F. Functional aspects of secondary carotenoids in Haematococcus lacustris [Girod] Rostafinski (Volvocales) IV. Protection from photodynamic damage. J. Photochem. Photobiol. B 1993, 20, 153–160. [Google Scholar] [CrossRef]
- Hagen, C.; Braune, W.; Björn, L.O. Functional aspects of secondary carotenoids in Haematococcus lacustris (Volvocales) III. Action as a “sunshade”. J. Phycol. 1994, 30, 241–248. [Google Scholar] [CrossRef]
- Fábregas, J.; Otero, A.; Maseda, A.; Domínguez, A. Two-stage cultures for the production of astaxanthin from Haematococcus pluvialis. J. Biotechnol. 2001, 89, 65–71. [Google Scholar] [CrossRef]
- Orosa, M.; Franqueira, D.; Cid, A.; Abalde, J. Analysis and enhancement of astaxanthin accumulation in Haematococcus pluvialis. Bioresour. Technol. 2005, 96, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Aflalo, C.; Meshulam, Y.; Zarka, A.; Boussiba, S. On the relative efficiency of two- vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Hata, N.; Ogbonna, J.C.; Hasegawa, Y.; Taroda, H.; Tanaka, H. Production of astaxanthin by Haematococcus pluvialis in a sequential heterotrophic-photoautotrophic culture. J. Appl. Phycol. 2001, 13, 395–402. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Sommerfeld, M.; Chen, F.; Hu, Q. Enhanced protection against oxidative stress in an astaxanthin-overproduction Haematococcus mutant (Chlorophyceae). Eur. J. Phycol. 2008, 43, 365–376. [Google Scholar] [CrossRef]
- Li, Y.; Sommerfeld, M.; Chen, F.; Hu, Q. Effect of photon flux densities on regulation of carotenogenesis and cell viability of Haematococcus pluvialis (Chlorophyceae). J. Appl. Phycol. 2010, 22, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kakizono, T.; Nishio, N.; Nagai, S.; Kurimura, K.; Tsuji, Y. Antioxidant role of astaxanthin in the green alga Haematococcus pluvialis. Appl. Microbiol. Biot. 1997, 48, 351–356. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kurimura, Y.; Tsuji, Y. Light-independent, astaxanthin production by the green microalga Haematococcus pluvialis under salt stress. Biotechnol. Lett. 1997, 19, 507–509. [Google Scholar] [CrossRef]
- Li, Y.; Sommerfeld, M.; Chen, F.; Hu, Q. Consumption of oxygen by astaxanthin biosynthesis: A protective mechanism against oxidative stress in Haematococcus pluvialis (Chlorophyceae). J. Plant Physiol. 2008, 165, 1783–1797. [Google Scholar] [CrossRef] [PubMed]
- Harker, M.; Tsavalos, A.J.; Young, A.J. Factors responsible for astaxanthin formation in the chlorophyte Haematococcus pluvialis. Bioresour. Technol. 1996, 55, 207–214. [Google Scholar] [CrossRef]
- Río, E.D.; Acién, F.G.; García-Malea, M.C.; Rivas, J.; Molina-Grima, E.; Guerrero, M.G. Efficient one-step production of astaxanthin by the microalga Haematococcus pluvialis in continuous culture. Biotechnol. Bioeng. 2005, 91, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, D.; Sommerfeld, M.R.; Lu, C.; Hu, Q. Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor. J. Appl. Phycol. 2013, 25, 253–260. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kurimura, Y.; Kakizono, T.; Nishio, N.; Tsuji, Y. Morphological changes in the life cycle of the green alga Haematococcus pluvialis. J. Biosci. Bioeng. 1997, 84, 94–97. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Hu, Q. Biology and commercial aspects of Haematococcus pluvialis. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; Blackwell: Hoboken, NJ, USA, 2013; pp. 388–405. [Google Scholar]
- Wayama, M.; Ota, S.; Matsuura, H.; Nango, N.; Hirata, A.; Kawano, S. Three-dimensional ultrastructural study of oil and astaxanthin accumulation during encystment in the green alga Haematococcus pluvialis. PLoS ONE 2013, 8, e53618. [Google Scholar] [CrossRef] [PubMed]
- Sarada, R.; Tripathi, U.; Ravishankar, G.A. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem. 2002, 37, 623–627. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Nishio, N.; Nagai, S. Effects of light intensity, light quality, and illumination cycle on astaxanthin formation in a green alga, Haematococcus pluvialis. J. Biosci. Bioeng. 1992, 74, 61–63. [Google Scholar] [CrossRef]
- Park, E.K.; Lee, C.G. Astaxanthin production by Haematococcus pluvialis under various light intensities and wavelengths. J. Microbiol. Biotechnol. 2001, 11, 1024–1030. [Google Scholar]
- Wang, B.; Zarka, A.; Trebst, A.; Boussiba, S. Astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae) as an active photoprotective process under high irradiance. J. Appl. Phycol. 2003, 39, 1116–1124. [Google Scholar] [CrossRef]
- Casano, L.M.; Zapata, J.M.; Martín, M.; Sabater, B. Chlororespiration and poising of cyclic electron transport: Plastoquinone as electron transporter between thylakoid NADH dehydrogenase and peroxidase. J. Biol. Chem. 2000, 275, 942–948. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sommerfeld, M.; Hu, Q. Occurrence and environmental stress responses of two plastid terminal oxidases in Haematococcus pluvialis (Chlorophyceae). Planta 2009, 230, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.Y.R.; Lee, Y.K. Do carotenoids play a photoprotective role in the cytoplasm of Haematococcus lacustris (Chlorophyta)? Phycologia 1991, 30, 257–261. [Google Scholar] [CrossRef]
- Recht, L.; Zarka, A.; Boussiba, S. Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Appl. Microbiol. Biot. 2012, 94, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Zhekisheva, M.; Boussiba, S.; Khozin-Goldberg, I.; Zarka, A.; Cohen, Z. Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J. Appl. Phycol. 2002, 38, 325–331. [Google Scholar] [CrossRef]
- Sun, H.; Liu, B.; Lu, X.; Cheng, K.W.; Chen, F. Staged cultivation enhances biomass accumulation in the green growth phase of Haematococcus pluvialis. Bioresour. Technol. 2017, 233, 326–331. [Google Scholar] [CrossRef] [PubMed]
| Strain | Major Cell Types | Light Intensity (μmol·m−2·s−1) | Cell Mortality Rate (ca. %) | References |
|---|---|---|---|---|
| CCMA-451 | Motile cells | 30 | 8 | This work |
| CCMA-451 | Motile cells | 80 | 22 | This work |
| CCMA-451 | Motile cells | 150 | 28 | This work |
| CCMA-451 | Nonmotile cells | 30 | 4 | This work |
| CCMA-451 | Nonmotile cells | 80 | 7 | This work |
| CCMA-451 | Nonmotile cells | 150 | 8 | This work |
| Light Intensity (μmol·m−2·s−1) | Cysts in Motile Cell Cultures | Cysts in Nonmotile Cell Cultures | ||||
|---|---|---|---|---|---|---|
| Average Cell Diameter (μm) | Biomass Content (ng·cell−1) | Astaxanthin Content (pg·cell−1) | Average Cell Diameter (μm) | Biomass Content (ng·cell−1) | Astaxanthin Content (pg·cell−1) | |
| 30 | 19.76 ± 2.66 | 0.93 ± 0.11 | 20.72 ± 3.49 | 28.71 ± 5.22 | 1.81 ± 0.07 | 38.49 ± 0.66 |
| 80 | 21.38 ± 3.44 | 1.13 ± 0.11 | 28.51 ± 3.26 | 33.07 ± 4.25 | 2.58 ± 0.15 | 86.21 ± 1.51 |
| 150 | 21.39 ± 3.85 | 1.31 ± 0.04 | 30.87 ± 0.56 | 33.97 ± 5.35 | 2.82 ± 0.14 | 108.61 ± 0.73 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Cai, M.; Lin, M.; Huang, X.; Wang, J.; Ke, H.; Zheng, X.; Chen, D.; Wang, C.; Wu, S.; et al. Differences between Motile and Nonmotile Cells of Haematococcus pluvialis in the Production of Astaxanthin at Different Light Intensities. Mar. Drugs 2019, 17, 39. https://doi.org/10.3390/md17010039
Li F, Cai M, Lin M, Huang X, Wang J, Ke H, Zheng X, Chen D, Wang C, Wu S, et al. Differences between Motile and Nonmotile Cells of Haematococcus pluvialis in the Production of Astaxanthin at Different Light Intensities. Marine Drugs. 2019; 17(1):39. https://doi.org/10.3390/md17010039
Chicago/Turabian StyleLi, Feng, Minggang Cai, Mingwei Lin, Xianghu Huang, Jun Wang, Hongwei Ke, Xuehong Zheng, Ding Chen, Chunhui Wang, Shaoting Wu, and et al. 2019. "Differences between Motile and Nonmotile Cells of Haematococcus pluvialis in the Production of Astaxanthin at Different Light Intensities" Marine Drugs 17, no. 1: 39. https://doi.org/10.3390/md17010039
APA StyleLi, F., Cai, M., Lin, M., Huang, X., Wang, J., Ke, H., Zheng, X., Chen, D., Wang, C., Wu, S., & An, Y. (2019). Differences between Motile and Nonmotile Cells of Haematococcus pluvialis in the Production of Astaxanthin at Different Light Intensities. Marine Drugs, 17(1), 39. https://doi.org/10.3390/md17010039





