Characterization and Hypoglycemic Activity of a Rhamnan-Type Sulfated Polysaccharide Derivative
Abstract
1. Introduction
2. Results and Discussion
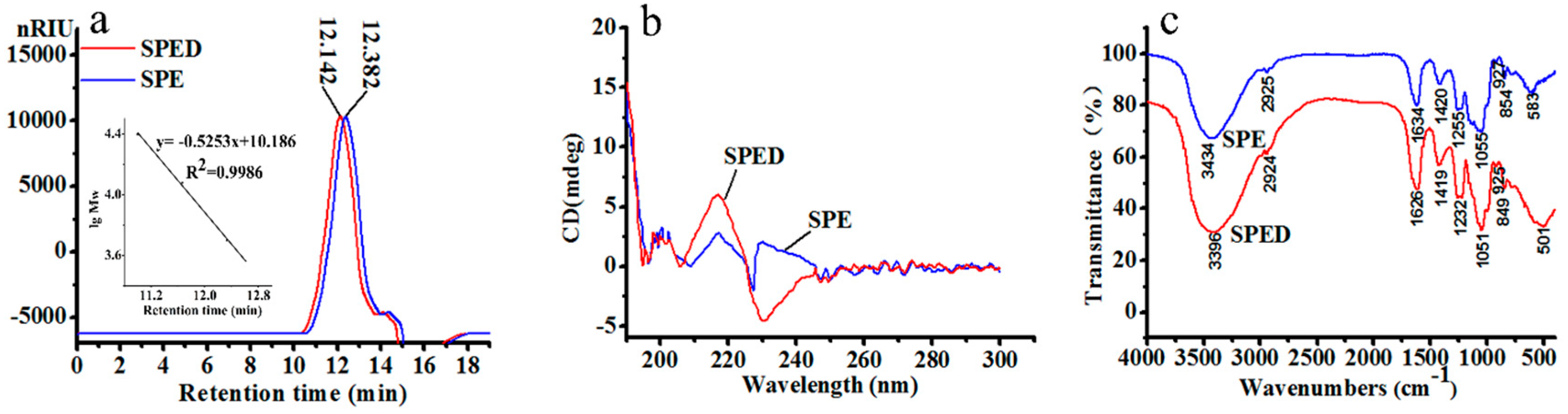
2.1. Characterization of SPE
2.2. Characterization of SPED
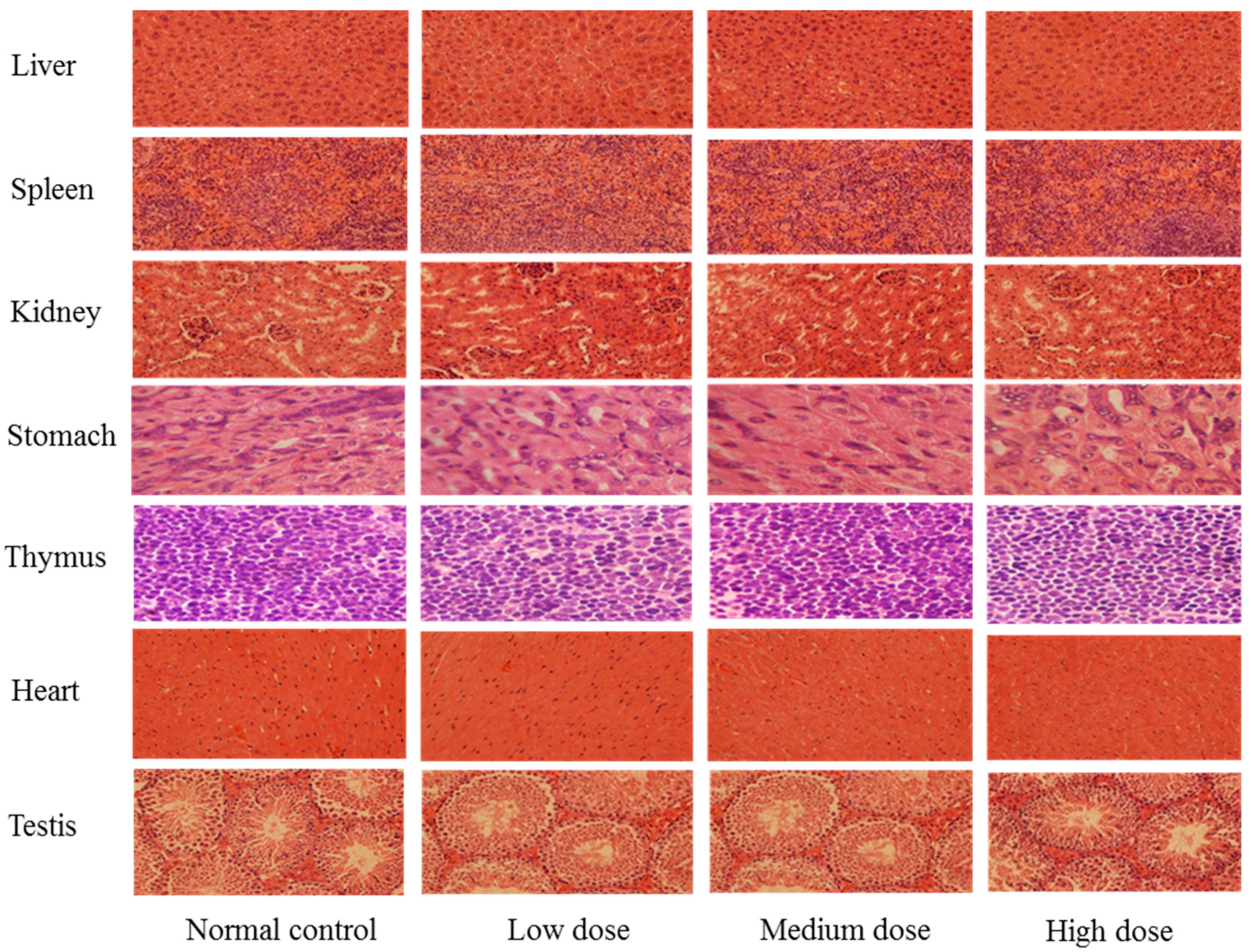
2.3. Safety Evaluation of SPED
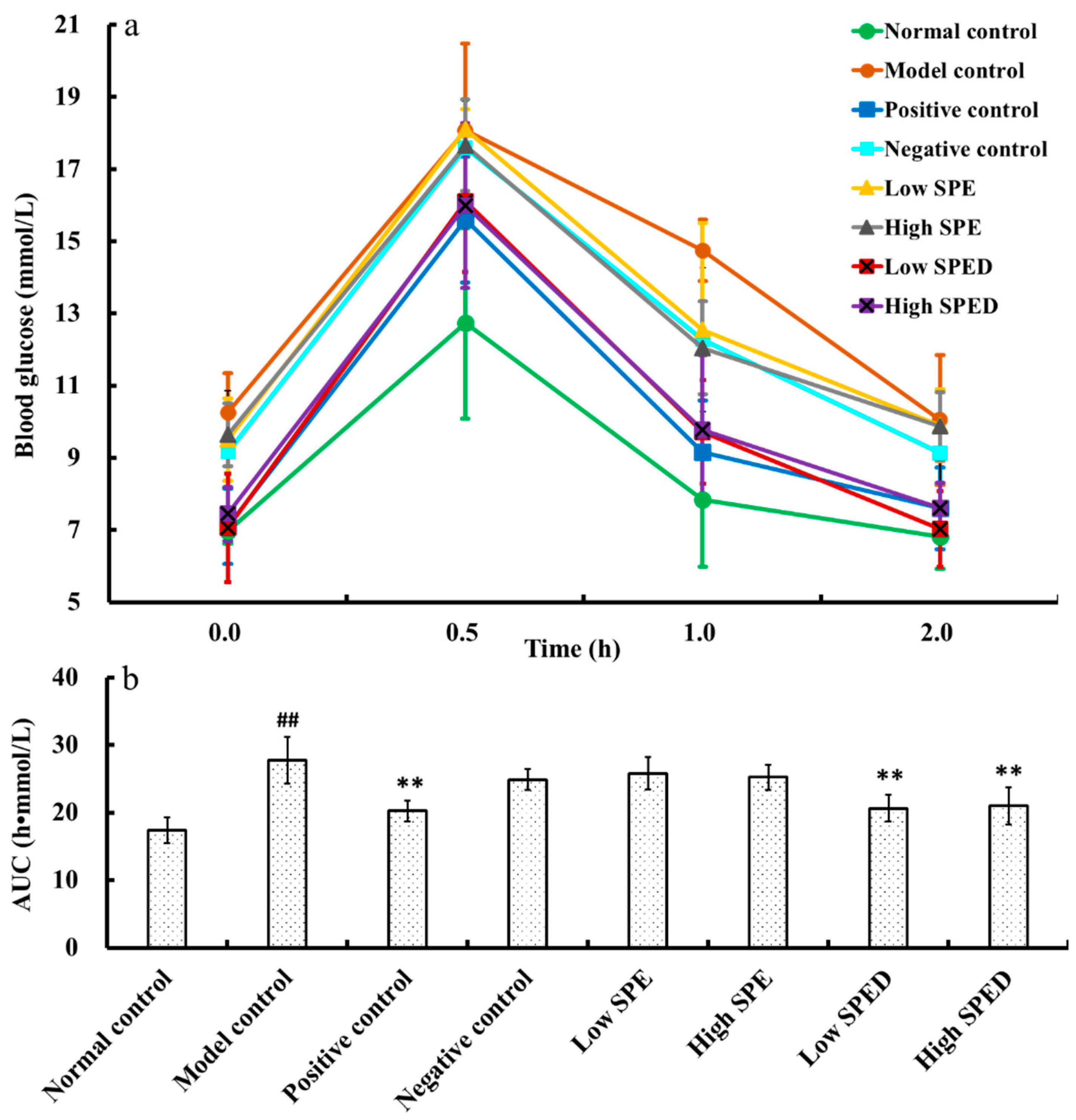
2.4. Anti-Diabetic Effects of SPED
2.4.1. Oral Glucose Tolerance Test
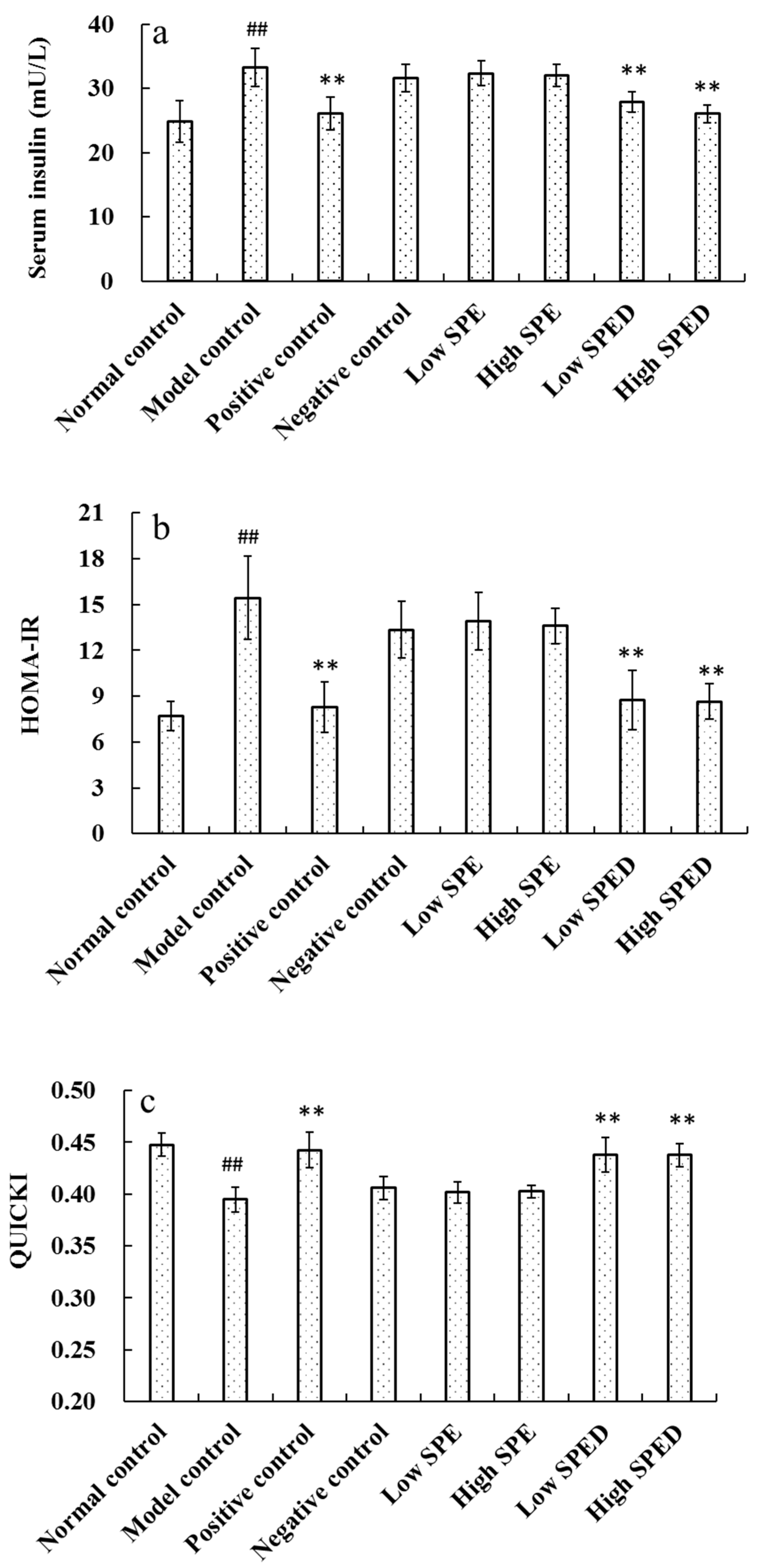
2.4.2. Serum Insulin Level
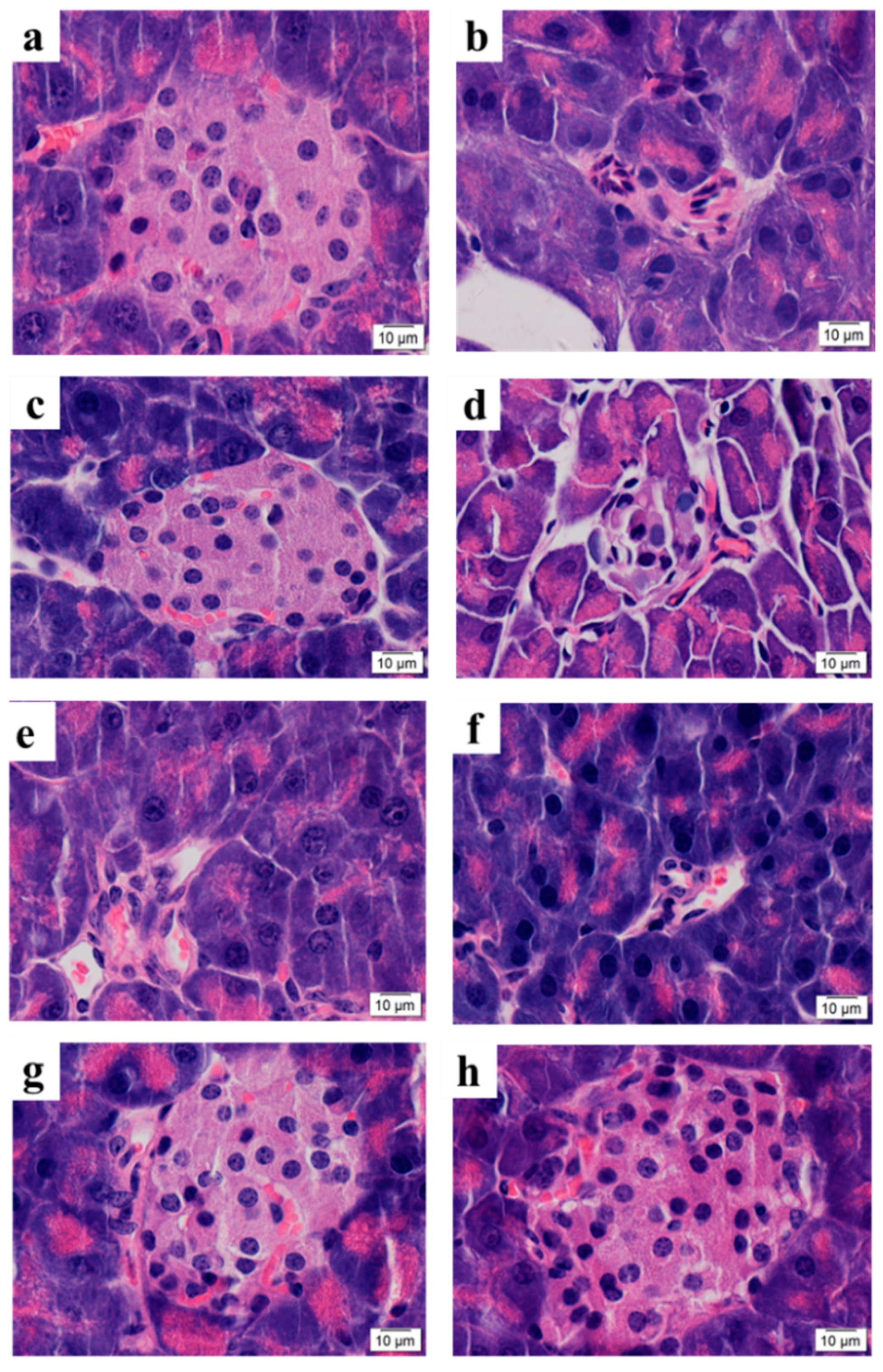
2.4.3. Microscopic Structures of Pancreas Islets
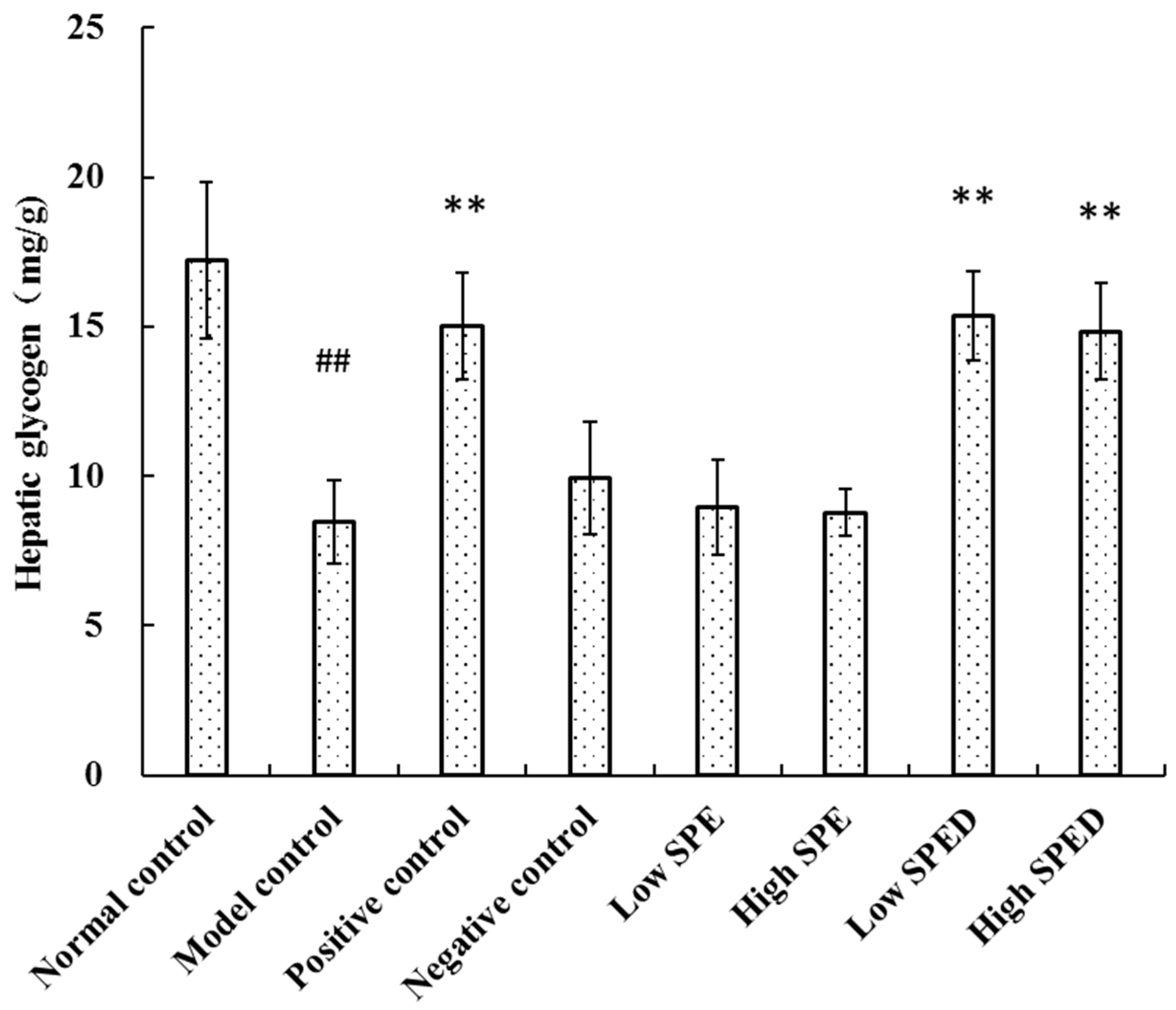
2.4.4. Hepatic Glycogen Content
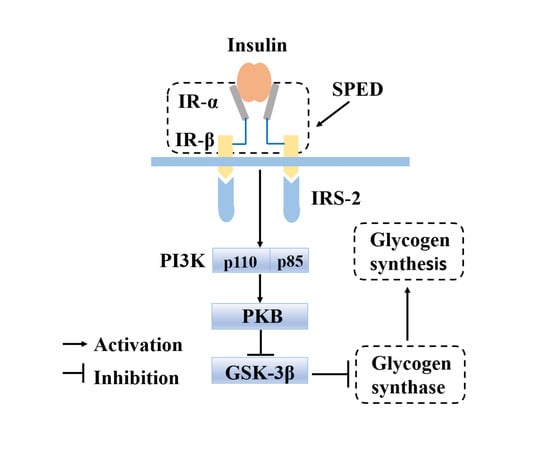
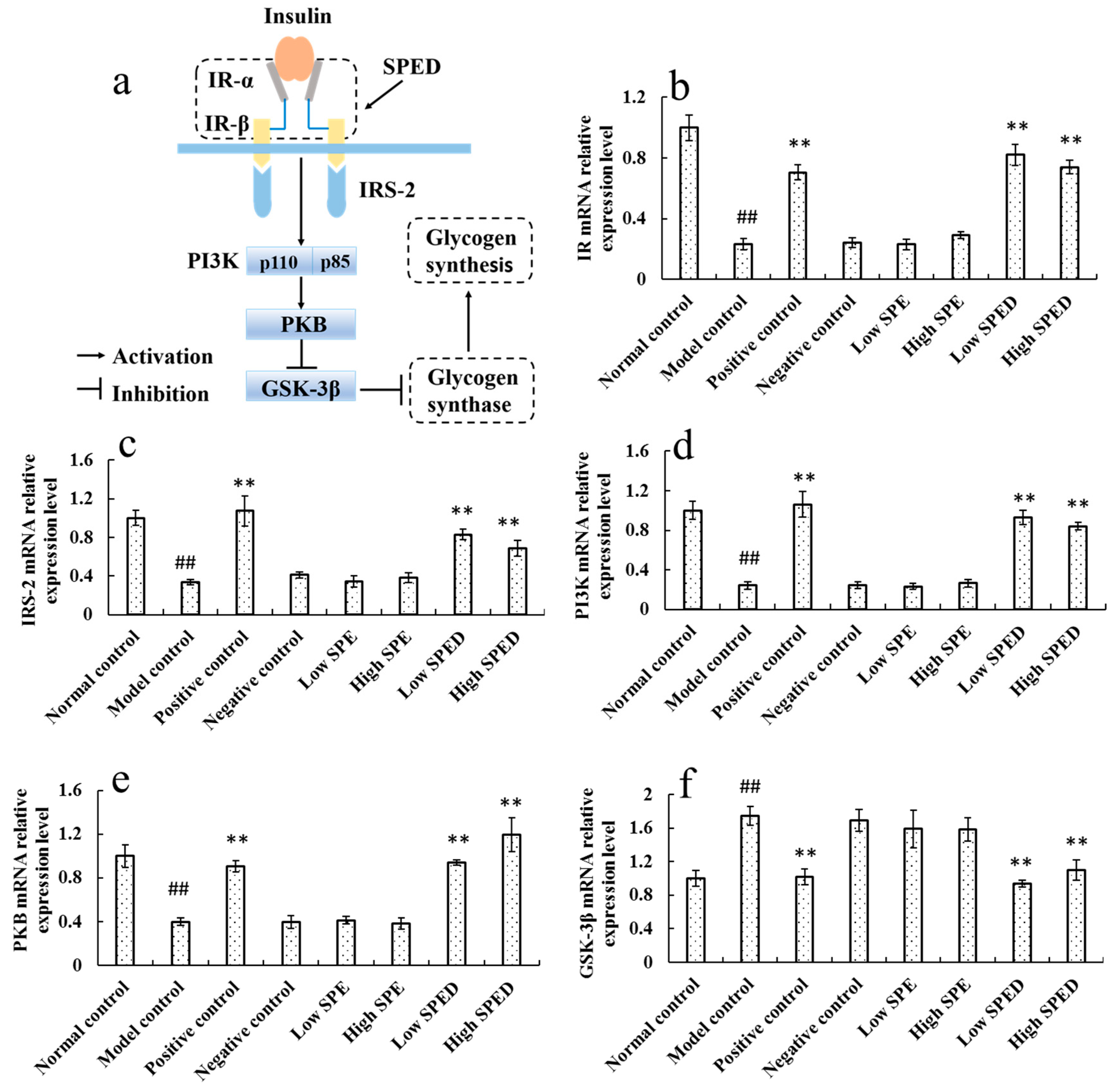
2.4.5. Quantitative Real-Time PCR Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation and Characterization of SPE
3.3. Synthesis and Characterization of the SPED
3.4. Animals
3.5. Safety Evaluation of the SPED
3.6. Induction of Type 2 Diabetic Mice and Experimental Design
3.6.1. Oral Glucose Tolerance Test
3.6.2. Evaluation of Serum Insulin Level
3.6.3. Microscopic Structure of Pancreas Islet
3.6.4. Evaluation of Hepatic Glycogen Content
3.6.5. Quantitative Real-Time PCR Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arrese, M. Nonalcoholic fatty liver disease: Liver disease: An overlooked complication of diabetes mellitus. Nat. Rev. Endocrinol. 2010, 6, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Habila, J.D.; Koorbanally, N.A.; Islam, M.S. Butanol fraction of Parkia biglobosa (Jacq.) G. Don leaves enhance pancreatic β-cell functions, stimulates insulin secretion and ameliorates other type 2 diabetes-associated complications in rats. J. Ethnopharmacol. 2016, 183, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lewicki, S.; Zdanowski, R.; Krzyzowska, M.; Lewicka, A.; Debski, B.; Niemcewicz, M.; Goniewicz, M. The role of chromium III in the organism and its possible use in diabetes and obesity treatment. Ann. Agric. Environ. Med. 2014, 21, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Yang, X. Controlling diabetes by chromium complexes: The role of the ligands. J. Inorg. Biochem. 2015, 146, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Y.; Zheng, S.S.; Zhang, M.H.; Feng, J.H.; Xie, P.; Bi, J.M. Study of oxidative damage in growing-finishing pigs with continuous excess dietary chromium picolinate intake. Biol. Trace Elem. Res. 2008, 126, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Gong, J.; Zhao, Y.; Zhuang, X.; Ye, Y.; Huang, F.; Lin, W. Sulfated polysaccharide from Enteromorpha prolifera suppresses SREBP-1c and ACC expression to lower serum triglycerides in high-fat diet-induced hyperlipidaemic rats. J. Funct. Foods 2018, 40, 722–728. [Google Scholar] [CrossRef]
- Qi, X.; Mao, W.; Gao, Y.; Chen, Y.; Chen, Y.; Zhao, C.; Li, N.; Wang, C.; Yan, M.; Lin, C.; et al. Chemical characteristic of an anticoagulant-active sulfated polysaccharide from Enteromorpha clathrate. Carbohydr. Polym. 2012, 90, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, Y.; Yu, P.; Zhan, Q.; Wang, J.; Chi, Y.; Wang, P. A novel low molecular weight Enteromorpha polysaccharide-iron (III) complex and its effect on rats with iron deficiency anemia (IDA). Int. J. Biol. Macromol. 2018, 108, 412–418. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Mo, X.; Lu, X.; Zhang, Y.; Qin, L. Modifcation, characterization and structure-anticoagulant activity relationships of persimmon polysaccharides. Carbohydr. Polym. 2010, 82, 515–520. [Google Scholar] [CrossRef]
- Combo, A.M.M.; Aguedo, M.; Quiévy, N.; Danthine, S.; Goffin, D.; Jacquet, N.; Blecker, C.; Devaux, J.; Paquot, M. Characterization of sugar beet pectic-derived oligosaccharides obtained by enzymatic hydrolysis. Int. J. Biol. Macromol. 2013, 52, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Cheng, C.; Zhao, H.; Jing, J.; Gong, N.; Lu, W. In vivo anti-radiation activities of the Ulva pertusa polysaccharides and polysaccharide-iron (III) complex. Int. J. Biol. Macromol. 2013, 60, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Z.; Pan, Y.; Gao, X.; Chen, H. Anti-diabetic effects of Inonotus obliquus polysaccharides-chromium (III) complex in type 2 diabetic mice and its sub-acute toxicity evaluation in normal mice. Food Chem. Toxicol. 2017, 108, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, B.; Gill, P. Circular dichroism techniques: Biomolecular and nano-structural analyses—A review. Chem. Biol. Drug Des. 2009, 74, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H.; Wang, Y.; Xing, L. Synthesis and characterization of a new Inonotus obliquus polysaccharide-iron (III) complex. Int. J. Biol. Macromol. 2015, 75, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Chakrabarti, B. Optical characteristics of carboxyl group in relation to the circular dichroic properties and dissociation constants of glycosamino-glycans. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 1978, 544, 667–675. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, G.; Guan, H.; Yue, N.; Zhang, Z.; Li, H. Preparation of low-molecular-weight polyguluronate sulfate and its anticoagulant and anti-inflammatory activities. Carbohydr. Polym. 2007, 69, 272–279. [Google Scholar] [CrossRef]
- Mao, W.J.; Fang, F.; Li, H.Y.; Qi, X.H.; Sun, H.H.; Chen, Y.; Guo, S.D. Heparinoid-active two sulfated polysaccharides isolated from marine green alage Monostroma nitidum. Carbohydr. Polym. 2008, 74, 834–839. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Du, C.; Mou, H.; Wang, P. Compositional and structural characteristics of sulfated polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2017, 165, 221–228. [Google Scholar] [CrossRef]
- Saheed, S.; Oladipipo, A.E.; Abdulazeez, A.A.; Olarewaju, S.A.; Ismaila, N.O.; Emmanuel, I.A.; Fatimaha, Q.D.; Aisha, A.Y. Toxicological evaluations of Stigma maydis (corn silk) aqueous extract on hematological and lipid parameters in Wistar rats. Toxicol. Rep. 2015, 2, 638–644. [Google Scholar] [CrossRef]
- Wu, X.Y.; Li, F.; Xu, W.D.; Zhao, J.L.; Zhao, T.; Liang, L.H.; Yang, L.Q. Anti-hyperglycemic activity of chromium (III) malate complex in alloxan-induced diabetic rats. Biol. Trace Elem. Res. 2011, 143, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, X.; Zhao, T.; Zhang, M.; Zhao, J.; Mao, G.; Yang, L. Anti-diabetic properties of chromium citrate complex in alloxan-induced diabetic rats. J. Trace Elem. Med. Biol. 2011, 25, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Stallings, D.M.; Hepburn, D.D.D.; Hannah, M.; Vincent, J.B.; O’Donnell, J. Nutritional supplement chromium picolinate generates chromosomal aberrations and impedes progeny development in Drosophila melanogaster. Mutat. Res. 2006, 610, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, D.D.D.; Vincent, J.B. Tissue and subcellular distribution of chromium picolinate with time after entering the bloodstream. J. Inorg. Biochem. 2003, 94, 86–93. [Google Scholar] [CrossRef]
- Mazzola, N. Review of current and emerging therapies in type 2 diabetes mellitus. Am. J. Manag. Care 2012, 18, S17–S26. [Google Scholar]
- Khan, H.B.; Vinayaqam, K.S.; Palanivelu, S.; Panchanadham, S. Ameliorating effect of Semecarpus anacardium Linn. nut milk extract on altered glucose metabolism in high fat diet STZ induced type 2 diabetic rats. Asian Pac. J. Trop. Med. 2012, 5, 956–961. [Google Scholar] [CrossRef]
- Dabhi, B.; Mistry, K.N. Oxidative stress and its association with TNF-α-308 G/C and IL-1α-889C/T gene polymorphisms in patients with diabetes and diabetic nephropathy. Gene 2015, 562, 197–202. [Google Scholar] [CrossRef]
- El-Azab, M.F.; Attia, F.M.; El-Mowafy, A.M. Novel role of curcumin combined with bone marrow transplantation in reversing experimental diabetes: Effects on pancreatic islet regeneration, oxidative stress, and inflammatory cytokines. Eur. J. Pharmacol. 2011, 658, 41–48. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Bai, W. Characterization of Momordica charantia L. polysaccharide and its protective effect on pancreatic cells injury in STZ-induced Diabetic mice. Int. J. Biol. Macromol. 2018, 115, 45–52. [Google Scholar] [CrossRef]
- Hu, S.; Chang, Y.; Wang, J.; Xue, C.; Li, Z.; Wang, Y. Fucosylated chondroitin sulfate from sea cucumber in combination with rosiglitazone improved glucose metabolism in the liver of the insulin-resistant mice. Biosci. Biotechnol. Biochem. 2013, 77, 2263–2268. [Google Scholar] [CrossRef]
- Jung, U.J.; Baek, N.I.; Chung, H.G.; Bang, M.H.; Yoo, J.S.; Jeong, T.S.; Lee, K.T.; Kang, Y.J.; Lee, M.K.; Kim, H.J.; et al. The anti-diabetic effects of ethanol extract from two variants of Artemisia princeps Pampanini in C57BL/KsJ-db/db mice. Food Chem. Toxicol. 2007, 45, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, H.; Liu, Y.; Shui, W.; Wang, J.; Cao, P.; Wang, H.; You, R.; Zhang, Y. Dendrobium officinale polysaccharide attenuates type 2 diabetes mellitus via the regulation of PI3K/Akt-mediated glycogen synthesis and glucose metabolism. Funct. Foods 2018, 40, 261–271. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signaling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chiang, S.H.; Saltiel, A.R. Insulin signaling and the regulation of glucose transport. Mol. Med. 2004, 10, 65–71. [Google Scholar] [PubMed]
- Coops, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, Y.; Wang, S.; Chi, Y.; Hwang, H.; Wang, P. Directional preparation of anticoagulant-active sulfated polysaccharides from Enteromorpha prolifera using artificial neural networks. Sci. Rep. 2018, 8, 3062. [Google Scholar] [CrossRef]
- Terho, T.T.; Hartiala, K. Method for determination of the sulfate content of glycosaminoglycans. Anal. Biochem. 1971, 41, 471–476. [Google Scholar] [CrossRef]
| Samples | Total Sugar % | Sulfate (%) | Rhamnose (mol/%) | Glucose (mol/%) | Xylose (mol/%) | Glucuronic Acid (mol/%) | Mw (kDa) |
|---|---|---|---|---|---|---|---|
| SPE | 70.1 | 25.9 | 59.8 | 3.1 | 17.9 | 19.1 | 4.8 |
| SPED | 71.6 | 24.4 | 58.2 | 4.3 | 16.9 | 20.5 | 6.4 |
| Groups | Samples | Dose (mg/kg) | Equivalent to Cr3+ (µg/kg) |
|---|---|---|---|
| Normal Control | NaCl (0.9%) | -- | |
| Model Control | NaCl (0.9%) | -- | |
| Positive Control | Picolinic | 4.5 | 560 |
| Negative Control | CrCl3·6H2O | 2.9 | 560 |
| Low SPE | SPE | 5 | |
| High SPE | SPE | 20 | |
| Low | SPED | 5 | 140 |
| High | SPED | 20 | 560 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.-F.; Ye, H.; Zhu, Y.-J.; Li, Y.-P.; Wang, J.-F.; Wang, P. Characterization and Hypoglycemic Activity of a Rhamnan-Type Sulfated Polysaccharide Derivative. Mar. Drugs 2019, 17, 21. https://doi.org/10.3390/md17010021
Cui J-F, Ye H, Zhu Y-J, Li Y-P, Wang J-F, Wang P. Characterization and Hypoglycemic Activity of a Rhamnan-Type Sulfated Polysaccharide Derivative. Marine Drugs. 2019; 17(1):21. https://doi.org/10.3390/md17010021
Chicago/Turabian StyleCui, Jie-Fen, Han Ye, Yu-Jie Zhu, Yin-Ping Li, Jing-Feng Wang, and Peng Wang. 2019. "Characterization and Hypoglycemic Activity of a Rhamnan-Type Sulfated Polysaccharide Derivative" Marine Drugs 17, no. 1: 21. https://doi.org/10.3390/md17010021
APA StyleCui, J.-F., Ye, H., Zhu, Y.-J., Li, Y.-P., Wang, J.-F., & Wang, P. (2019). Characterization and Hypoglycemic Activity of a Rhamnan-Type Sulfated Polysaccharide Derivative. Marine Drugs, 17(1), 21. https://doi.org/10.3390/md17010021