Natural Product Repertoire of the Genus Amphimedon
Abstract
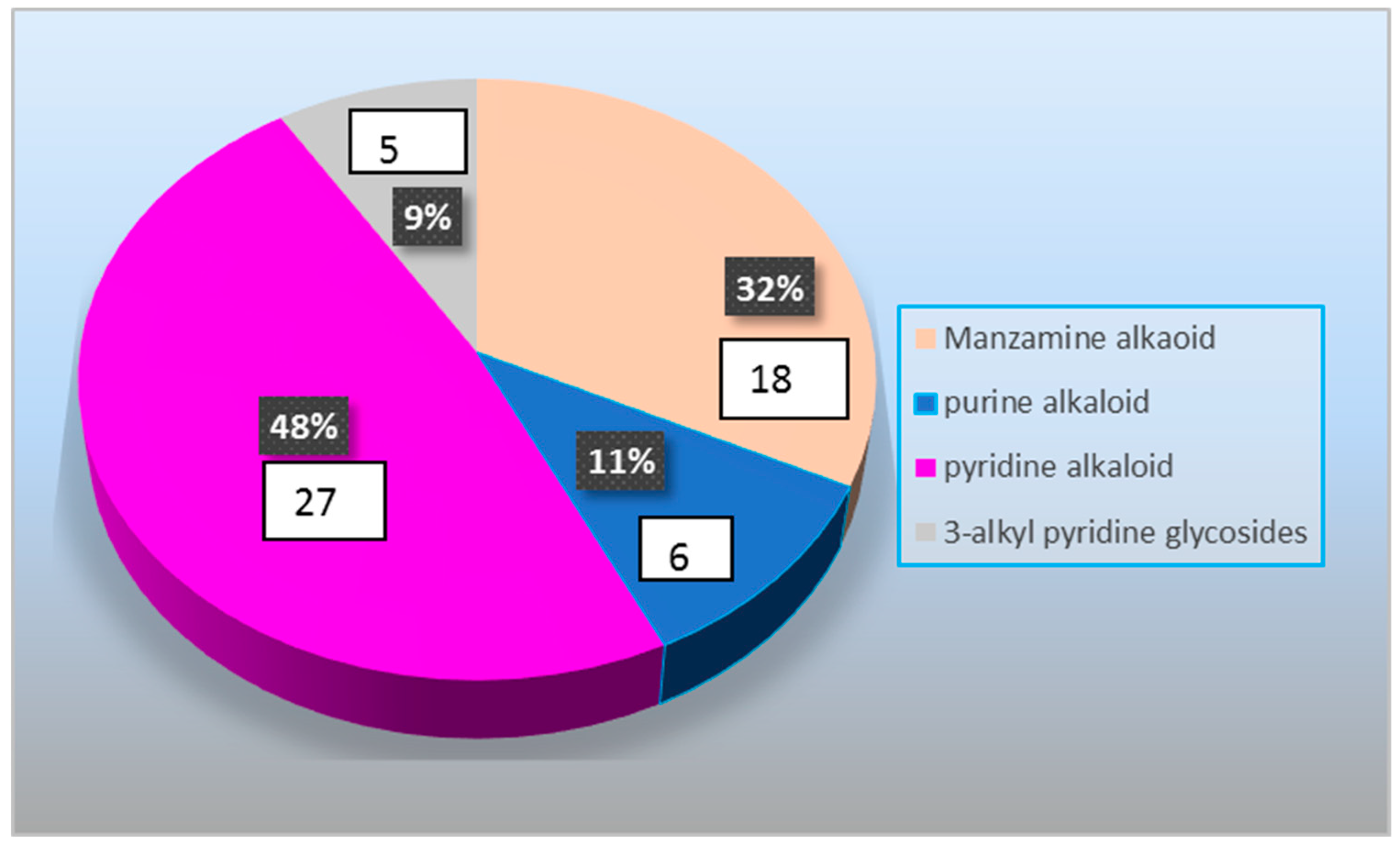
1. Introduction
2. Amphimedon compressa
3. Amphimedon viridis
4. Amphimedon complanata
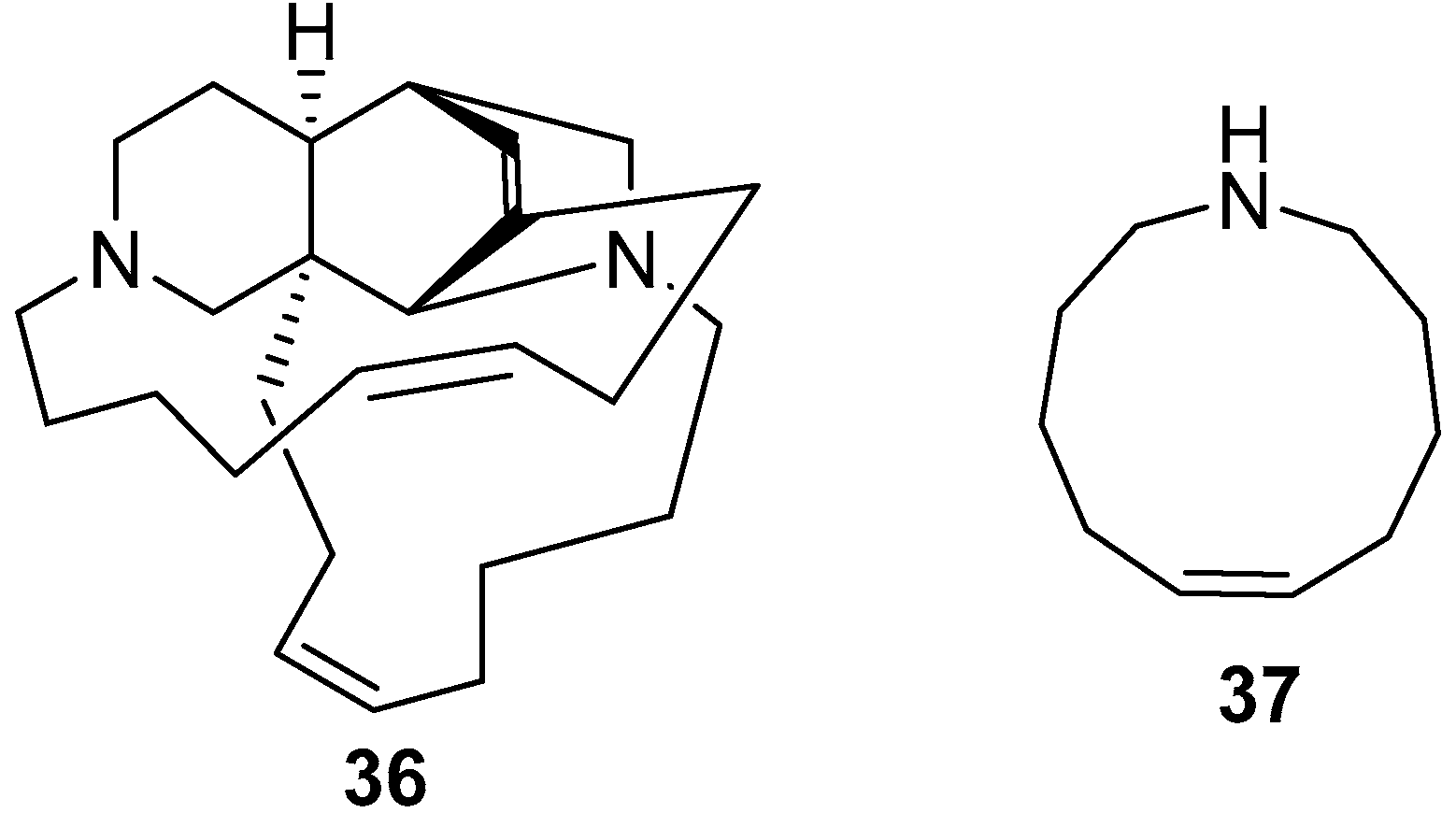
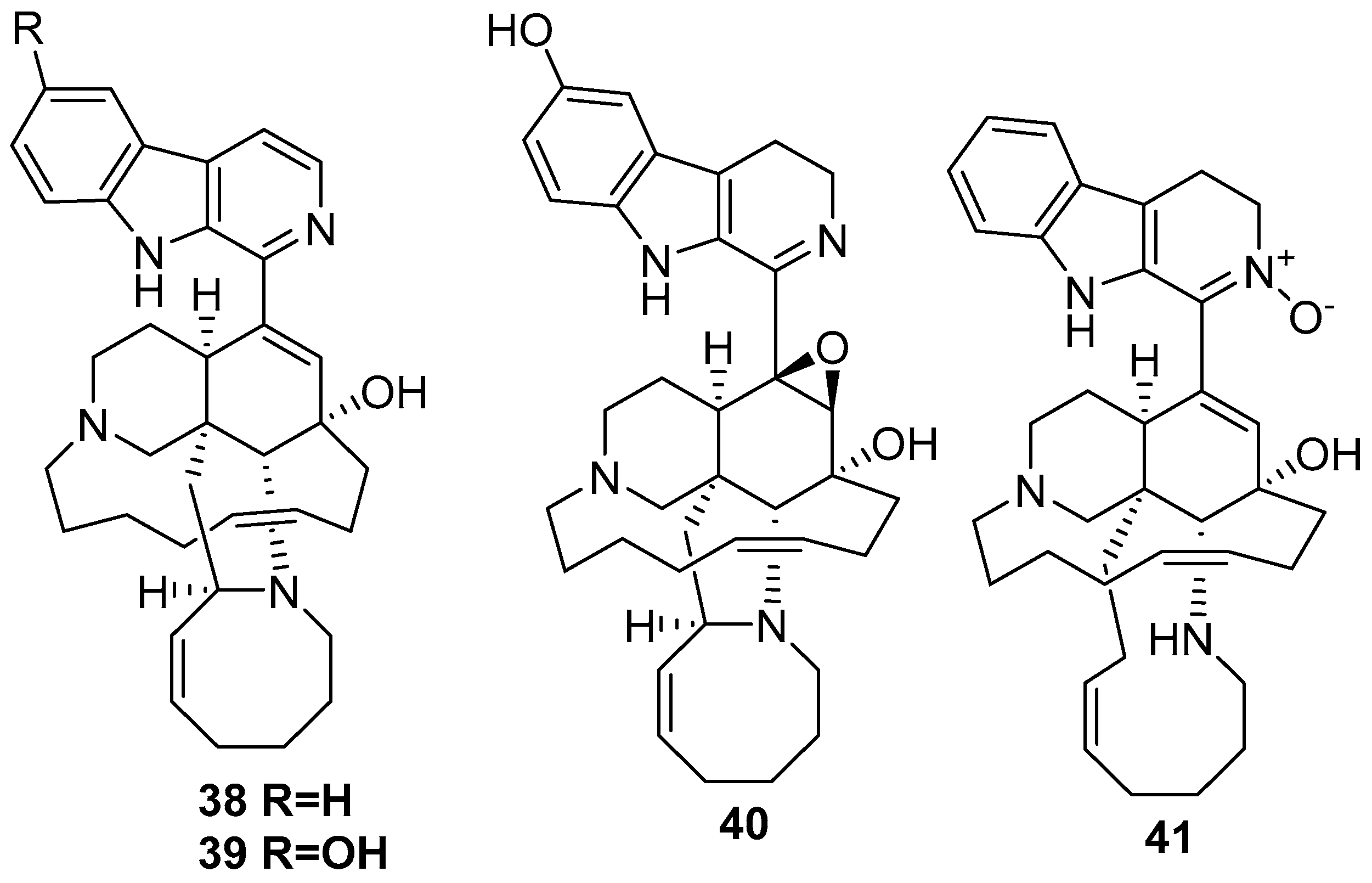
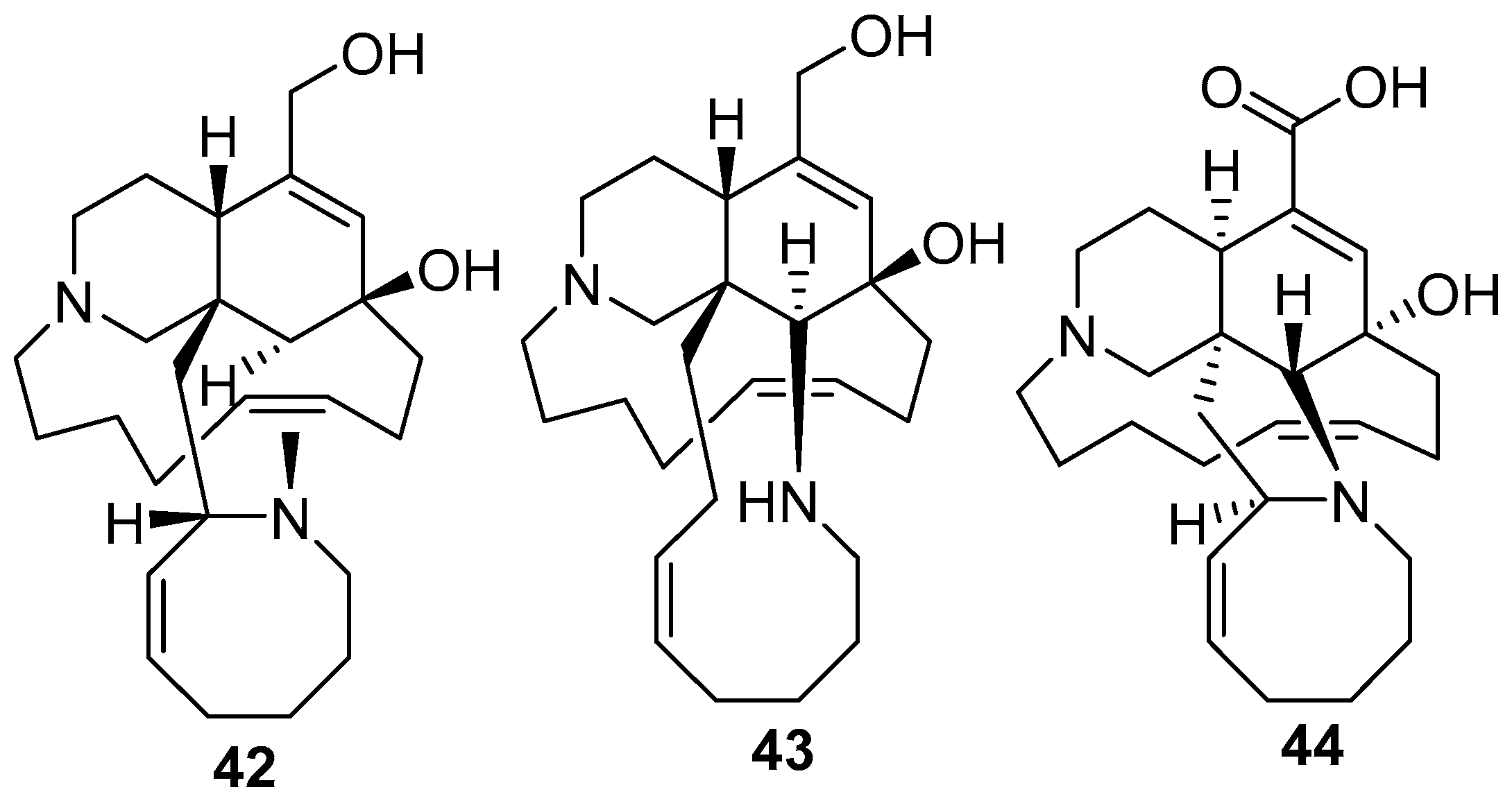
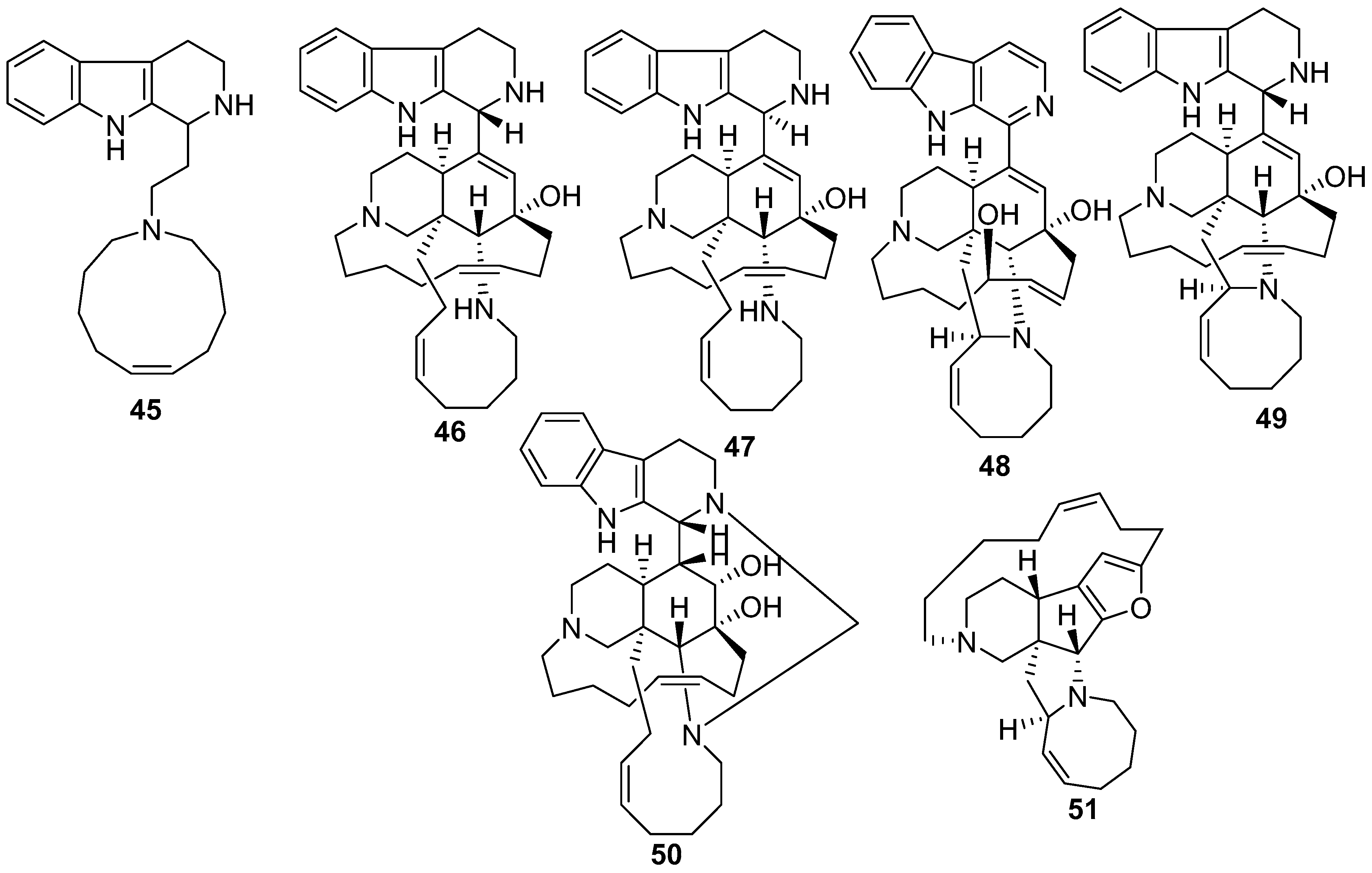
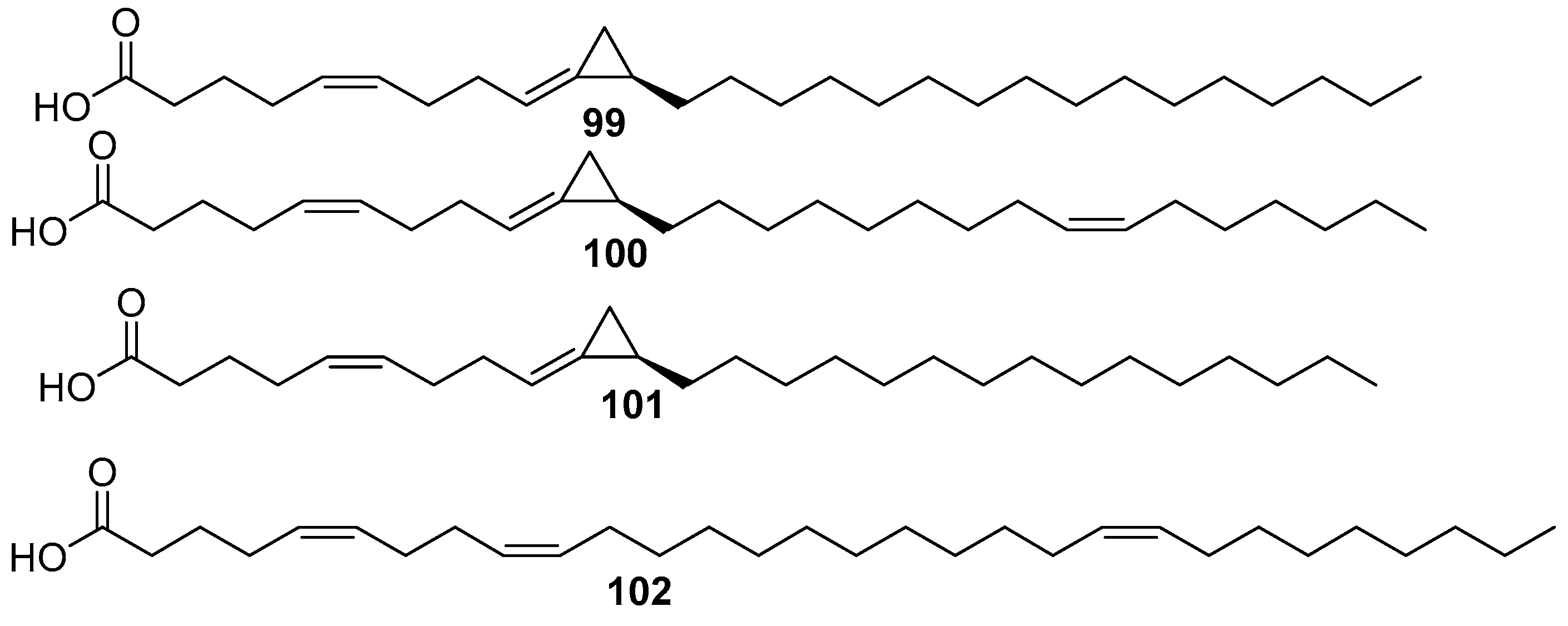
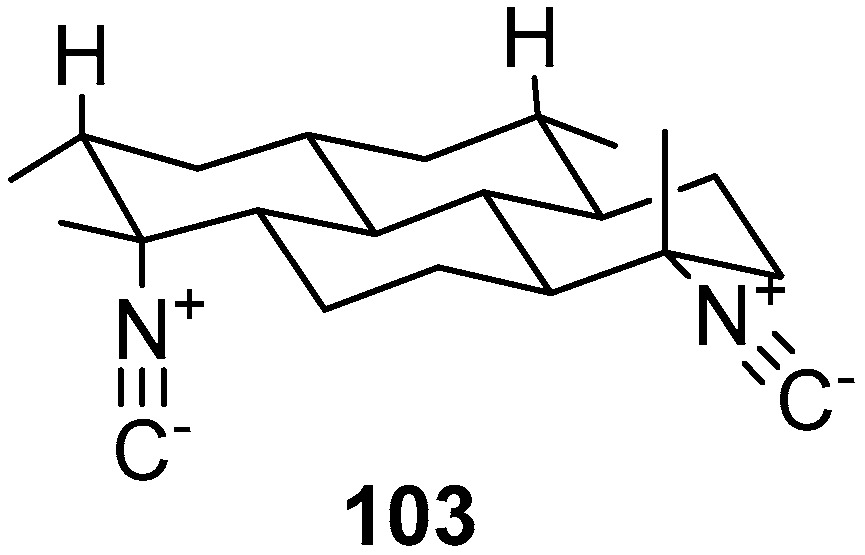
5. Amphimedon terpenensis
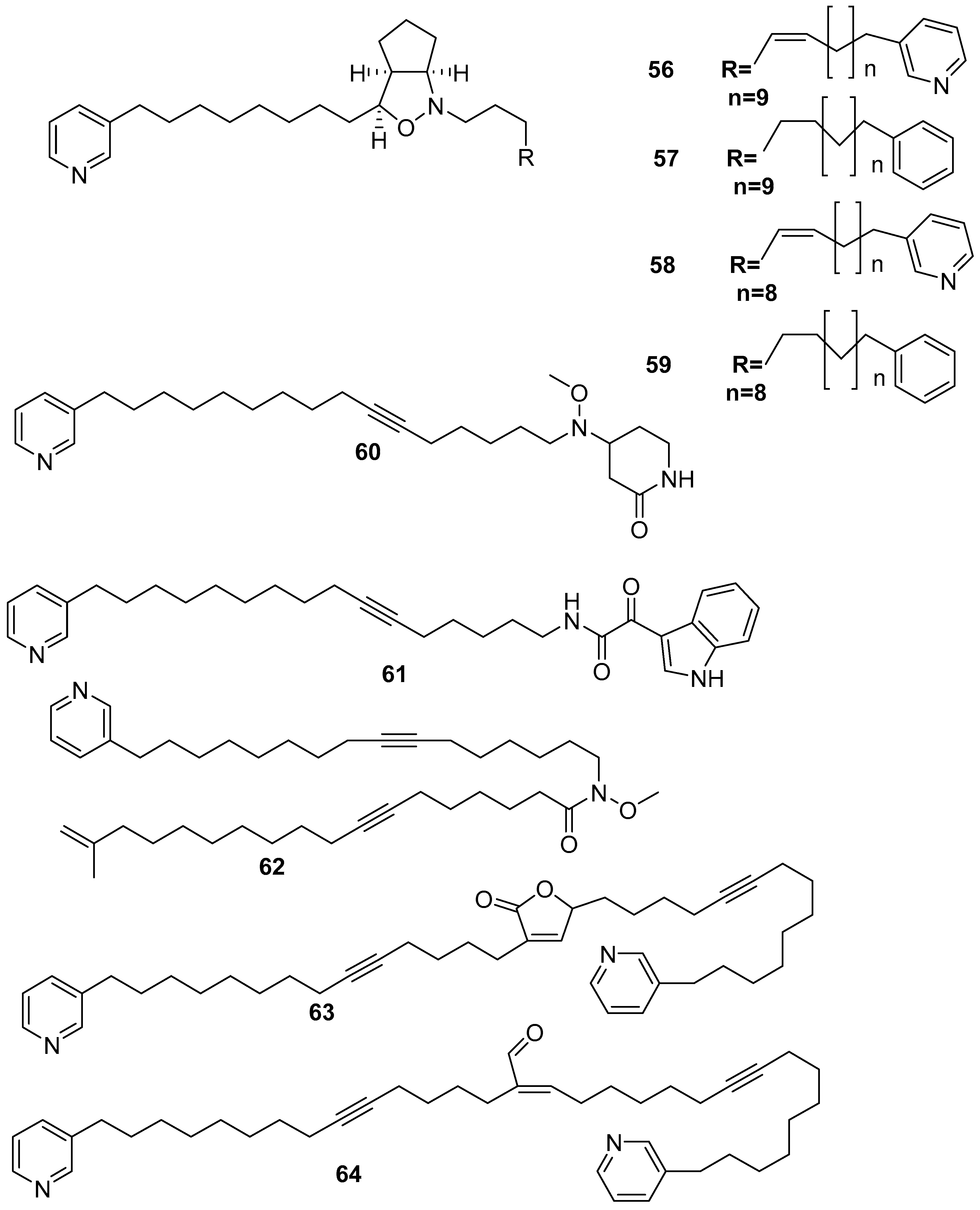
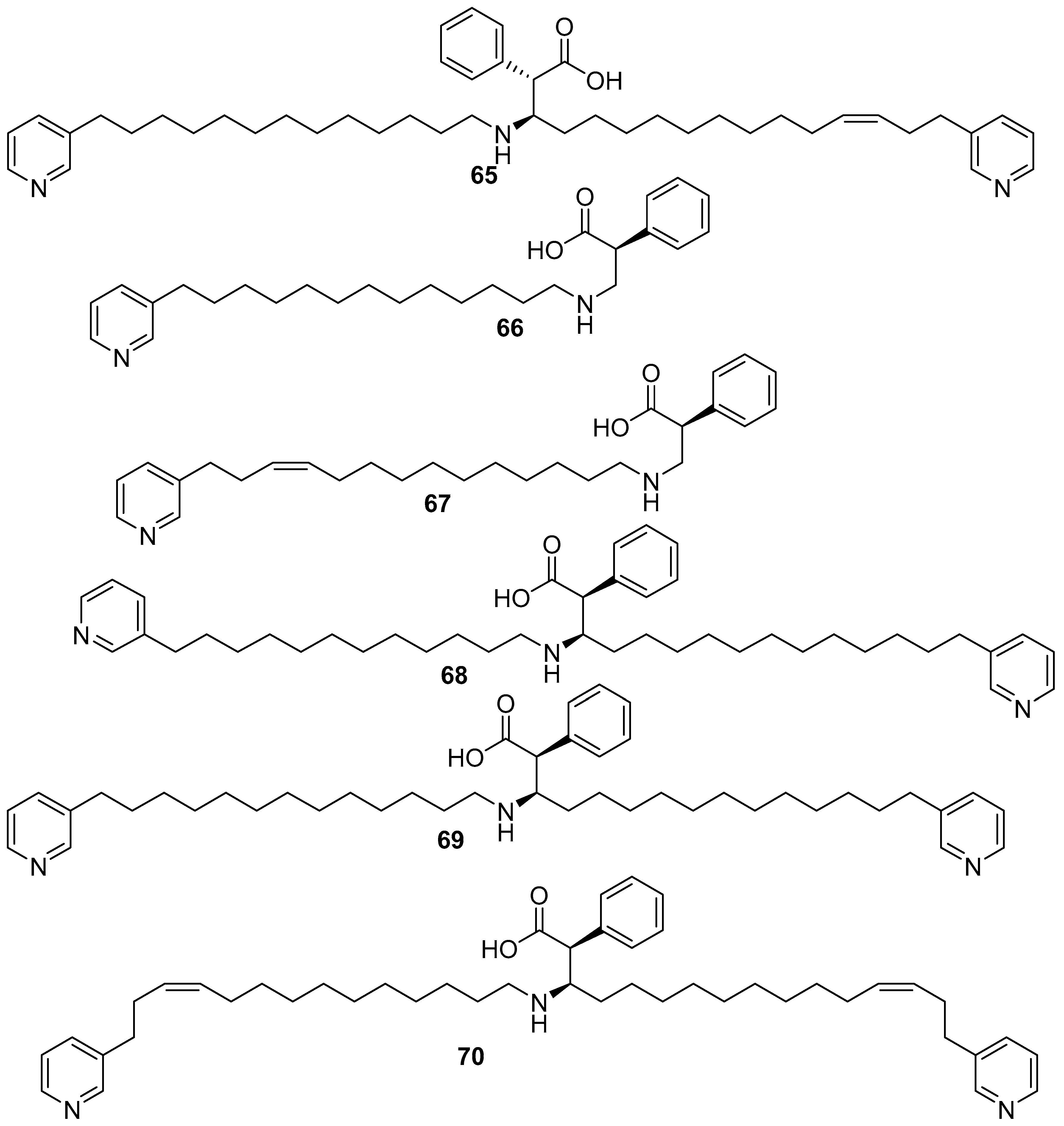
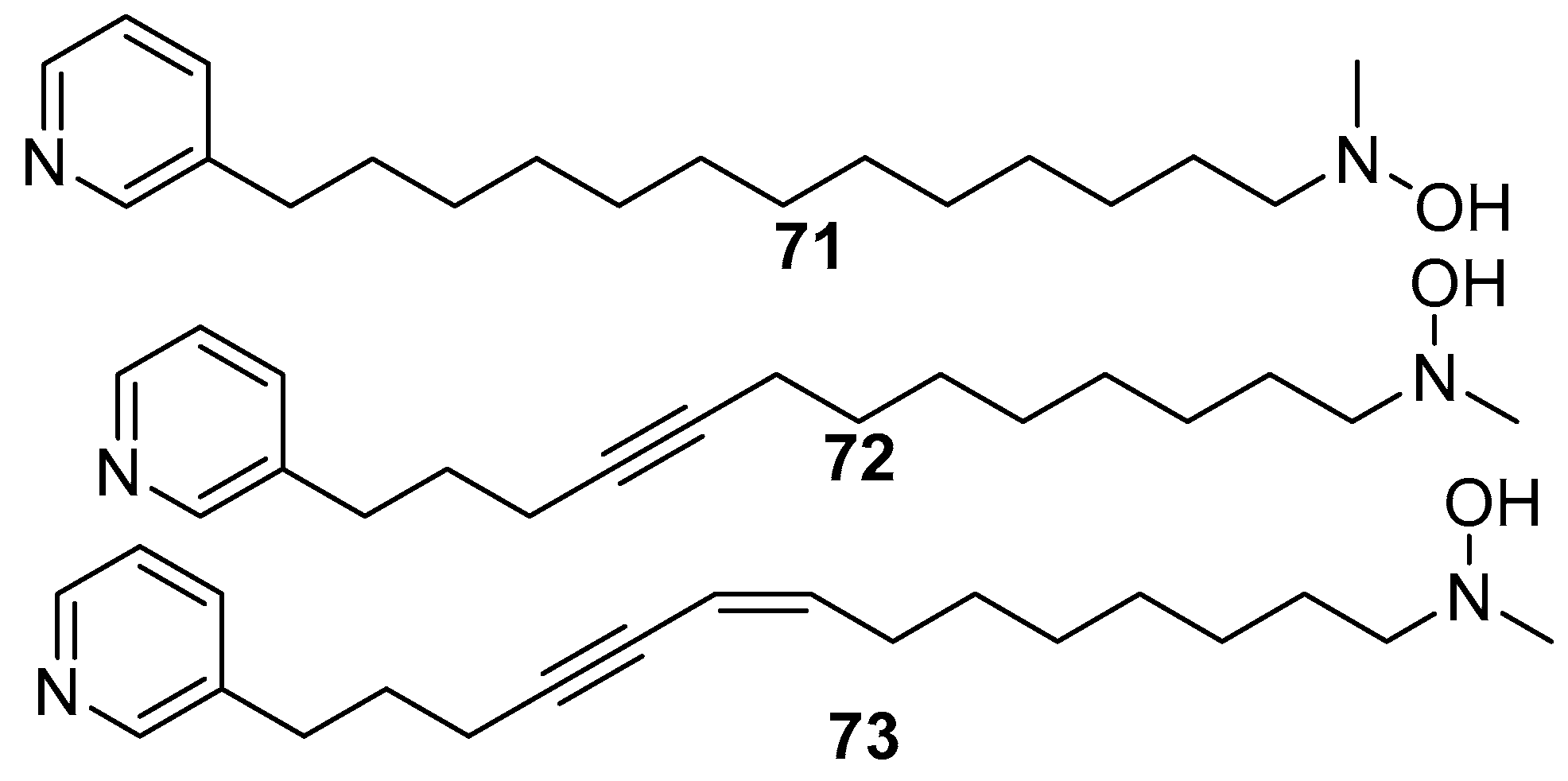
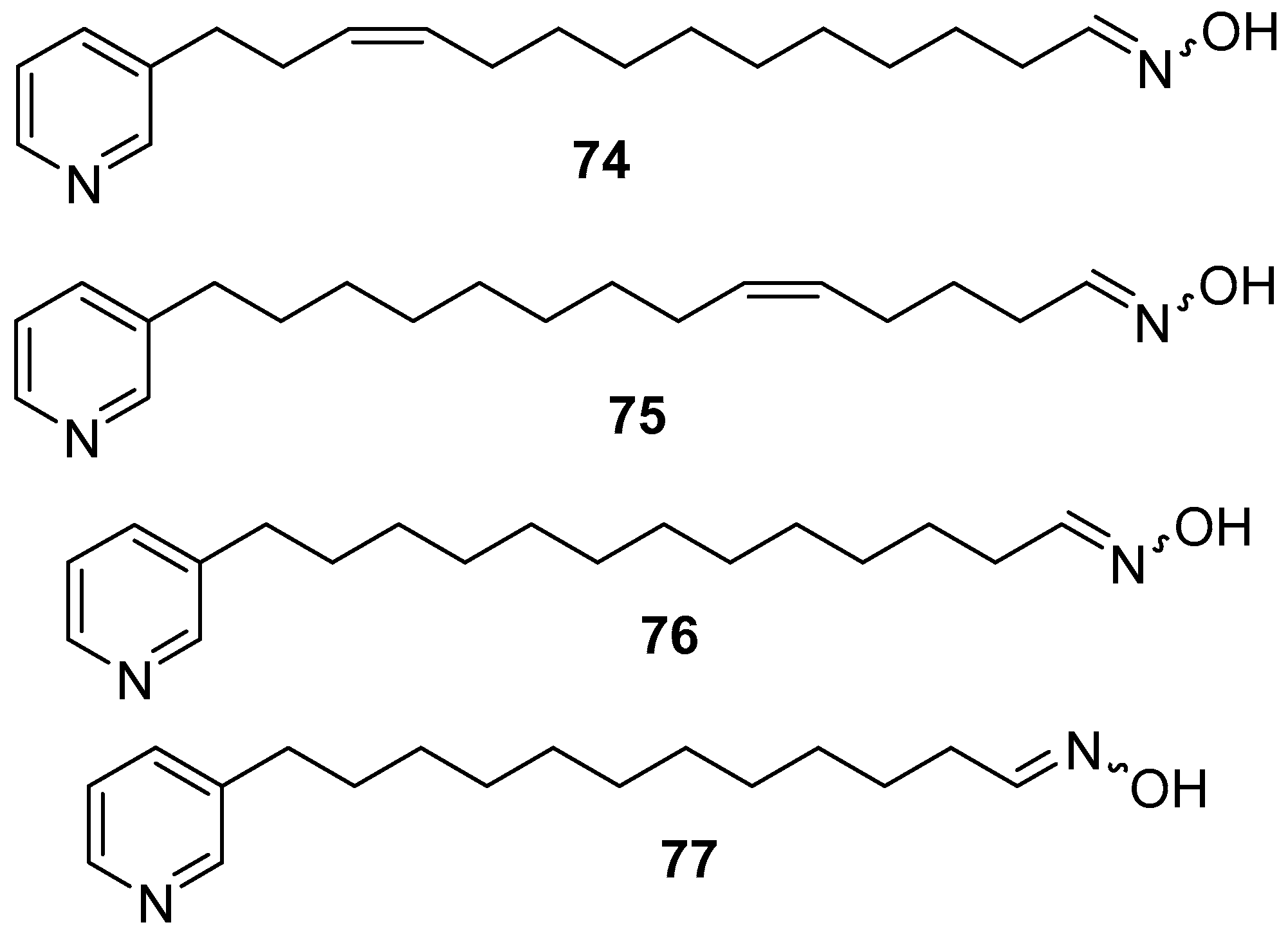
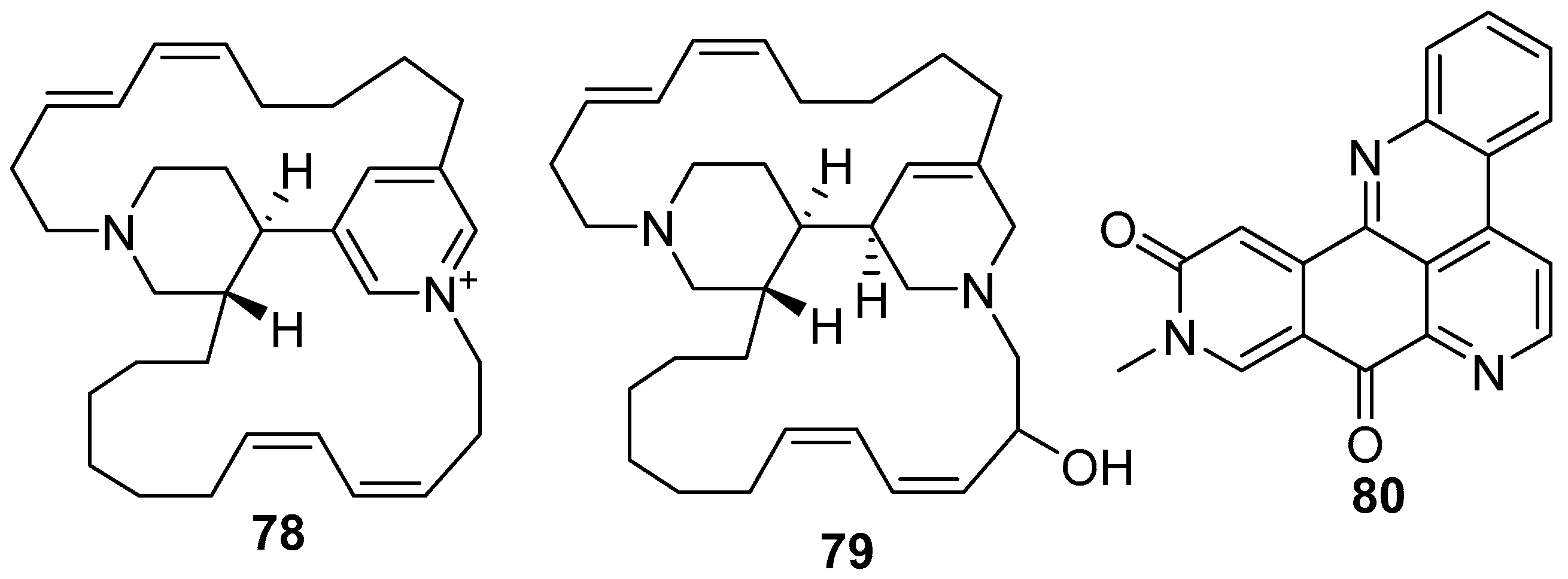
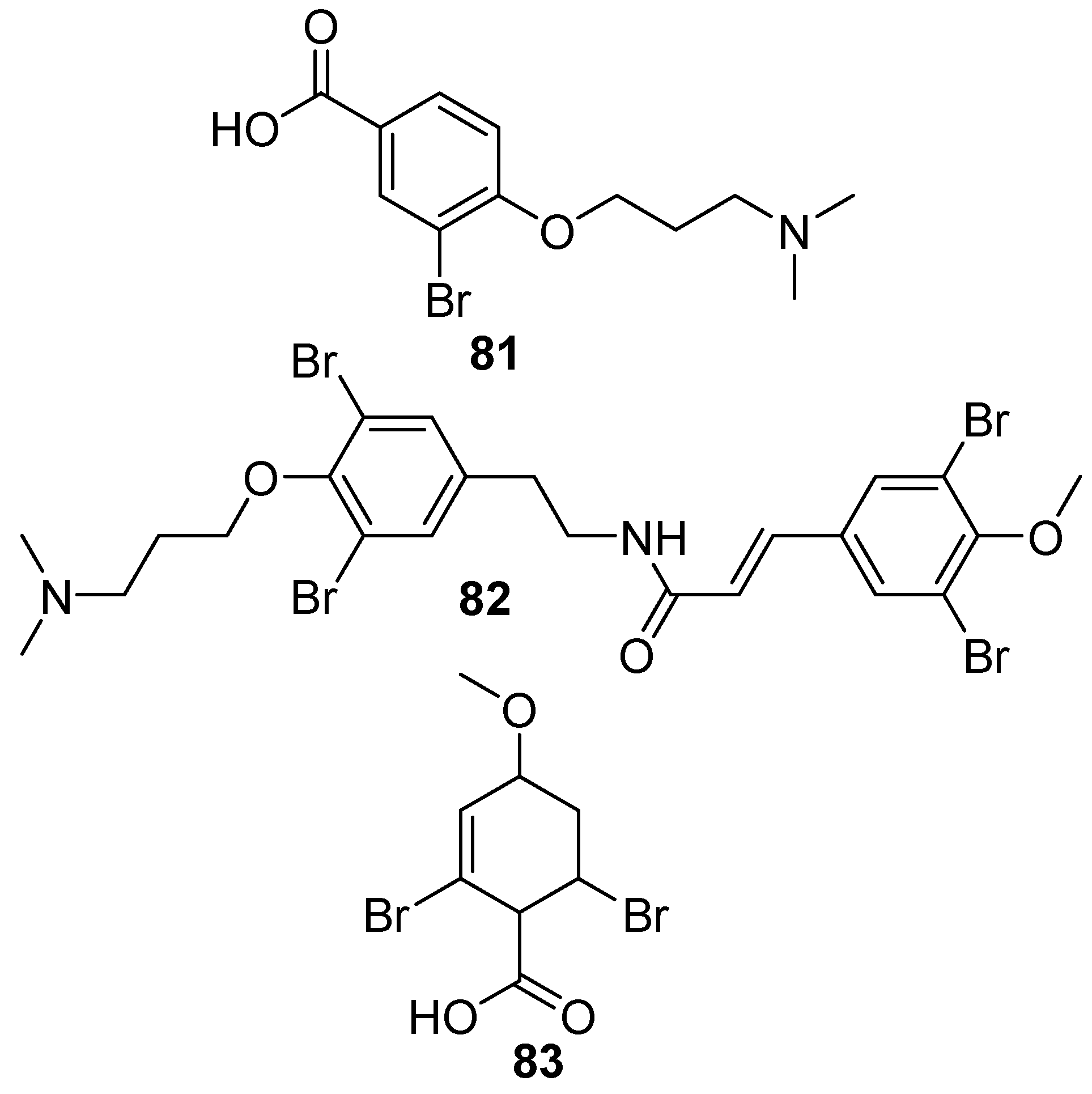
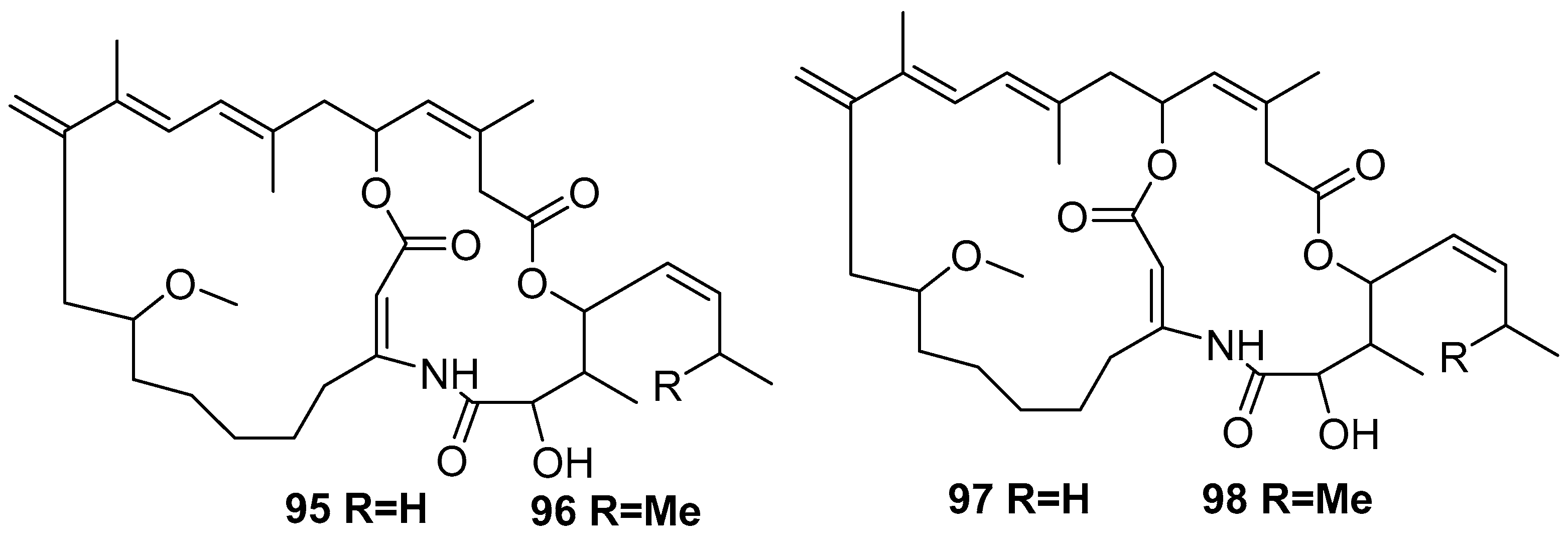
6. Undescribed Marine Sponges of the Genus Amphimedon
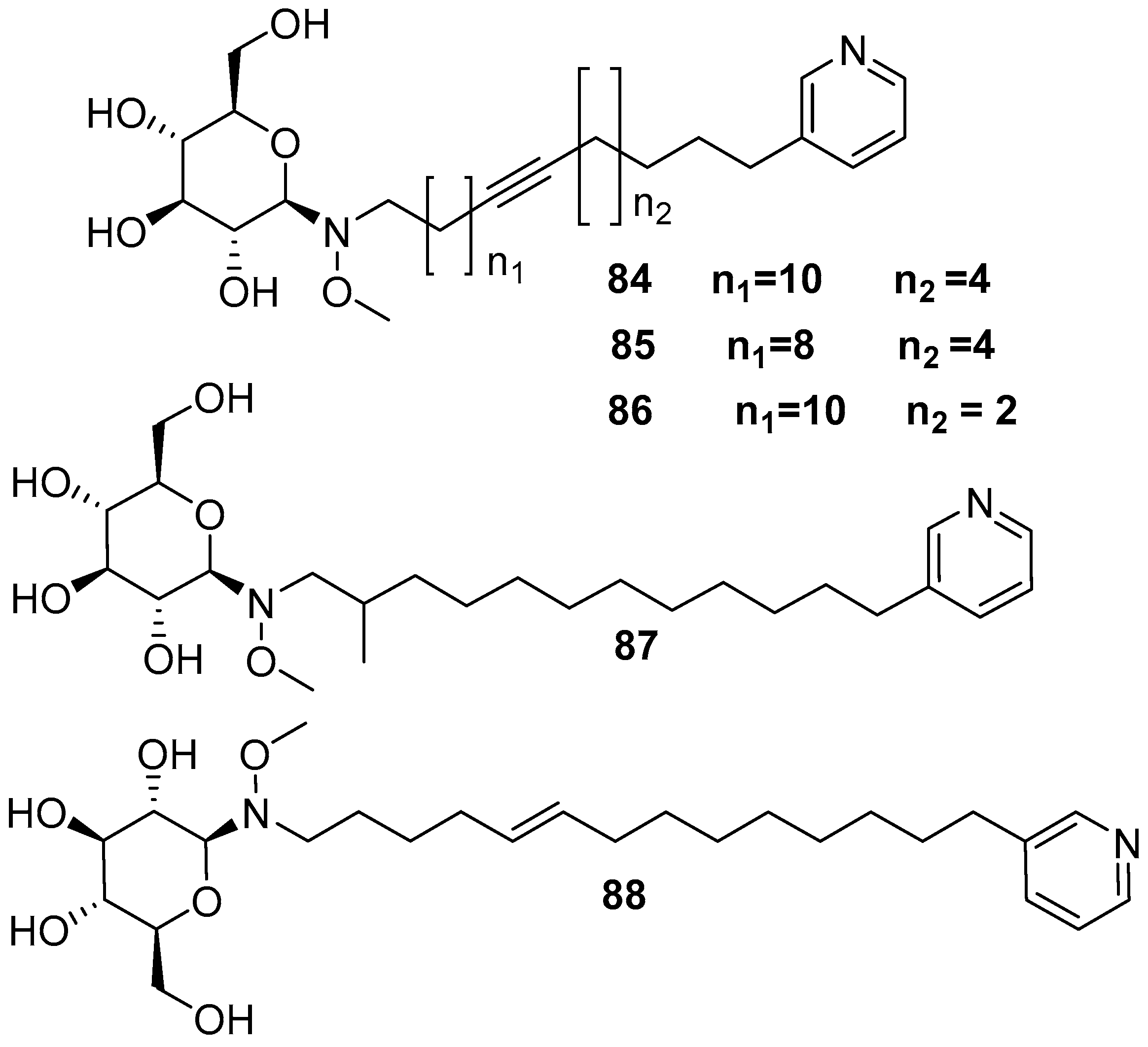
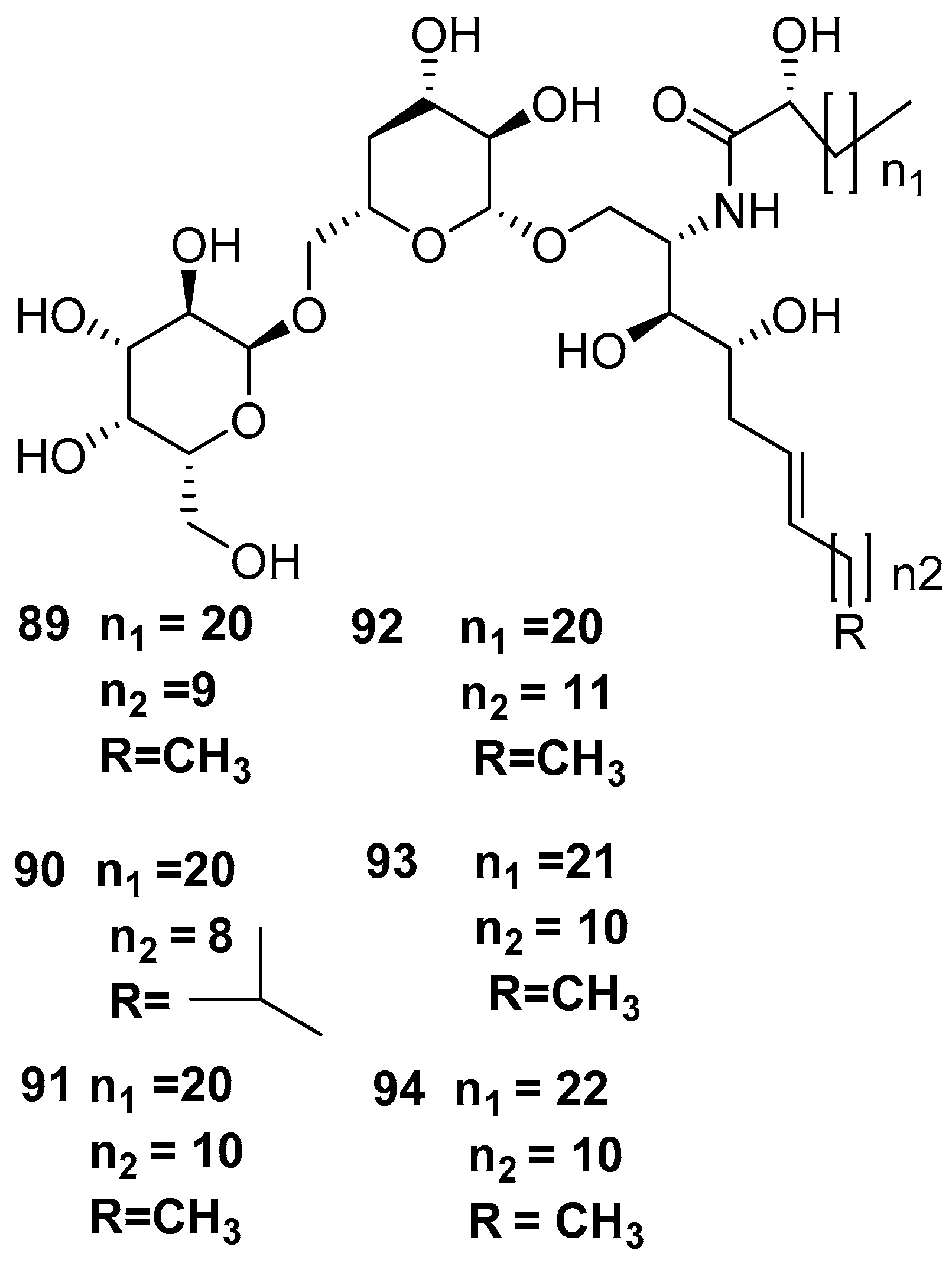
7. Bacteria Associated with the Genus Amphimedon
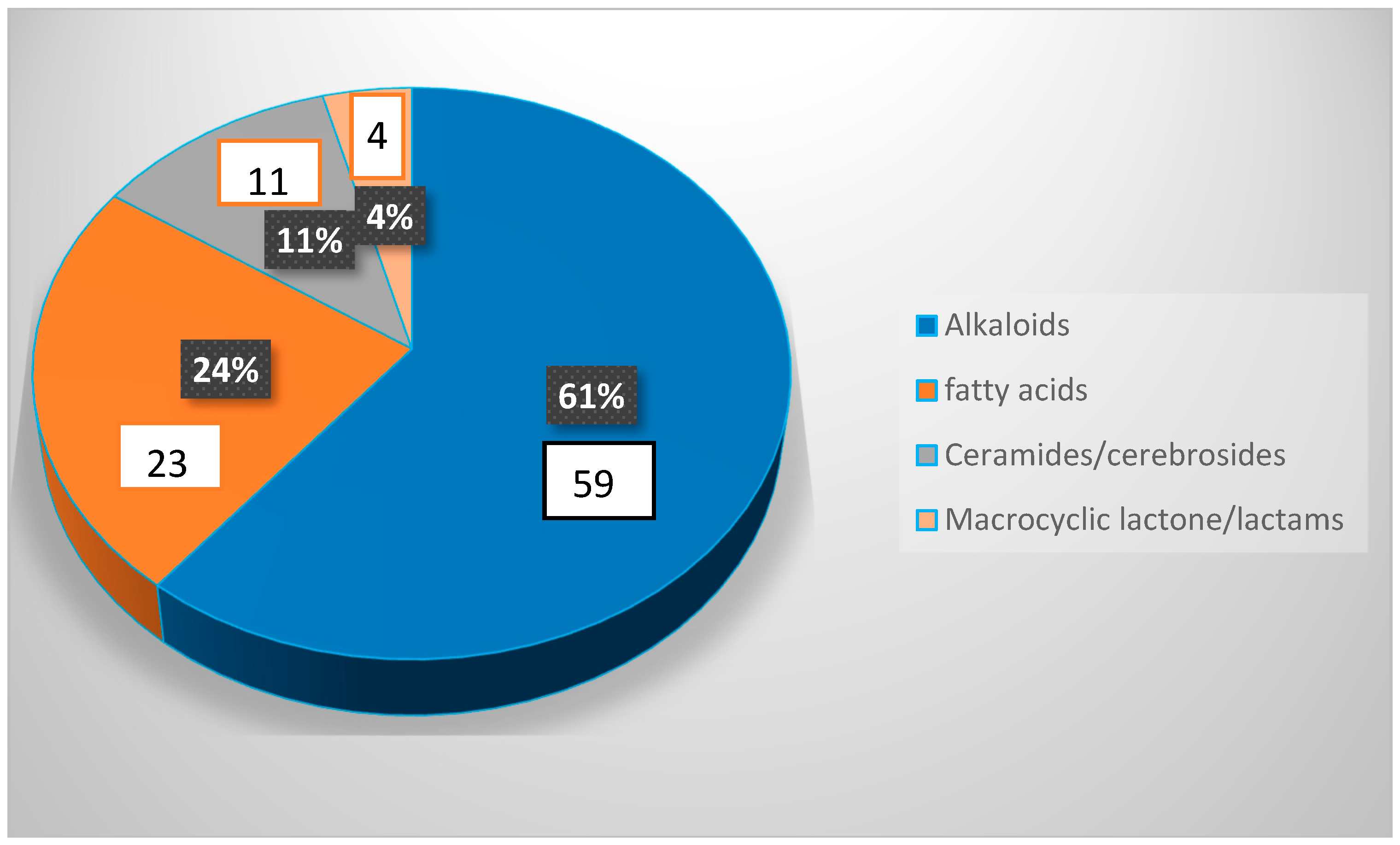
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, G.-P.; Yuan, J.; Sun, L.; She, Z.-G.; Wu, J.-H.; Lan, X.-J.; Zhu, X.; Lin, Y.-C.; Chen, S.-P. Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar. Drugs 2011, 9, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Hill, R.T. Microbes from marine sponges: A treasure trove of biodiversity for natural products discovery. In Microbial Diversity and Bioprospecting; American Society of Microbiology: Washington, DC, USA, 2004; pp. 177–190. [Google Scholar]
- Webster, N.S.; Taylor, M.W. Marine sponges and their microbial symbionts: Love and other relationships. Environ. Microbiol. 2012, 14, 335–346. [Google Scholar] [CrossRef]
- Mohamed, N.M.; Rao, V.; Hamann, M.T.; Kelly, M.; Hill, R.T. Monitoring bacterial diversity of the marine sponge Ircinia strobilina upon transfer into aquaculture. Appl. Environ. Microbiol. 2008, 74, 4133–4143. [Google Scholar] [CrossRef]
- Shieh, W.Y.; Lin, Y.M. Association of heterotrophic nitrogen-fixing bacteria with a marine sponge of Halichondria sp. Bull. Mar. Sci. 1994, 54, 557–564. [Google Scholar]
- Osinga, R.; Armstrong, E.; Burgess, J.G.; Hoffmann, F.; Reitner, J.; Schumann-Kindel, G. Sponge–microbe associations and their importance for sponge bioprocess engineering. Hydrobiologia 2001, 461, 55–62. [Google Scholar] [CrossRef]
- Kubota, T.; Kamijyo, Y.; Takahashi-Nakaguchi, A.; Fromont, J.; Gonoi, T.; Kobayashi, J.I. Zamamiphidin A, a new manzamine related alkaloid from an Okinawan marine sponge Amphimedon sp. Org. Lett. 2013, 15, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Kawasaki, N.; Kobayashi, J.I. Ircinols A and B, first antipodes of manzamine-related alkaloids from an Okinawan marine sponge. Tetrahedron 1994, 50, 7957–7960. [Google Scholar] [CrossRef]
- Sakurada, T.; Gill, M.B.; Frausto, S.; Copits, B.; Noguchi, K.; Shimamoto, K.; Swanson, G.T.; Sakai, R. Novel N-methylated 8-oxoisoguanines from Pacific sponges with diverse neuroactivities. J. Med. Chem. 2010, 53, 6089–6099. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Kubota, T.; Tsuda, M.; Mikami, Y.; Kobayashi, J.I. Pyrinodemins B–D, potent cytotoxic bis-pyridine alkaloids from marine sponge Amphimedon sp. Chem. Pharm. Bull. 2000, 48, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Takekawa, Y.; Matsunaga, S.; van Soest, R.W.; Fusetani, N. Amphimedosides, 3-alkylpyridine glycosides from a marine sponge Amphimedon sp. J. Nat. Prod. 2006, 69, 1503–1505. [Google Scholar] [CrossRef] [PubMed]
- Ovenden, S.P.; Capon, R.J.; Lacey, E.; Gill, J.H.; Friedel, T.; Wadsworth, D. Amphilactams A–D: Novel Nematocides from Southern Australian Marine Sponges of the Genus Amphimedon. J. Org. Chem. 1999, 64, 1140–1144. [Google Scholar] [CrossRef]
- Emura, C.; Higuchi, R.; Miyamoto, T.; Van Soest, R.W. Amphimelibiosides A–F, Six New Ceramide Dihexosides Isolated from a Japanese Marine Sponge Amphimedon sp. J. Org. Chem. 2005, 70, 3031–3038. [Google Scholar] [CrossRef]
- Nemoto, T.; Ojika, M.; Sakagami, Y. Amphimic acids, novel unsaturated C28 fatty acids as DNA topoisomerase I inhibitors from an Australian sponge Amphimedon sp. Tetrahedron Lett. 1997, 38, 5667–5670. [Google Scholar] [CrossRef]
- Tsuda, M.; Inaba, K.; Kawasaki, N.; Honma, K.; Kobayashi, J.I. Chiral resolution of (±)-keramaphidin B and isolation of manzamine L, a new β-carboline alkaloid from a sponge Amphimedon sp. Tetrahedron 1996, 52, 2319–2324. [Google Scholar] [CrossRef]
- Kubota, T.; Nakamura, K.; Kurimoto, S.-I.; Sakai, K.; Fromont, J.; Gonoi, T.; Kobayashi, J.I. Zamamidine D, a manzamine alkaloid from an Okinawan Amphimedon sp. marine sponge. J. Nat. Prod. 2017, 80, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Takahashi, Y.; Kubota, T.; Fromont, J.; Ishiyama, A.; Otoguro, K.; Yamada, H.; Ōmura, S.; Kobayashi, J.I. Corrigendum to Zamamidine C, 3,4-dihydro-6-hydroxy-10,11-epoxymanzamine A, and 3,4-dihydromanzamine J N-oxide, new manzamine alkaloids from sponge Amphimedon sp. Tetrahedron 2009, 31, 6263. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Takahashi, M.; Matsunaga, S.; Fusetani, N.; Van Soest, R.W. Hachijodines A–G: Seven New Cytotoxic 3-Alkylpyridine Alkaloids from Two Marine Sponges of the Genera Xestospongia and Amphimedon. J. Nat. Prod. 2000, 63, 682–684. [Google Scholar] [CrossRef] [PubMed]
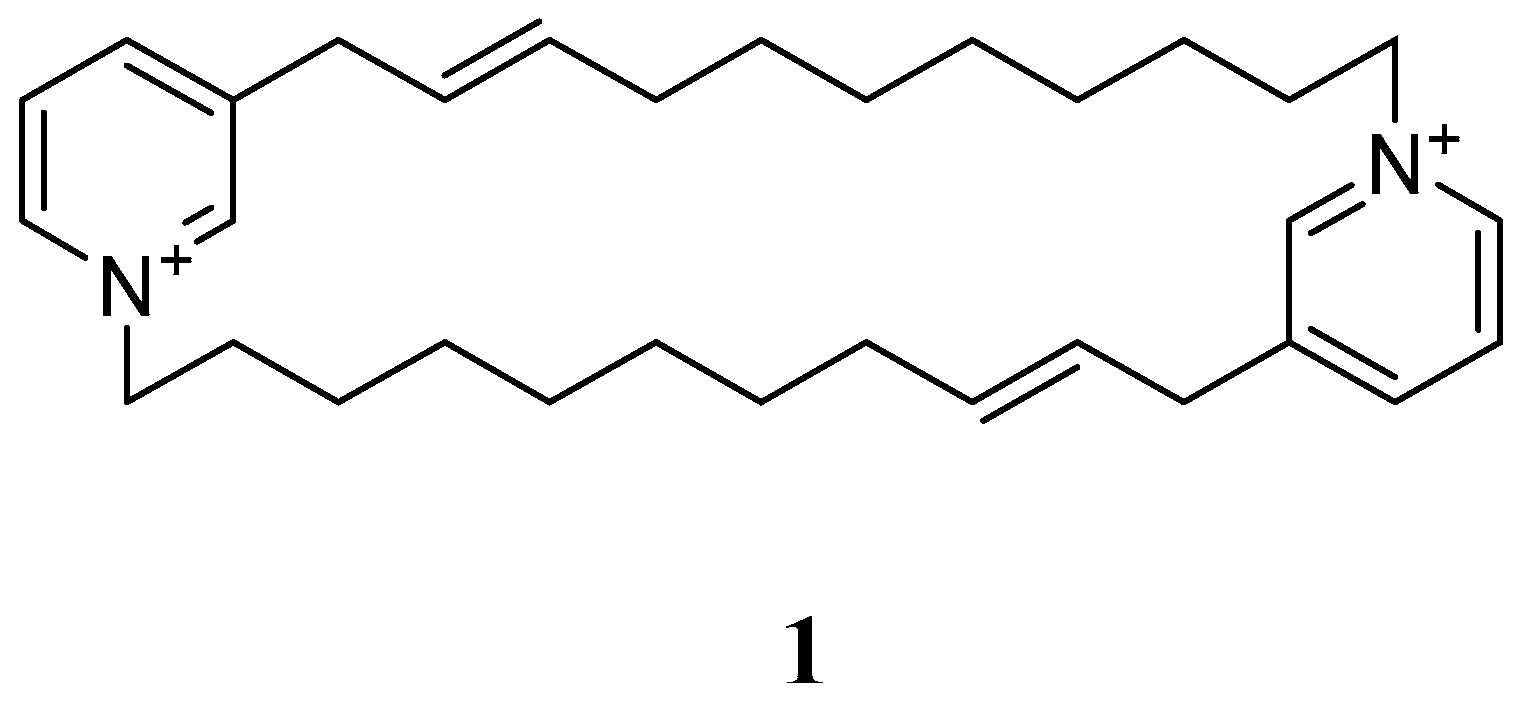
- Xu, N.J.; Sun, X.; Yan, X.J. A new cyclostellettamine from sponge Amphimedon compressa. Chin. Chem. Lett. 2007, 18, 947–950. [Google Scholar] [CrossRef]
- Albrizio, S.; Ciminiello, P.; Fattorusso, E.; Magno, S.; Pawlik, J.R. Amphitoxin, a new high molecular weight antifeedant pyridinium salt from the Caribbean sponge Amphimedon compressa. J. Nat. Prod. 1995, 58, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.N.; Gallimore, W.A.; Townsend, M.M.; Chambers, N.A.; Williams, L.A. Bioactivity of amphitoxin, the major constituent of the Jamaican sponge Amphimedon compressa. Chem. Biodivers. 2010, 7, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
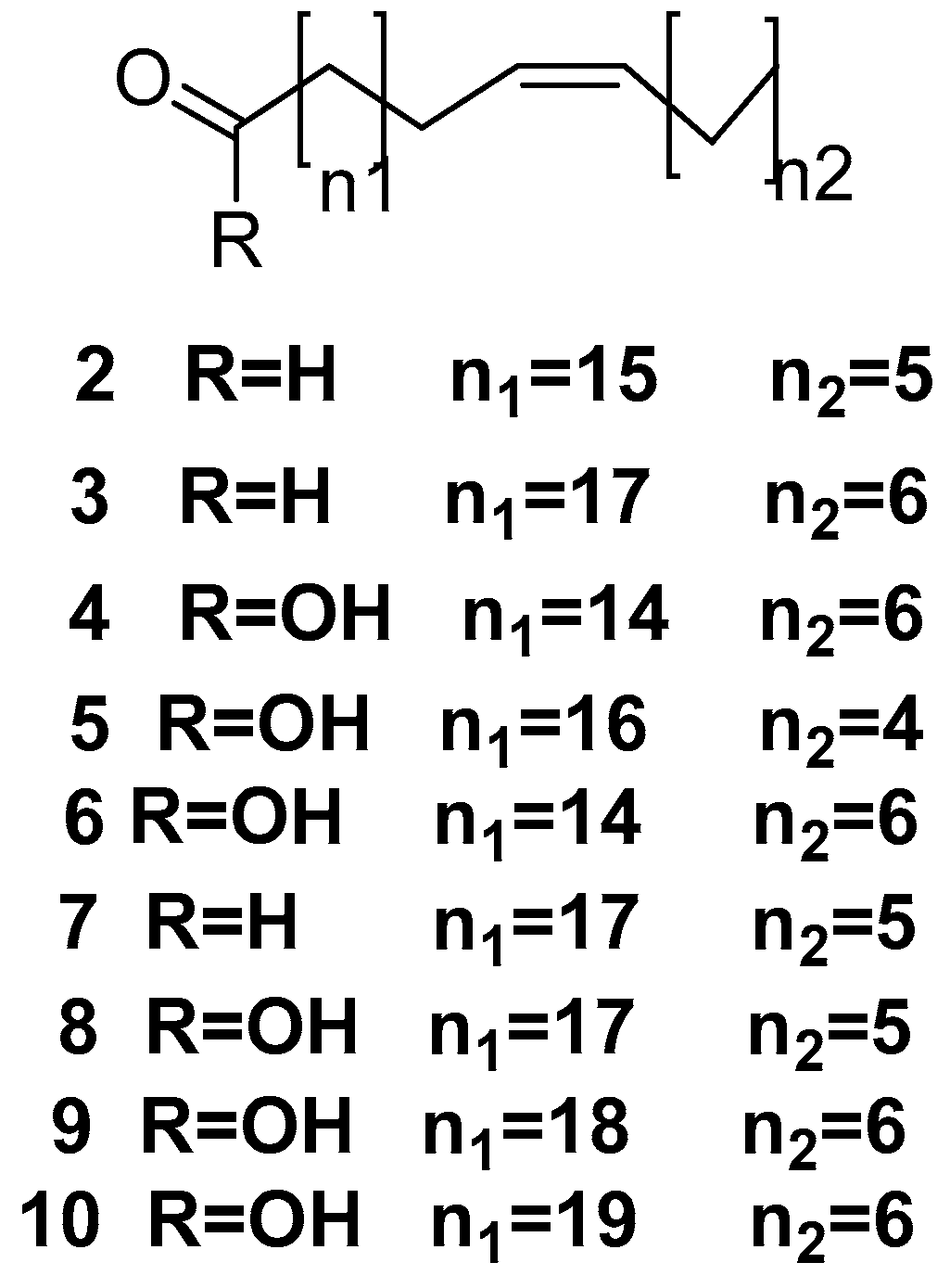
- Carballeira, N.M.; Negrón, V.; Reyes, E.D. Novel monounsaturated fatty acids from the sponges Amphimedon compressa and Mycale laevis. J. Nat. Prod. 1992, 55, 333–339. [Google Scholar] [CrossRef]
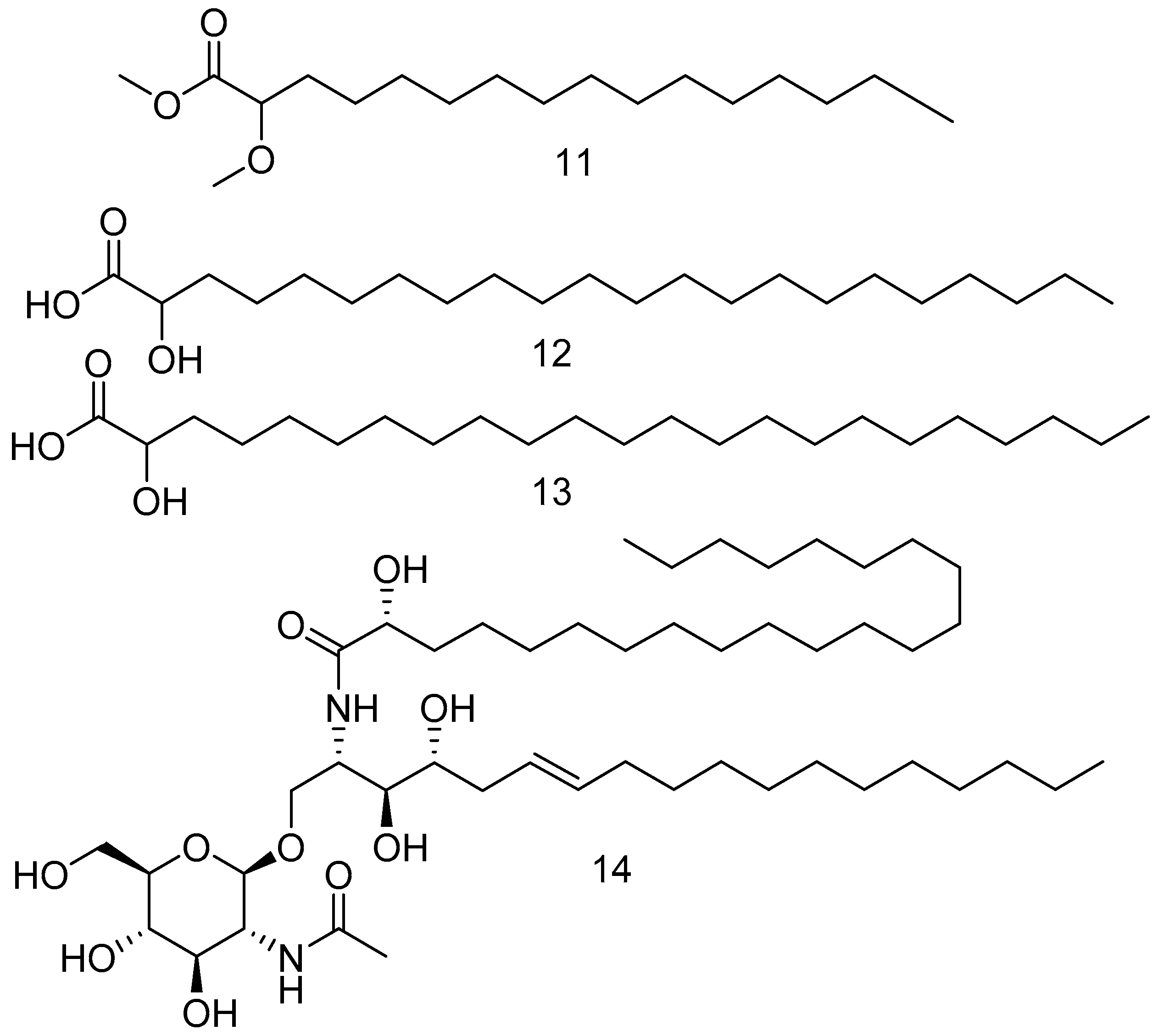
- Carballeira, N.M.; Lopez, M.R. On the Isolation of 2-Hydroxydocosanoic and 2-Hydroxytricosanoic Acids from the marine Sponge Amphimedon compressa. Lipids 1989, 24, 89–91. [Google Scholar] [CrossRef]
- Carballeira, N.M.; Colón, R.; Emiliano, A. Identification of 2-methoxyhexadecanoic acid in Amphimedon compressa. J. Nat. Prod. 1998, 61, 675–676. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Imperatore, C.; Mangoni, A.; Teta, R. Amphiceramide A and B, novel glycosphingolipids from the marine sponge Amphimedon compressa. Eur. J. Org. Chem. 2009, 2009, 2112–2119. [Google Scholar] [CrossRef]
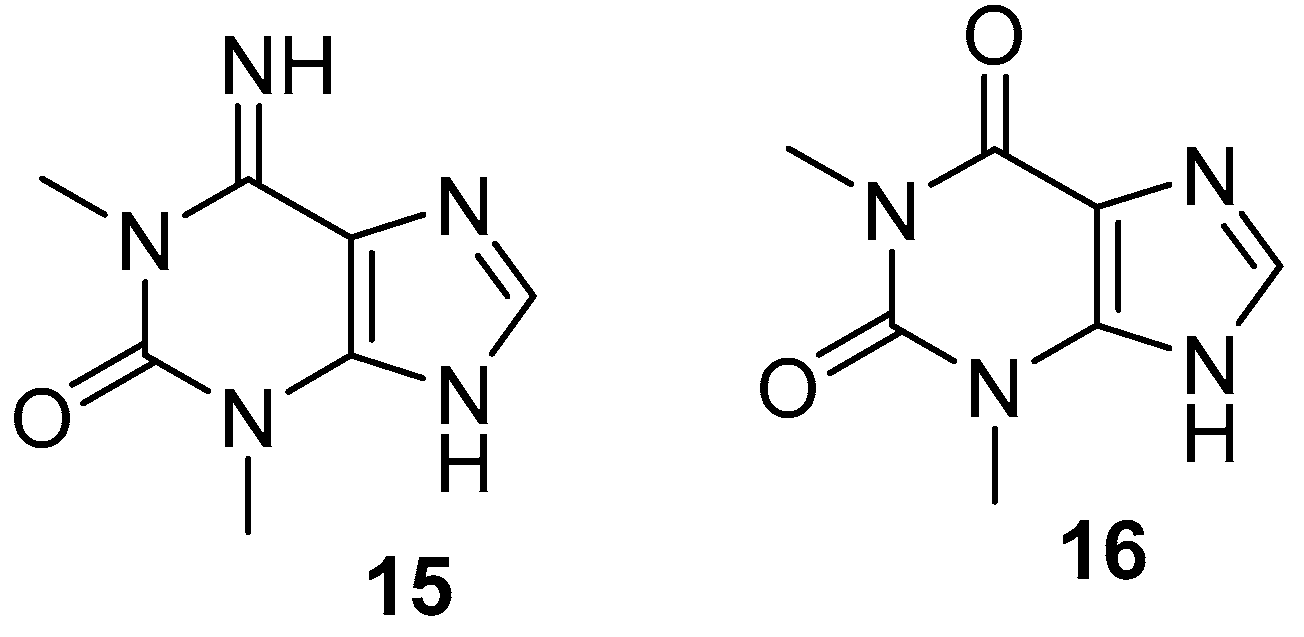
- Mitchell, S.S.; Whitehill, A.B.; Trapido-Rosenthal, H.G.; Ireland, C.M. Isolation and characterization of 1,3-dimethylisoguanine from the Bermudian sponge Amphimedon viridis. J. Nat. Prod. 1997, 60, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Chehade, C.C.; Dias, R.L.; Berlinck, R.G.; Ferreira, A.G.; Costa, L.V.; Rangel, M.; Malpezzi, E.L.; de Freitas, J.C.; Hajdu, E. 1,3-Dimethylisoguanine, a new purine from the marine sponge Amphimedon viridis. J. Nat. Prod. 1997, 60, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Sharangi, A.B. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.)—A review. Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Hirsch, S.; Kashman, Y. New glycosphingolipids from marine organisms. Tetrahedron 1989, 45, 3897–3906. [Google Scholar] [CrossRef]
- Carballeira, N.M.; Restituyo, J. Identification of the new 11,15-icosadienoic acid and related acids in the sponge Amphimedon complanata. J. Nat. Prod. 1991, 54, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, N.M.; Alicea, J. The first naturally occurring α-methoxylated branched-chain fatty acids from the phospholipids of Amphimedon complanata. Lipids 2001, 36, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Higa, T.; Jefford, C.W.; Bernardinelli, G. Manzamine A, a novel antitumor alkaloid from a sponge. J. Am. Chem. Soc. 1986, 108, 6404–6405. [Google Scholar] [CrossRef]
- Sakai, R.; Kohmoto, S.; Higa, T.; Jefford, C.W.; Bernardinelli, G. Manzamine B and C, two novel alkaloids from the sponge Haliclona sp. Tetrahedron Lett. 1987, 28, 5493–5496. [Google Scholar] [CrossRef]
- Kondo, K.; Shigemori, H.; Kikuchi, Y.; Ishibashi, M.; Sasaki, T.; Kobayashi, J. Ircinals A and B from the Okinawan marine sponge Ircinia sp.: Plausible biogenetic precursors of manzamine alkaloids. J. Org. Chem. 1992, 57, 2480–2483. [Google Scholar] [CrossRef]
- Radwan, M.; Hanora, A.; Khalifa, S.; Abou-El-Ela, S.H. Manzamines: A potential for novel cures. Cell Cycle 2012, 11, 1765–1772. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Dunbar, D.C.; Perry, T.L.; Wilkins, S.P.; Hamann, M.T.; Greenplate, J.T.; Wideman, M.A. Marine natural products as prototype insecticidal agents. J. Agric. Food Chem. 1997, 45, 2735–2739. [Google Scholar] [CrossRef]
- Kobayashi, J.I.; Tsuda, M.; Kawasaki, N.; Sasaki, T.; Mikami, Y. 6-Hydroxymanzamine A and 3,4-dihydromanzamine A, new alkaloids from the Okinawan marine sponge Amphimedon sp. J. Nat. Prod. 1994, 57, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Gunasekera, S.P.; Pomponi, S.A.; Sennett, S.H. Anti-Inflammatory Uses of Manzamines. U.S. Patent 6,387,916, 14 May 2002. [Google Scholar]
- Takahashi, Y.; Kubota, T.; Fromont, J.; Kobayashi, J.I. Zamamidines A and B, new manzamine alkaloids from the sponge Amphimedon species. Org. Lett. 2008, 11, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Takahashi, Y.; Kubota, T.; Fromont, J.; Ishiyama, A.; Otoguro, K.; Yamada, H.; Ōmura, S.; Kobayashi, J.I. Zamamidine C, 3,4-dihydro-6-hydroxy-10,11-epoxymanzamine A, and 3,4-dihydromanzamine J N-oxide, new manzamine alkaloids from sponge Amphimedon sp. Tetrahedron 2009, 65, 2313–2317. [Google Scholar] [CrossRef]
- Tsuda, M.; Kawasaki, N.; Kobayashi, J. i. Keramaphidin C and keramamine C plausible biogenetic precursors of manzamine C from an Okinawan marine sponge. Tetrahedron Lett. 1994, 35, 4387–4388. [Google Scholar] [CrossRef]
- Kobayashi, J.I.; Tsuda, M.; Kawasaki, N.; Matsumoto, K.; Adachi, T. Keramaphidin B, a novel pentacyclic alkaloid from a marine sponge Amphimedon sp.: A plausible biogenetic precursor of manzamine alkaloids. Tetrahedron Lett. 1994, 35, 4383–4386. [Google Scholar] [CrossRef]
- Watanabe, D.; Tsuda, M.; Kobayashi, J.I. Three new manzamine congeners from Amphimedon sponge. J. Nat. Prod. 1998, 61, 689–692. [Google Scholar] [CrossRef]
- Kobayashi, J.I.; Watanabe, D.; Kawasaki, N.; Tsuda, M. Nakadomarin A, a novel hexacyclic manzamine-related alkaloid from Amphimedon sponge. J. Org. Chem. 1997, 62, 9236–9239. [Google Scholar] [CrossRef]
- Tsuda, M.; Hirano, K.; Kubota, T.; Kobayashi, J.I. Pyrinodemin A, a cytotoxic pyridine alkaloid with an isoxazolidine moiety from sponge Amphimedon sp. Tetrahedron Lett. 1999, 40, 4819–4820. [Google Scholar] [CrossRef]
- Kura, K.I.; Kubota, T.; Fromont, J.; Kobayashi, J.I. Pyrinodemins E and F, new 3-alkylpyridine alkaloids from sponge Amphimedon sp. Bioorg. Med. Chem. Lett. 2011, 21, 267–270. [Google Scholar] [CrossRef]
- Kubota, T.; Kura, K.I.; Fromont, J.; Kobayashi, J.I. Pyrinodemins G–I, new bis-3-alkylpyridine alkaloids from a marine sponge Amphimedon sp. Tetrahedron 2013, 69, 96–100. [Google Scholar] [CrossRef]
- Kubota, T.; Nishi, T.; Fukushi, E.; Kawabata, J.; Fromont, J.; Kobayashi, J. Nakinadine A, a novel bis-pyridine alkaloid with a β-amino acid moiety from sponge Amphimedon sp. Tetrahedron Lett. 2007, 48, 4983–4985. [Google Scholar] [CrossRef]
- Nishi, T.; Kubota, T.; Fromont, J.; Sasaki, T.; Kobayashi, J.I. Nakinadines B–F: New pyridine alkaloids with a β-amino acid moiety from sponge Amphimedon sp. Tetrahedron 2008, 64, 3127–3132. [Google Scholar] [CrossRef]
- Matsunaga, S.; Miyata, Y.; van Soest, R.W.; Fusetani, N. Tetradehydrohalicyclamine A and 22-hydroxyhalicyclamine A, new cytotoxic bis-piperidine alkaloids from a marine sponge Amphimedon sp. J. Nat. Prod. 2004, 67, 1758–1760. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.J.; Agarwal, S.K.; Gunasekera, S.P.; Schmidt, P.G.; Shoolery, J.N. Amphimedine, new aromatic alkaloid from a pacific sponge, Amphimedon sp. Carbon connectivity determination from natural abundance carbon-13-carbon-13 coupling constants. J. Am. Chem. Soc. 1983, 105, 4835–4836. [Google Scholar] [CrossRef]
- Campos, P.-E.; Wolfender, J.-L.; Queiroz, E.F.; Marcourt, L.; Al-Mourabit, A.; De Voogd, N.; Illien, B.; Gauvin-Bialecki, A. Amphimedonoic acid and psammaplysene E, novel brominated alkaloids from Amphimedon sp. Tetrahedron Lett. 2017, 58, 3901–3904. [Google Scholar] [CrossRef]
- Nemoto, T.; Yoshino, G.; Ojika, M.; Sakagami, Y. Amphimic acids and related long-chain fatty acids as DNA topoisomerase I inhibitors from an Australian sponge, Amphimedon sp.: Isolation, structure, synthesis, and biological evaluation. Tetrahedron 1997, 53, 16699–16710. [Google Scholar] [CrossRef]
- Garson, M.J.; Thompson, J.E.; Larsen, R.M.; Battershill, C.N.; Murphy, P.T.; Bergquist, P.R. Terpenes in sponge cell membranes: Cell separation and membrane fractionation studies with the tropical marine sponge Amphimedon sp. Lipids 1992, 27, 378–388. [Google Scholar] [CrossRef]
- Sathiyanarayanan, G.; Saibaba, G.; Kiran, G.S.; Yang, Y.-H.; Selvin, J. Marine sponge-associated bacteria as a potential source for polyhydroxyalkanoates. Crit. Rev. Microbiol. 2017, 43, 294–312. [Google Scholar] [CrossRef]
- Kelman, D.; Kashman, Y.; Hill, R.T.; Rosenberg, E.; Loya, Y. Chemical warfare in the sea: The search for antibiotics from Red Sea corals and sponges. Pure Appl. Chem. 2009, 81, 1113–1121. [Google Scholar] [CrossRef]
- Kelman, D.; Kashman, Y.; Rosenberg, E.; Ilan, M.; Ifrach, I.; Loya, Y. Antimicrobial activity of the reef sponge Amphimedon viridis from the Red Sea: Evidence for selective toxicity. Aquat. Microb. Ecol. 2001, 24, 9–16. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Bayer, K.; Hentschel, U. Diversity, abundance and natural products of marine sponge-associated actinomycetes. Nat. Prod. Rep. 2014, 31, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Gauthier, M.E.; Degnan, B.M.; Ng, Y.K.; Hewavitharana, A.K.; Shaw, P.N.; Fuerst, J.A. Diversity of Mycobacterium species from marine sponges and their sensitivity to antagonism by sponge-derived rifamycin-synthesizing actinobacterium in the genus Salinispora. FEMS Microbiol. Lett. 2010, 313, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T. Polyketide and nonribosomal peptide antibiotics: Modularity and versatility. Science 2004, 303, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.F.; Hamann, M.T.; Hill, R.; Kelly, M. The manzamine alkaloids. Alkaloids Chem. Biol. 2003, 60, 207–286. [Google Scholar]
- Carballeira, N.M.; Shalabi, F.; Maldonado, M.E. Identification of the new 18-hexacosenoic acid in the sponge Thalysias juniperina. Lipids 1990, 25, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Garson, M.J.; Zimmermann, M.P.; Hoberg, M.; Larsen, R.M.; Battershill, C.N.; Murphy, P.T. Isolation of brominated long-chain fatty acids from the phospholipids of the tropical marine sponge Amphimedon terpenensis. Lipids 1993, 28, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Garson, M.J.; Partali, V.; Liaaen-Jensen, S.; Stoilov, I.L. Isoprenoid biosynthesis in a marine sponge of the Amphimedon genus: Incorporation studies with [1-14C] acetate, [4-14C] cholesterol and [2-14C] mevalonate. Comp. Biochem. Physiol. B 1988, 91, 293–300. [Google Scholar] [CrossRef]
- Karuso, P.; Scheuer, P.J. Biosynthesis of isocyanoterpenes in sponges. J. Org. Chem. 1989, 54, 2092–2095. [Google Scholar] [CrossRef]
- Young, I.S.; Kerr, M.A. Total synthesis of (+)-nakadomarin A. J. Am.Chem. Soc. 2007, 129, 1465–1469. [Google Scholar] [CrossRef]
- Chavda, J.K.; Procopiou, P.A.; Horton, P.N.; Coles, S.J.; Porter, M.J. Synthetic Studies Towards the Core Structure of Nakadomarin A by a Thioamide-Based Strategy. Eur. J. Org. Chem. 2014, 2014, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, K.A.; Crabtree, S.R.; Mander, L.N. A formal total synthesis of the marine diterpenoid, diisocyanoadociane. In Strategies and Tactics in Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 2008; Volume 7, pp. 35–58. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shady, N.H.; Fouad, M.A.; Salah Kamel, M.; Schirmeister, T.; Abdelmohsen, U.R. Natural Product Repertoire of the Genus Amphimedon. Mar. Drugs 2019, 17, 19. https://doi.org/10.3390/md17010019
Shady NH, Fouad MA, Salah Kamel M, Schirmeister T, Abdelmohsen UR. Natural Product Repertoire of the Genus Amphimedon. Marine Drugs. 2019; 17(1):19. https://doi.org/10.3390/md17010019
Chicago/Turabian StyleShady, Nourhan Hisham, Mostafa A. Fouad, Mohamed Salah Kamel, Tanja Schirmeister, and Usama Ramadan Abdelmohsen. 2019. "Natural Product Repertoire of the Genus Amphimedon" Marine Drugs 17, no. 1: 19. https://doi.org/10.3390/md17010019
APA StyleShady, N. H., Fouad, M. A., Salah Kamel, M., Schirmeister, T., & Abdelmohsen, U. R. (2019). Natural Product Repertoire of the Genus Amphimedon. Marine Drugs, 17(1), 19. https://doi.org/10.3390/md17010019





