Stress-Induced Mucus Secretion and Its Composition by a Combination of Proteomics and Metabolomics of the Jellyfish Aurelia coerulea
Abstract
1. Introduction
2. Results
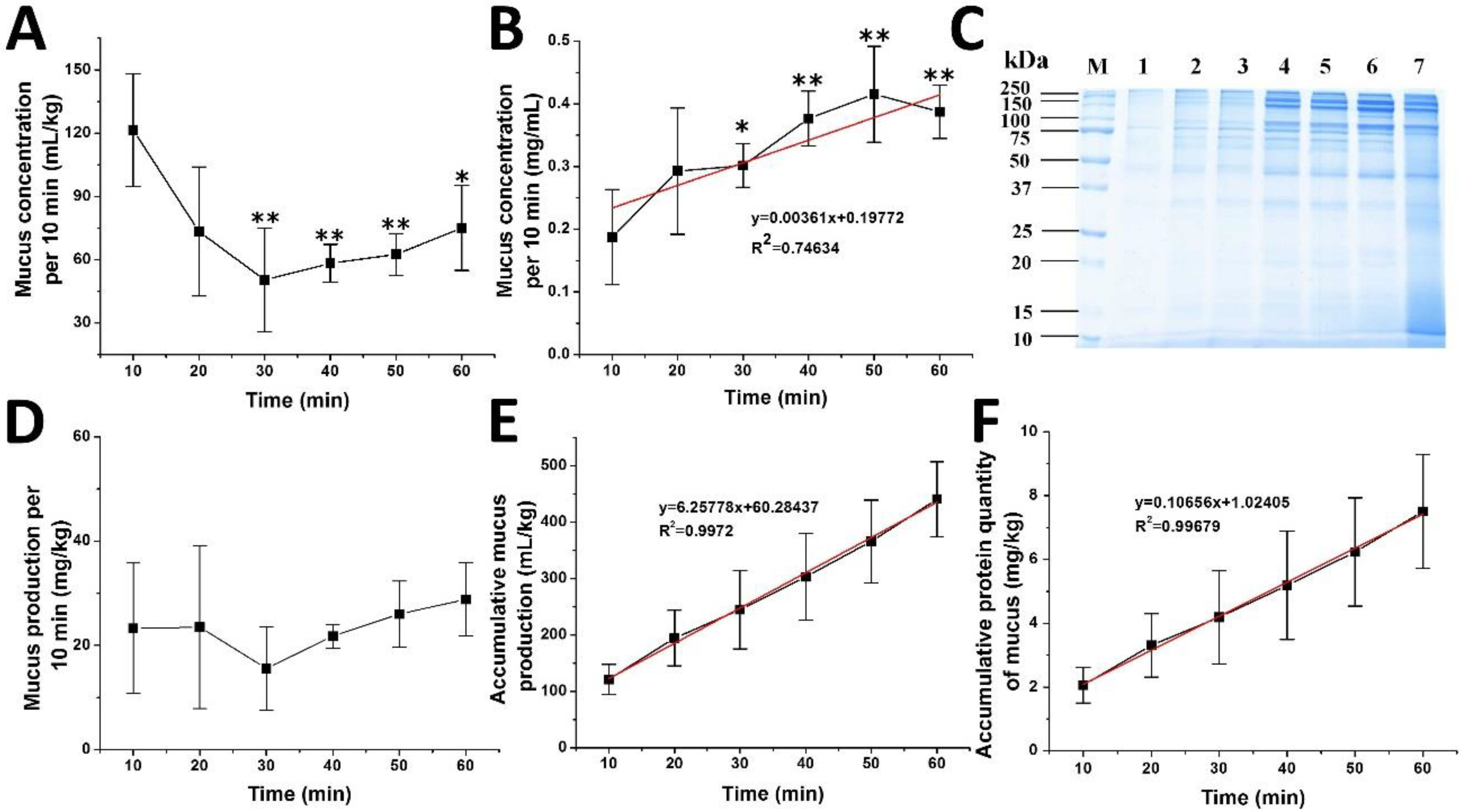
2.1. Stress-Induced Mucus Secretion and Autolysis of A. coerulea
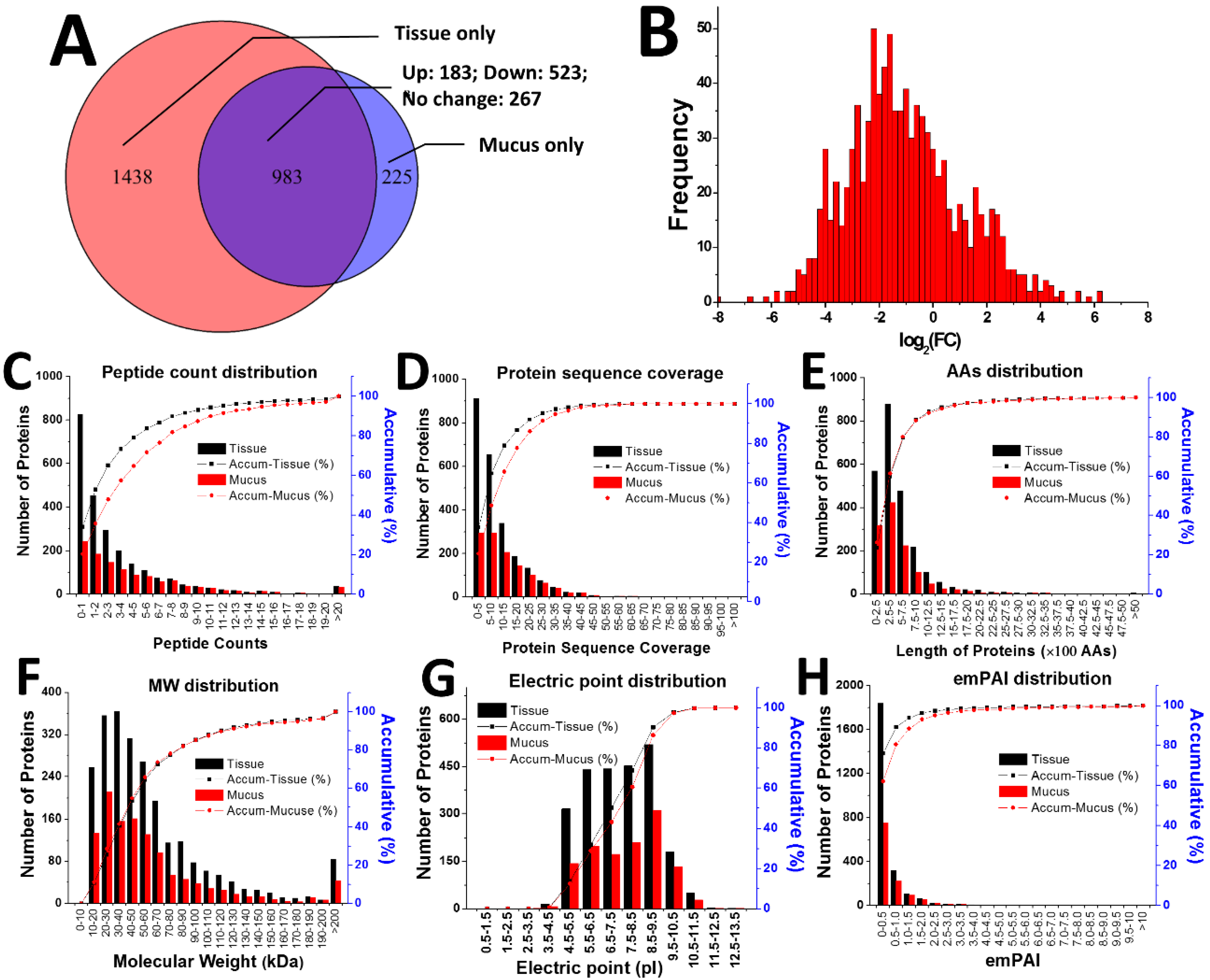
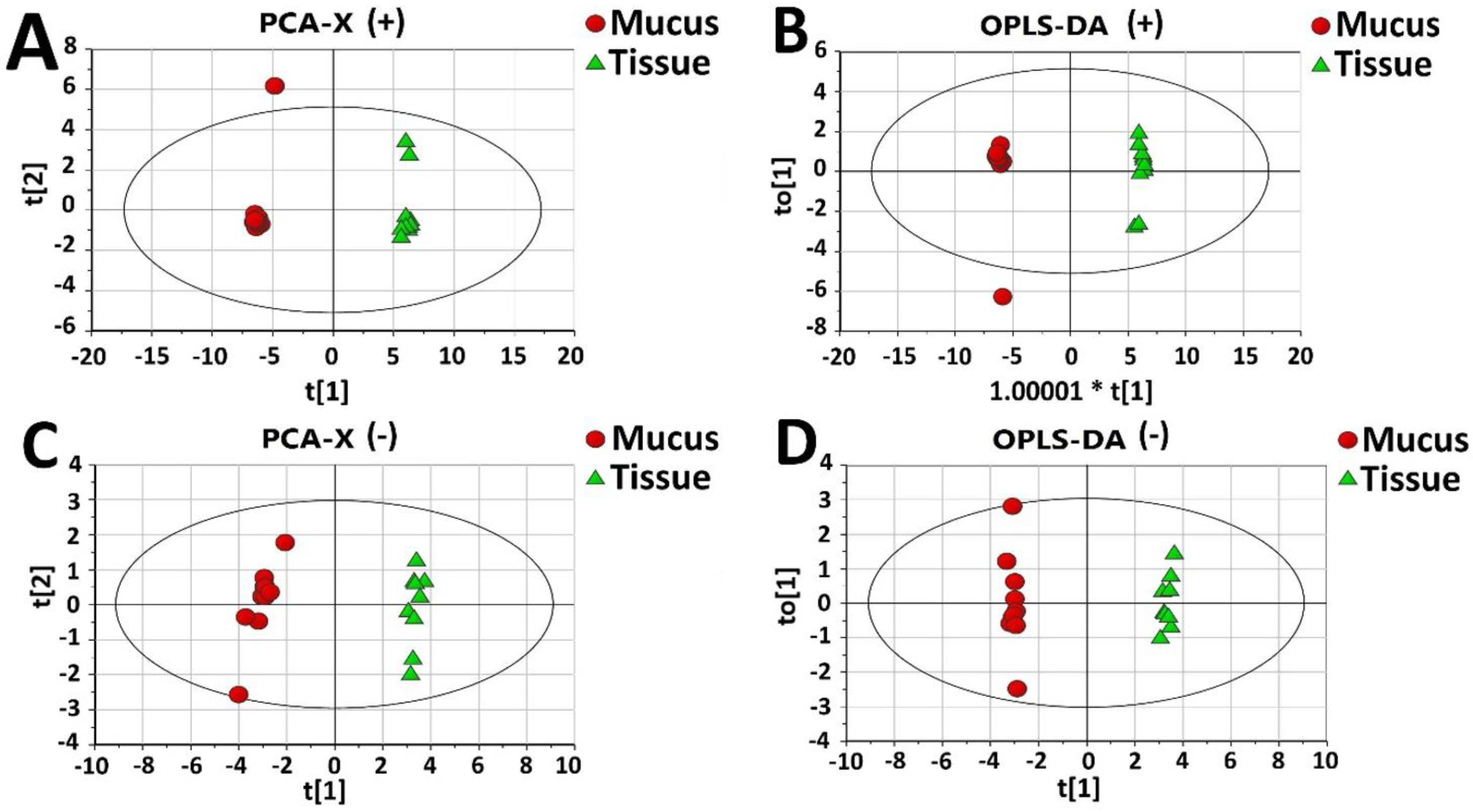
2.2. Proteomic Comparison of Secreted Mucus and Tissue Homogenate
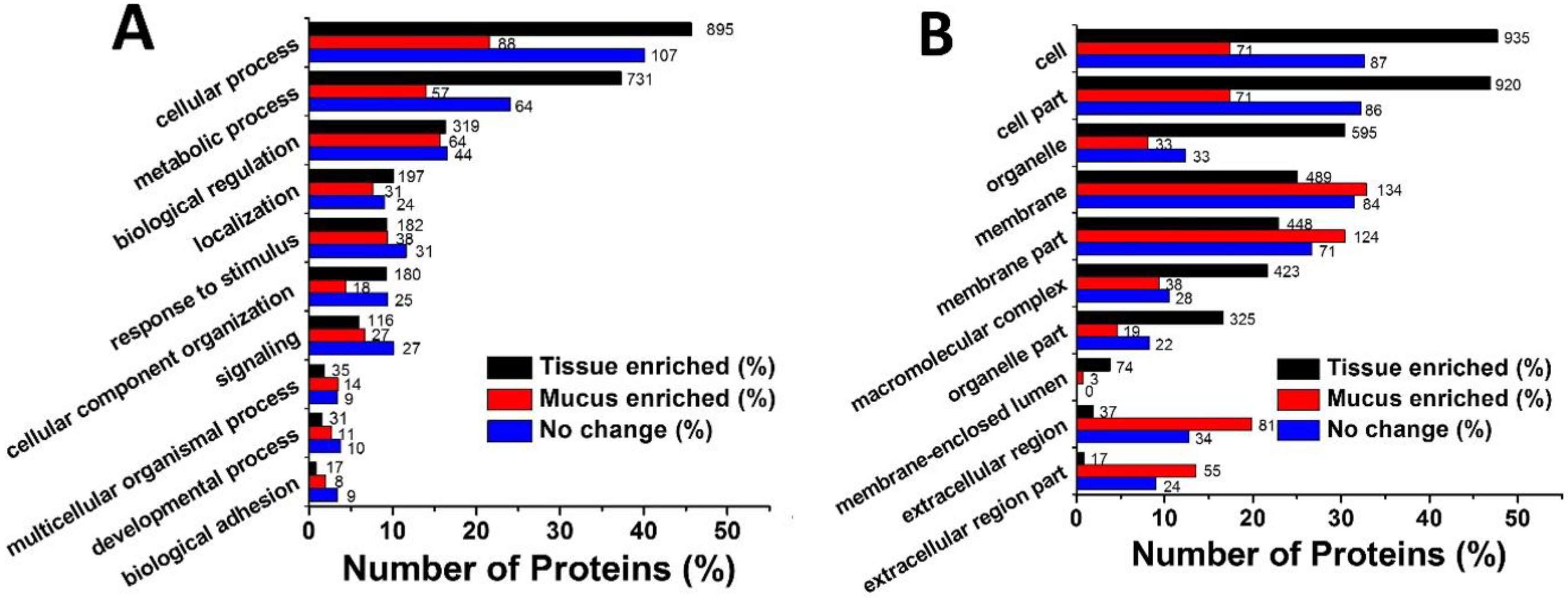
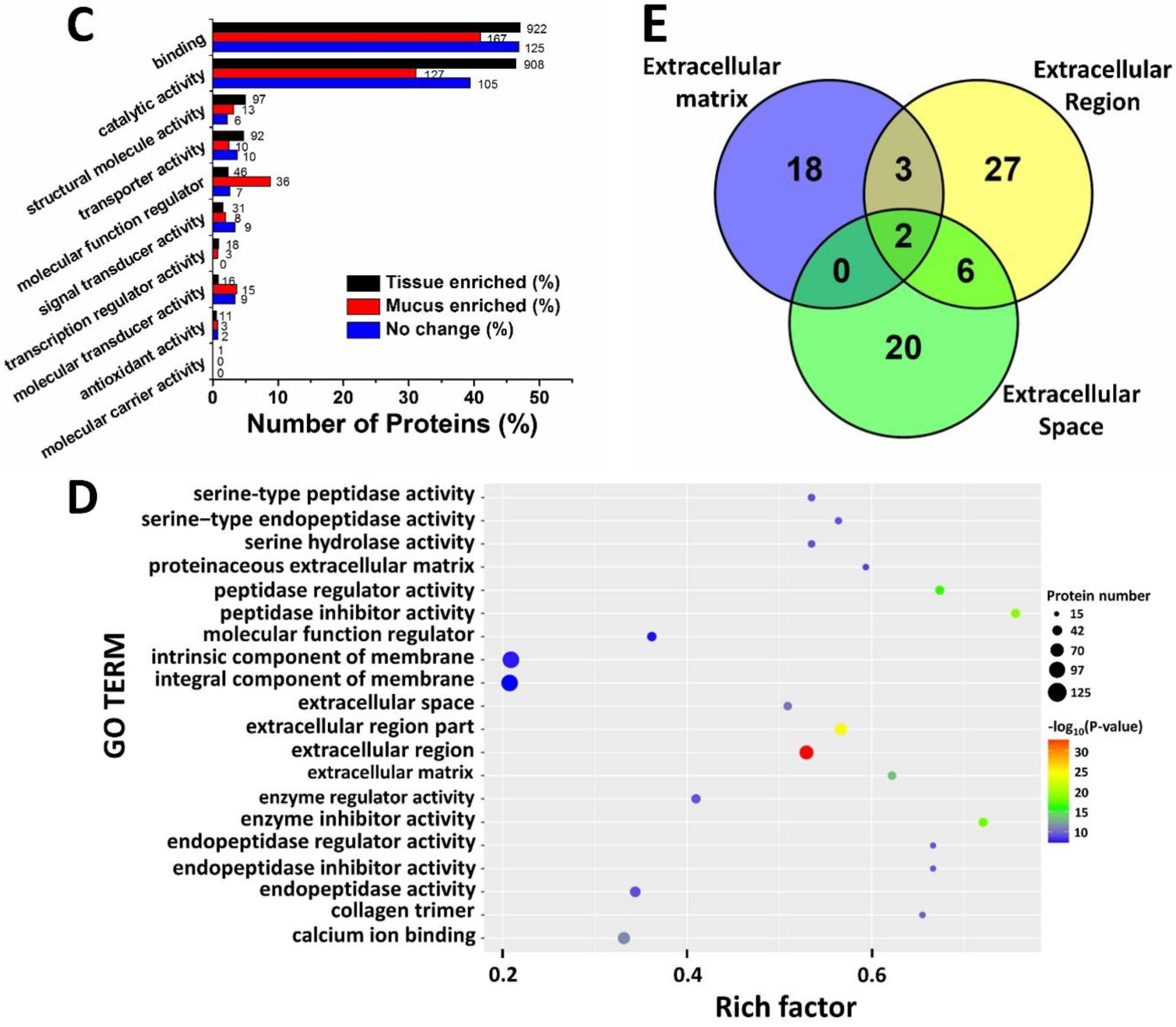
2.3. Gene Ontology Analysis
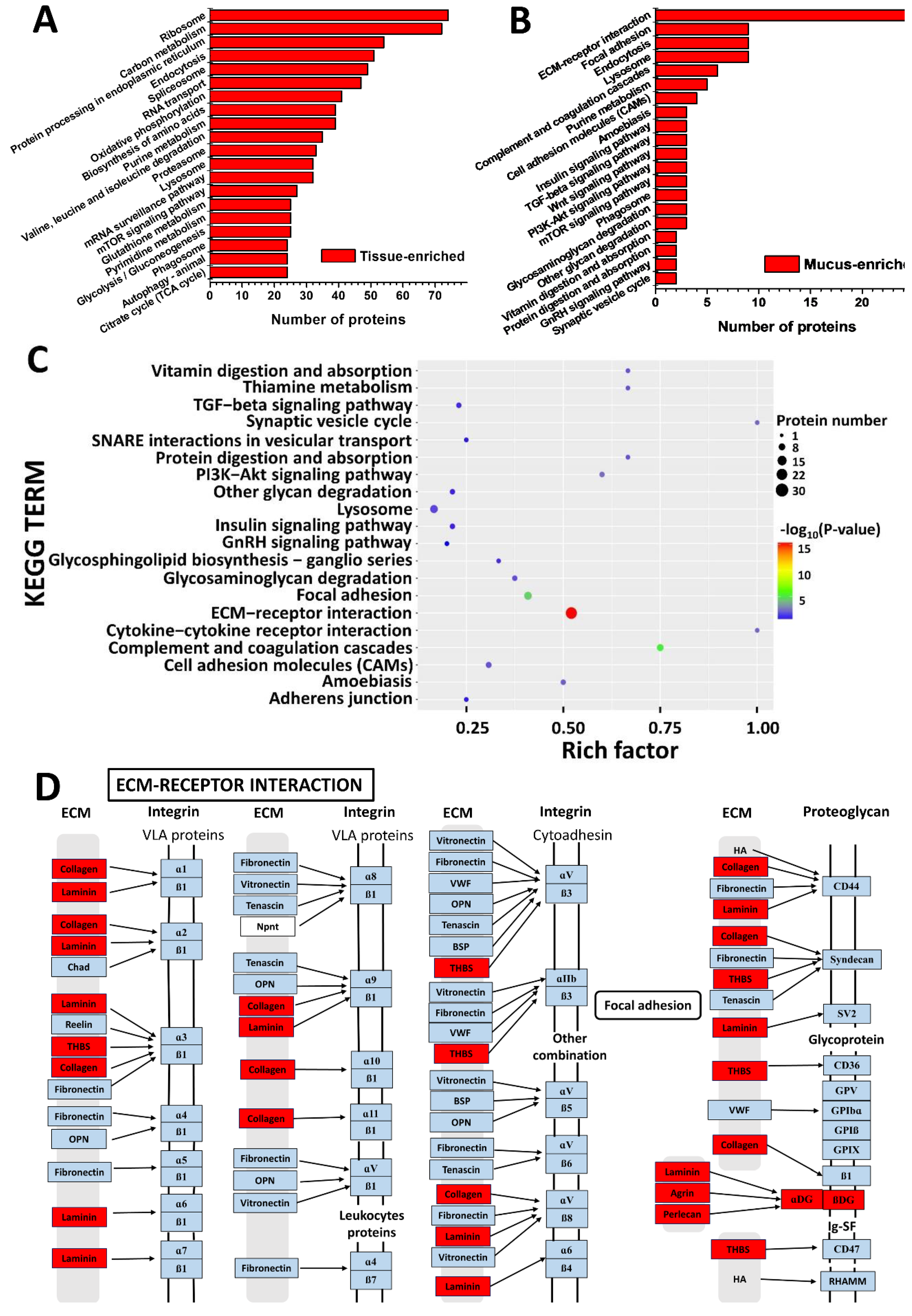
2.4. KEGG Pathway Analysis
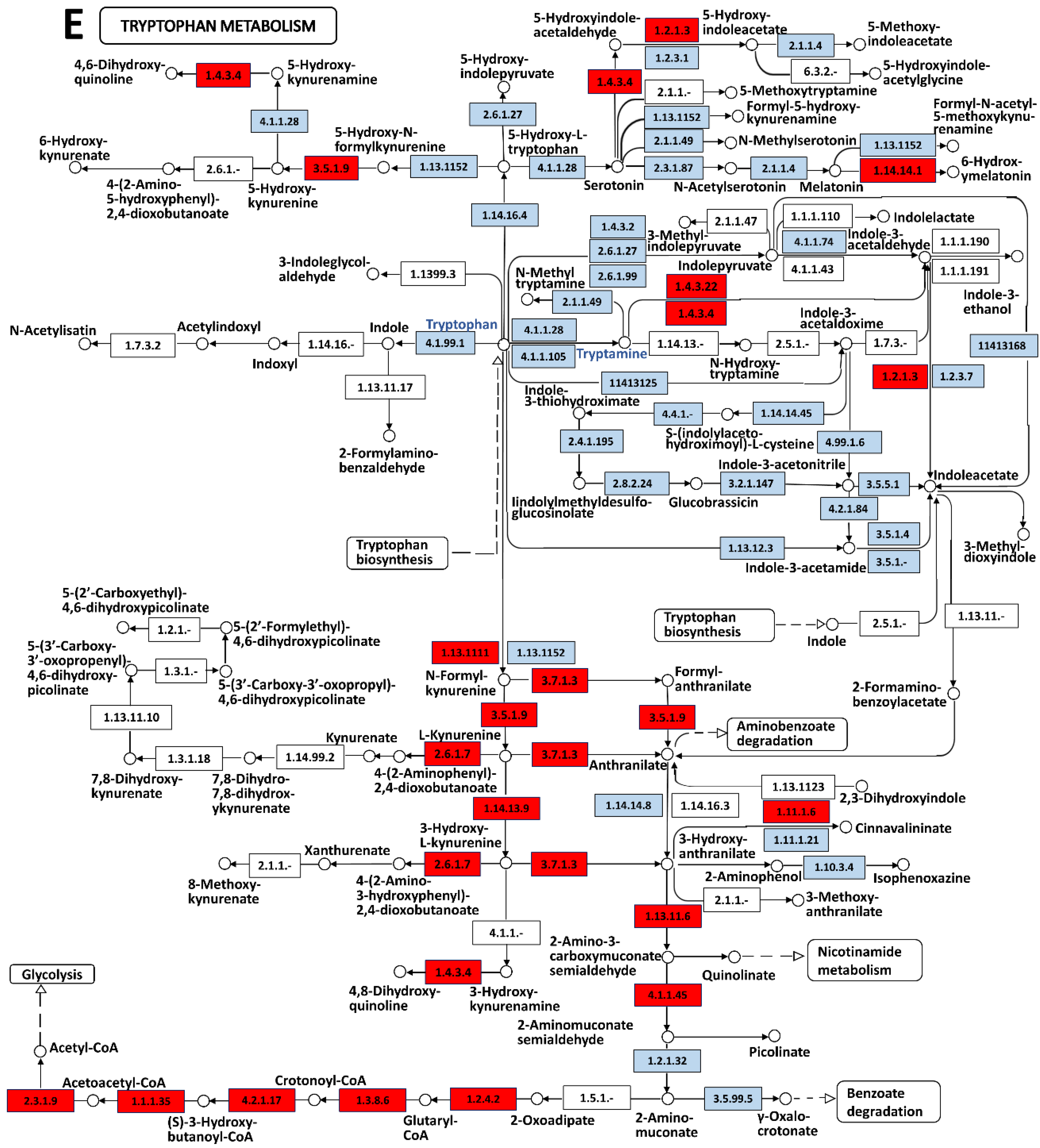
2.5. Metabolomics
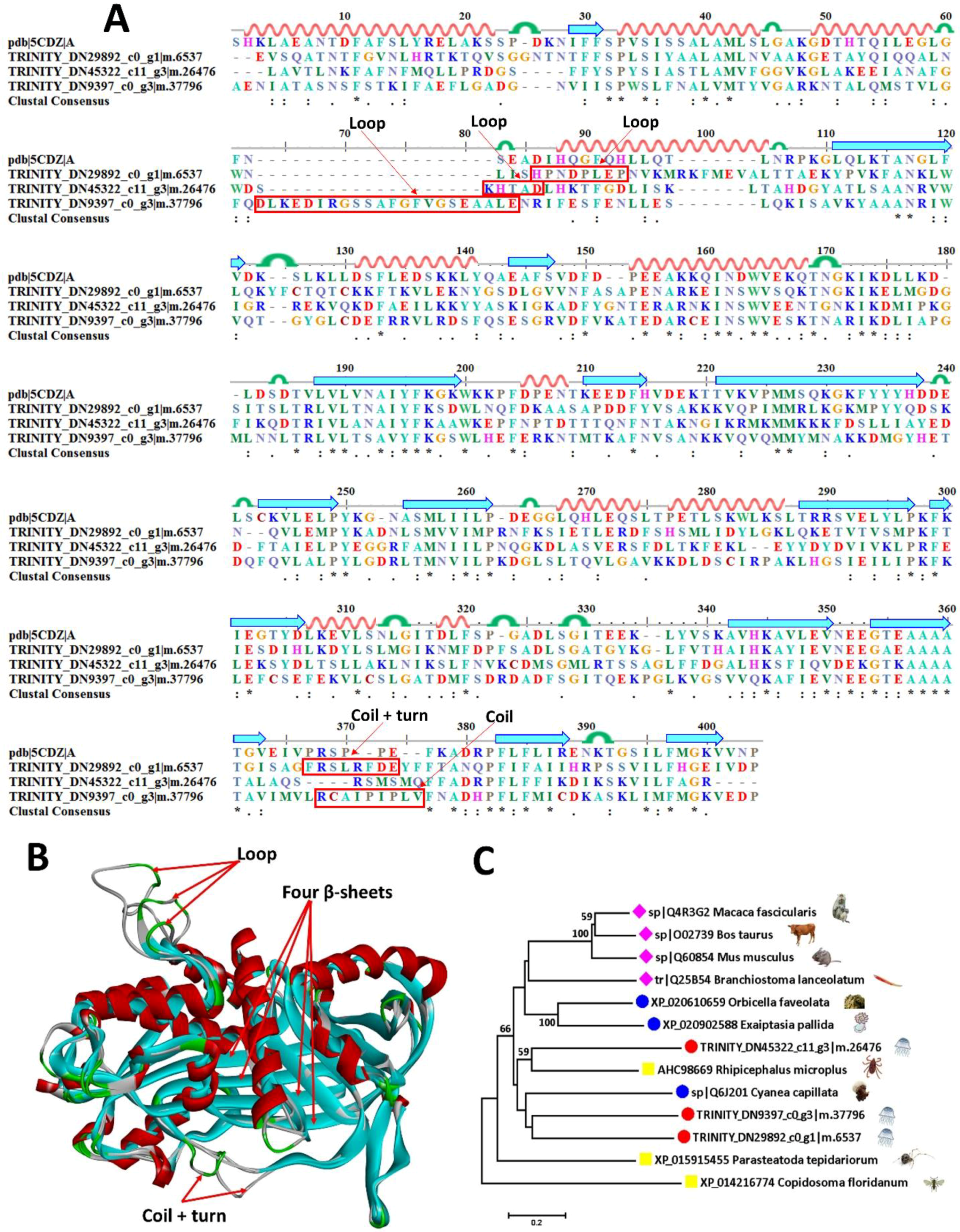
2.6. Self-Protective Proteins
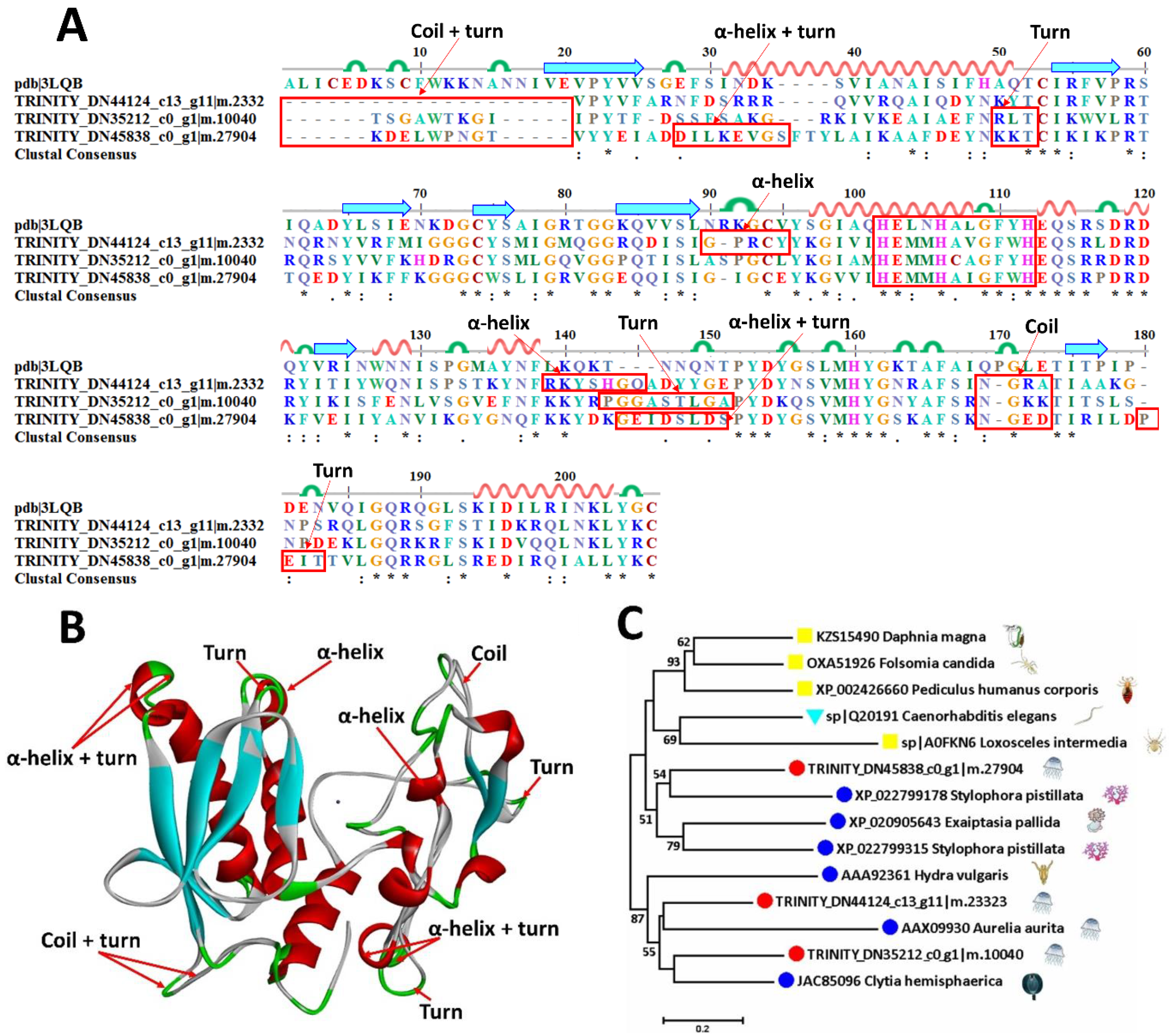
2.6.1. Metalloproteinases
2.6.2. Serine Protease Inhibitors
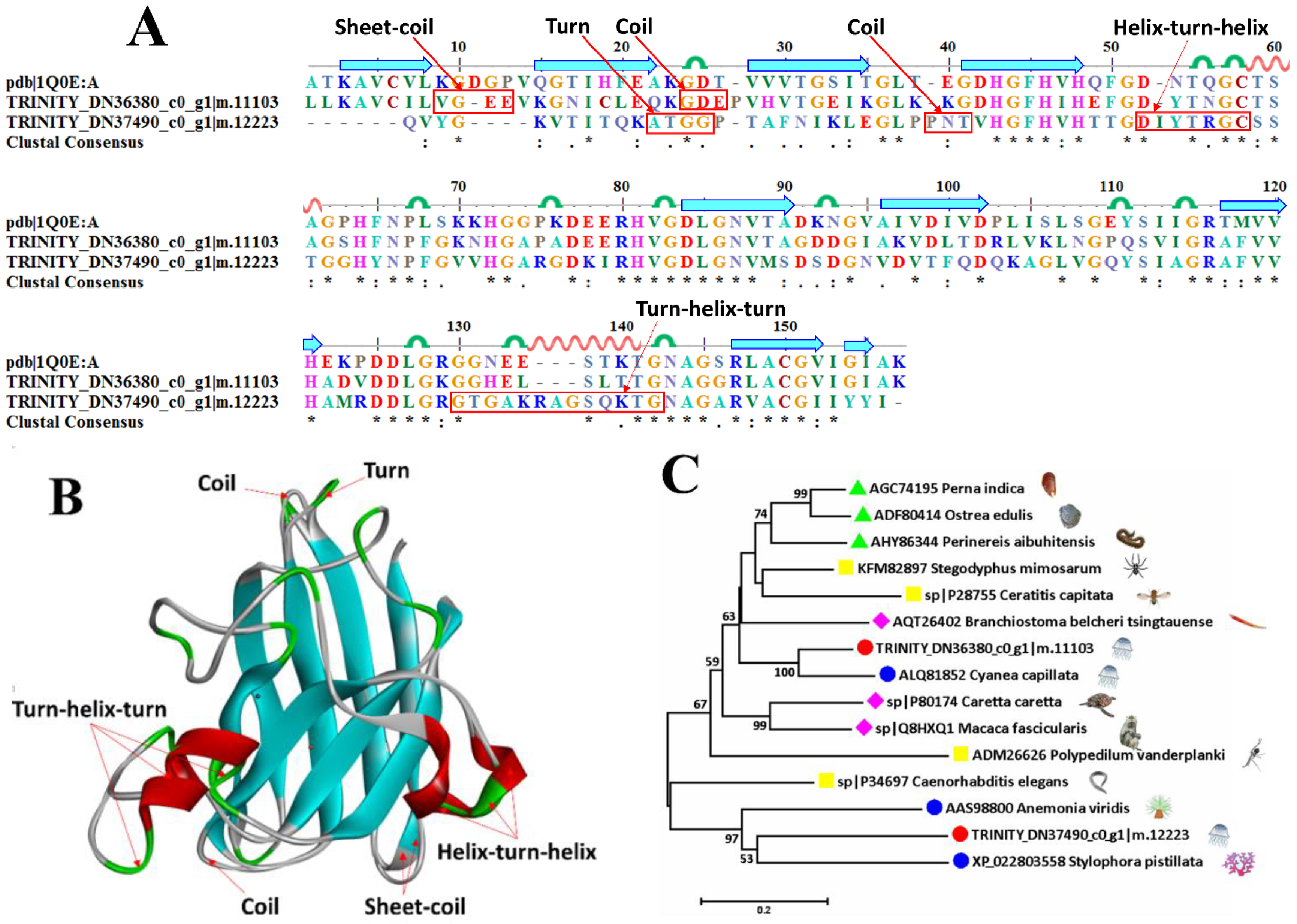
2.6.3. Superoxide Dismutase
3. Discussion
3.1. Jellyfish Mucus Is a Rich Library for the Discovery of Novel Bioactive Compounds
3.2. Tryptamine Release Indicates an Elevated Stress of Jellyfish When Stimulated
3.3. Self-Protective Proteins Play a Key Role in Innate Immunity of Jellyfish
4. Materials and Methods
4.1. A. coerulea Samples
4.2. Transcriptome Sequencing and Its Annotation
4.3. Mucus Collection and Tissue Homogenate Preparation
4.4. SDS-PAGE
4.5. Proteomics
4.5.1. Protein Sample Preparation
4.5.2. LC-MS/MS
4.5.3. Database Search and Bioinformatics Analysis
4.6. Metabolomics
4.6.1. Sample Preparation
4.6.2. Metabolites Acquisition
4.6.3. Data Reduction and Pattern Recognitio
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zoccarato, L.; Celussi, M.; Pallavicini, A.; Fonda Umani, S. Aurelia aurita Ephyrae Reshape a Coastal Microbial Community. Front. Microbiol. 2016, 7, 749. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Xue, W.; Yue, Y.; Liu, S.; Xing, R.; Li, P. Jellyfish venomics and venom gland transcriptomics analysis of Stomolophus meleagris to reveal the toxins associated with sting. J. Proteom. 2014, 106, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.L.; Headlam, J.L.; Doyle, T.K.; Yanagihara, A.A. Assessing the Efficacy of First-Aid Measures in Physalia sp. Envenomation, Using Solution- and Blood Agarose-Based Models. Toxins 2017, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, Q.; Wang, Q.; Zhang, B.; Wang, B.; Xu, F.; Wang, T.; Xiao, L.; Zhang, L. Intracellular Ca2+ overload induced by extracellular Ca2+ entry plays an important role in acute heart dysfunction by tentacle extract from the jellyfish Cyanea capillata. Cardiovasc. Toxicol. 2014, 14, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Lazcanopérez, F.; Arellano, R.O.; Garay, E.; Arreguínespinosa, R.; Sánchezrodríguez, J. Electrophysiological activity of a neurotoxic fraction from the venom of box jellyfish Carybdea marsupialis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 191, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, J.; Torrens, E.; Segura-Puertas, L. Partial purification and characterization of a novel neurotoxin and three cytolysins from box jellyfish (Carybdea marsupialis) nematocyst venom. Arch. Toxicol. 2006, 80, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Liu, G.; Guo, Y.; Zhou, Y.; Wang, Q.; Chang, Y.; Wang, B.; Zheng, J.; Zhang, L. First report of a thioredoxin homologue in jellyfish: Molecular cloning, expression and antioxidant activity of CcTrx1 from Cyanea capillata. PLoS ONE 2014, 9, e97509. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Maeda, T.; Hasegawa, Y.; Tokunaga, T.; Ogawa, S.; Fukuda, K.; Nagatsuka, N.; Nagao, K.; Ueno, S. Antioxidant activity of the giant jellyfish Nemopilema nomurai measured by the oxygen radical absorbance capacity and hydroxyl radical averting capacity methods. Mol. Med. Rep. 2011, 4, 919–922. [Google Scholar] [PubMed]
- Cassoli, J.S.; Verano-Braga, T.; Oliveira, J.S.; Montandon, G.G.; Cologna, C.T.; Peigneur, S.; Pimenta, A.M.; Kjeldsen, F.; Roepstorff, P.; Tytgat, J.; et al. The proteomic profile of Stichodactyla duerdeni secretion reveals the presence of a novel O-linked glycopeptide. J. Proteom. 2013, 87, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Schirosi, R.; Parisi, M.G.; Piraino, S.; Cammarata, M. The Mucus of Actinia equina (Anthozoa, Cnidaria): An Unexplored Resource for Potential Applicative Purposes. Mar. Drugs 2015, 13, 5276–5296. [Google Scholar] [CrossRef] [PubMed]
- Provan, F.; Nilsen, M.M.; Larssen, E.; Uleberg, K.E.; Sydnes, M.O.; Lyng, E.; Oysaed, K.B.; Baussant, T. An evaluation of coral lophelia pertusa mucus as an analytical matrix for environmental monitoring: A preliminary proteomic study. J. Toxicol. Environ. Health Part A 2016, 79, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Salinas, E.M.; Cebada, J.; Valdes, A.; Garateix, A.; Aneiros, A.; Alvarez, J.L. Effects of a toxin from the mucus of the Caribbean sea anemone (Bunodosoma granulifera) on the ionic currents of single ventricular mammalian cardiomyocytes. Toxicon 1997, 35, 1699–1709. [Google Scholar] [CrossRef]
- Patwa, A.; Thiéry, A.; Lombard, F.; Lilley, M.K.; Boisset, C.; Bramard, J.F.; Bottero, J.Y.; Barthélémy, P. Accumulation of nanoparticles in “jellyfish” mucus: A bio-inspired route to decontamination of nano-waste. Sci. Rep. 2015, 5, 11387. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, K.I.; Ohno, H.; Wada, N.; Ueno, S.; Goessler, W.; Kuehnelt, D.; Schlagenhaufen, C.; Kaise, T.; Irgolic, K.J. Occurrence of organo-arsenicals in jellyfishes and their mucus. Chemosphere 2001, 44, 743–749. [Google Scholar] [CrossRef]
- Patel, D.M.; Brinchmann, M.F. Skin mucus proteins of lumpsucker (Cyclopterus lumpus). Biochem. Biophys. Rep. 2017, 9, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jia, Z.; Li, X.; Geng, X.; Sun, J. Calmodulin is a stress and immune response gene in Chinese mitten crab Eriocheir sinensis. Fish. Shellfish Immunol. 2014, 40, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Rajan, B.; Fernandes, J.M.; Caipang, C.M.; Kiron, V.; Rombout, J.H.; Brinchmann, M.F. Proteome reference map of the skin mucus of Atlantic cod (Gadus morhua) revealing immune competent molecules. Fish. Shellfish Immunol. 2011, 31, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Elise, H.; Baptiste, L.; Ruddy, W.; Peter, L. An integrated transcriptomic and proteomic analysis of sea star epidermal secretions identifies proteins involved in defense and adhesion. J. Proteom. 2015, 128, 83–91. [Google Scholar]
- Jatkar, A.A.; Brown, B.E.; Bythell, J.C.; Guppy, R.; Morris, N.J.; Pearson, J.P. Coral mucus: The properties of its constituent mucins. Biomacromolecules 2010, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Shanks, A.L.; Graham, W.M. Chemical defense in a scyphomedusa. Mar. Ecol. Prog. Ser. 1988, 45, 81–86. [Google Scholar] [CrossRef]
- Heeger, T.; Möller, H. Ultrastructural observations on prey capture and digestion in the scyphomedusa Aurelia aurita. Mar. Biol. 1987, 96, 391–400. [Google Scholar] [CrossRef]
- Bonnet, D.; Molinero, J.C.; Schohn; Yahia, M.D. Seasonal changes in the population dynamics of Aurelia aurita in Thau lagoon. Cah. De Biol. Mar. 2012, 53, 343–347. [Google Scholar]
- Scorrano, S.; Aglieri, G.; Boero, F.; Dawson, M.N.; Piraino, S. Unmasking Aurelia species in the Mediterranean Sea: An integrative morphometric and molecular approach. Zool. J. Linn. Soc. 2016, 180, 243–267. [Google Scholar]
- Wang, Y.; Jiang, H.; Meng, H.; Li, J.; Yang, X.J.; Zhao, B.; Sun, Y.; Bao, T. Antidepressant Mechanism Research of Acupuncture—Insights from an Genome-wide Transcriptome Analysis of Frontal Cortex in Rats with Chronic Restraint Stress. Evid. Based Complement. Altern. Med. 2017, 2017, 1676808. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Takeya, H.; Iwanaga, S. Snake venom metalloproteinases: Structure, function and relevance to the mammalian ADAM/ADAMTS family proteins. Biochim. Biophys. Acta 2012, 1824, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Cristina, S.M.; Tamires, L.S.; Vieira, S.M.; Martins, M.C.; Maria, S.F.; Cortes, F.K.; Fábio, O.; Patriarca, M.T.W.; Roberto, M.J. Interaction between TNF and BmooMP-Alpha-I, a Zinc Metalloprotease Derived fromBothrops moojeni Snake Venom, Promotes Direct Proteolysis of This Cytokine: Molecular Modeling and Docking at a Glance. Toxins 2016, 8, 223. [Google Scholar] [CrossRef]
- Jacob-Ferreira, A.L.; Menaldo, D.L.; Sartim, M.A.; Riul, T.B.; Dias-Baruffi, M.; Sampaio, S.V. Antithrombotic activity of Batroxase, a metalloprotease from Bothrops atrox venom, in a model of venous thrombosis. Int. J. Biol. Macromol. 2016, 95, 263. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Han, D.Y.; Park, K.I.; Pyo, M.J.; Heo, Y.; Lee, H.; Kim, G.S.; Kim, E. Characterization and neutralization of Nemopilema nomurai (Scyphozoa: Rhizostomeae) jellyfish venom using polyclonal antibody. Toxicon 2014, 86, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhou, Y.; Liu, D.; Wang, Q.; Ruan, Z.; He, Q.; Zhang, L. Global Transcriptome Analysis of the Tentacle of the Jellyfish Cyanea capillata Using Deep Sequencing and Expressed Sequence Tags: Insight into the Toxin- and Degenerative Disease-Related Transcripts. PLoS ONE 2015, 10, e0142680. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chiang, D.Y.; Wang, S.; Wang, Q.; Sun, L.; Voigt, N.; Respress, J.L.; Ather, S.; Skapura, D.G.; Jordan, V.K.; et al. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation 2014, 129, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, P.; Libert, C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J. Leukocyte Biol. 2007, 82, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Brocker, C.N.; Vasiliou, V.; Nebert, D.W. Evolutionary divergence and functions of the ADAM and ADAMTS gene families. Hum. Genom. 2009, 4, 43–55. [Google Scholar] [CrossRef]
- Wolfsberg, T.G.; Straight, P.D.; Gerena, R.L.; Huovila, A.P.; Primakoff, P.; Myles, D.G.; White, J.M. ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloprotease domain. Dev. Biol. 1995, 169, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Apte, S.S. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: The ADAMTS family. Int. J. Biochem. Cell. Biol. 2004, 36, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J. Evolutionary families of metallopeptidases. Methods Enzymol. 1995, 248, 183–228. [Google Scholar] [PubMed]
- Manzetti, S.; McCulloch, D.R.; Herington, A.C.; van der Spoel, D. Modeling of enzyme-substrate complexes for the metalloproteases MMP-3, ADAM-9 and ADAM-10. J. Comput. Aided Mol. Des. 2003, 17, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Hunt, L.T.; Dayhoff, M.O. A surprising new protein superfamily containing ovalbumin, antithrombin-III, and alpha 1-proteinase inhibitor. Biochem. Biophys. Res. Commun. 1980, 95, 864–871. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Tolle, D.P.; Barrett, A.J. Evolutionary families of peptidase inhibitors. Biochem. J. 2004, 378, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Morjen, M.; Kallechziri, O.; Bazaa, A.; Othman, H.; Mabrouk, K.; Zouarikessentini, R.; Sanz, L.; Calvete, J.J.; Srairiabid, N.; El Ayeb, M. PIVL, a new serine protease inhibitor from Macrovipera lebetina transmediterranea venom, impairs motility of human glioblastoma cells. Matrix Biol. J. Int. Soc. Matrix Biol. 2013, 32, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Fujimura, Y.; Titani, K. Snake venom proteases affecting hemostasis and thrombosis. Biochim. Biophys. Acta. 2000, 1477, 146–156. [Google Scholar] [CrossRef]
- Mourão, C.B.; Schwartz, E.F. Protease Inhibitors from Marine Venomous Animals and Their Counterparts in Terrestrial Venomous Animals. Mar. Drugs 2013, 11, 2069–2112. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Kim, K.Y.; Sang, Y.L.; Cho, Y.S.; Bang, I.C.; Kim, K.H.; Dong, S.K.; Nam, Y.K. Molecular characterization and mRNA expression during metal exposure and thermal stress of copper/zinc- and manganese-superoxide dismutases in disk abalone, Haliotis discus discus. Fish. Shellfish Immunol. 2007, 23, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dong, S.; Jiang, Q.; Si, Q.; Liu, X.; Yang, J. Characterization and expression of cytoplasmic copper/zinc superoxide dismutase (CuZn SOD) gene under temperature and hydrogen peroxide (H2O2) in rotifer Brachionus calyciflorus. Gene 2013, 518, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Umasuthan, N.; Bathige, S.D.; Revathy, K.S.; Lee, Y.; Whang, I.; Choi, C.Y.; Park, H.C.; Lee, J. A manganese superoxide dismutase (MnSOD) from Ruditapes philippinarum: Comparative structural- and expressional-analysis with copper/zinc superoxide dismutase (Cu/ZnSOD) and biochemical analysis of its antioxidant activities. Fish. Shellfish Immunol. 2012, 33, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Broxton, C.N.; Culotta, V.C. SOD Enzymes and Microbial Pathogens: Surviving the Oxidative Storm of Infection. PLoS Pathog. 2016, 12, e1005295. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, C.; Liu, B. The role of Cu/Zn-SOD and Mn-SOD in the immune response to oxidative stress and pathogen challenge in the clam Meretrix meretrix. Fish. Shellfish Immunol. 2015, 42, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Bakshani, C.R.; Morales-Garcia, A.L.; Althaus, M.; Wilcox, M.D.; Pearson, J.P.; Bythell, J.C.; Burgess, J.G. Evolutionary conservation of the antimicrobial function of mucus: A first defence against infection. NPJ Biofilms Microbiomes 2018, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Abrams, M.J.; Goentoro, L. Symmetrization in jellyfish: Reorganization to regain function, and not lost parts. Zoology (Jena) 2016, 119, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Sjovall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Steukers, L.; Forier, K.; Xiong, R.; Braeckmans, K.; Reeth, K.V.; Nauwynck, H. A Beneficiary Role for Neuraminidase in Influenza Virus Penetration through the Respiratory Mucus. PLoS ONE 2014, 9, e110026. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.K.; Becher, N.; Bastholm, S.; Glavind, J.; Ramsing, M.; Kim, C.J.; Romero, R.; Jensen, J.S.; Uldbjerg, N. The cervical mucus plug inhibits, but does not block, the passage of ascending bacteria from the vagina during pregnancy. Acta Obstet. Gynecol Scand. 2013, 93, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Casado, B.; Pannell, L.K.; Iadarola, P.; Baraniuk, J.N. Identification of human nasal mucous proteins using proteomics. Proteomics 2010, 5, 2949–2959. [Google Scholar] [CrossRef] [PubMed]
- Panicker, G.; Ye, Y.; Wang, D.; Unger, E.R. Characterization of the Human Cervical Mucous Proteome. Clin. Proteom. 2010, 6, 18–28. [Google Scholar] [CrossRef] [PubMed]
- De, D.A.; Idolo, A.; Bagordo, F.; Grassi, T.; Leomanni, A.; Serio, F.; Guido, M.; Canitano, M.; Zampardi, S.; Boero, F. Impact of stinging jellyfish proliferations along south Italian coasts: Human health hazards, treatment and social costs. Int. J. Environ. Res. Public Health 2014, 11, 2488–2503. [Google Scholar]
- Wang, B.; Zhang, L.; Zheng, J.; Wang, Q.; Wang, T.; Lu, J.; Wen, X.; Zhang, B.; Liu, G.; Zhang, W.; et al. Multiple organ dysfunction: A delayed envenomation syndrome caused by tentacle extract from the jellyfish Cyanea capillata. Toxicon 2013, 61, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Takuwa, K.; Nakao, M.; Ito, E.; Miyake, M.; Noda, M.; Nakajima, T. Novel proteinaceous toxins from the box jellyfish (sea wasp) Carybdea rastoni. Biochem. Biophys. Res. Commun. 2000, 275, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Takuwa, K.; Nakao, M.; Sakamoto, B.; Crow, G.L.; Nakajima, T. Isolation and characterization of a novel protein toxin from the Hawaiian box jellyfish (sea wasp) Carybdea alata. Biochem. Biophys. Res. Commun. 2000, 275, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.; Burnell, J. Identification, cloning and sequencing of two major venom proteins from the box jellyfish, Chironex fleckeri. Toxicon 2007, 50, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.L.; Konstantakopoulos, N.; McInerney, B.V.; Mulvenna, J.; Seymour, J.E.; Isbister, G.K.; Hodgson, W.C. Chironex fleckeri (box jellyfish) venom proteins: Expansion of a cnidarian toxin family that elicits variable cytolytic and cardiovascular effects. J. Biol. Chem. 2014, 289, 4798–4812. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.L.; Burnell, J.N. Biochemical and molecular characterisation of cubozoan protein toxins. Toxicon 2009, 54, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.L.; Jia, X.; Potriquet, J.; Kumar, D.; Dash, D.; Kvaskoff, D.; Mulvenna, J. Transcriptome and venom proteome of the box jellyfish Chironex fleckeri. BMC Genom. 2015, 16, 407. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Yue, Y.; Liu, S.; Xing, R.; Chen, X.; Li, P. Combined proteomics and transcriptomics identifies sting-related toxins of jellyfish Cyanea nozakii. J. Proteom. 2016, 148, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Xie, G.; Jia, W. Metabonomics of human colorectal cancer: New approaches for early diagnosis and biomarker discovery. J. Proteome Res. 2014, 13, 3857–3870. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Li, H.; Peng, X.X. Functional metabolomics: From biomarker discovery to metabolome reprogramming. Protein Cell 2015, 6, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Quinones, M.P.; Kaddurah-Daouk, R. Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiol. Dis. 2009, 35, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.Y.; Carlson, T.L.; Carrier, R.L. Mucus models to evaluate the diffusion of drugs and particles. Adv. Drug Deliv. Rev. 2018, 124, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Murgia, X.; Loretz, B.; Hartwig, O.; Hittinger, M.; Lehr, C.M. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv. Drug Deliv. Rev. 2018, 124, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Tittarelli, R.; Mannocchi, G.; Pantano, F.; Romolo, F.S. Recreational Use, Analysis and Toxicity of Tryptamines. Curr. Neuropharmacol. 2015, 13, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Goddijn, O.J.; Lohman, F.P.; de Kam, R.J.; Schilperoort, R.A.; Hoge, J.H. Nucleotide sequence of the tryptophan decarboxylase gene of Catharanthus roseus and expression of tdc-gusA gene fusions in Nicotiana tabacum. Mol. Gen. Genet. 1994, 242, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Guenter, J.; Lenartowski, R. Molecular characteristic and physiological role of DOPA-decarboxylase. Postepy Higieny i Medycyny Doswiadczalnej 2016, 70, 1424–1440. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Freeman, S.; McGagh, P.; Abdul-Halim, N.; Alder, J.F. An analytical perspective on favoured synthetic routes to the psychoactive tryptamines. J. Pharm. Biomed. Anal. 2004, 36, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Mayorova, T.D.; Kosevich, I.A.; Melekhova, O.P. On some features of embryonic development and metamorphosis of Aurelia aurita (Cnidaria, Scyphozoa). Russ. J. Dev. Biol. 2012, 43, 271–285. [Google Scholar] [CrossRef]
- Fossat, P.; Bacque-Cazenave, J.; De Deurwaerdere, P.; Delbecque, J.P.; Cattaert, D. Comparative behavior. Anxiety-like behavior in crayfish is controlled by serotonin. Science 2014, 344, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Valdivia, C.R.; Gurrola, G.B.; Powers, P.P.; Willis, B.C.; Moss, R.L.; Jalife, J.; Valdivia, H.H. Arrhythmogenesis in a catecholaminergic polymorphic ventricular tachycardia mutation that depresses ryanodine receptor function. Proc. Natl. Acad. Sci. USA 2015, 112, E1669–E1677. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, R.B.; Wille, A.C.; Chaim, O.M.; Appel, M.H.; Silva, D.T.; Franco, C.R.; Toma, L.; Mangili, O.C.; Gremski, W.; Dietrich, C.P.; et al. Identification, cloning, expression and functional characterization of an astacin-like metalloprotease toxin from Loxosceles intermedia (brown spider) venom. Biochem. J. 2007, 406, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Molehin, A.J.; Gobert, G.N.; McManus, D.P. Serine protease inhibitors of parasitic helminths. Parasitology 2012, 139, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.R.; Xu, Y.H.; Jiang, H.S.; Xu, S.; Zhao, X.F.; Wang, J.X. Antibacterial activity of serine protease inhibitor 1 from kuruma shrimp Marsupenaeus japonicus. Dev. Comp. Immunol. 2014, 44, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell. Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.Z.; Kitchen, S.A.; Weis, V.M. The Role of Complement in Cnidarian-Dinoflagellate Symbiosis and Immune Challenge in the Sea Anemone Aiptasia pallida. Front. Microbiol. 2016, 7, 519. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Sakaguchi, E.; Nonaka, M. Multi-component complement system of Cnidaria: C3, Bf, and MASP genes expressed in the endodermal tissues of a sea anemone, Nematostella vectensis. Immunobiology 2009, 214, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, D.; Yang, A.; Dong, Y.; Chen, Z.; Wang, X.; Guan, X.; Jiang, B.; Wang, B. Molecular characterization and expression analysis of a complement component 3 in the sea cucumber (Apostichopus japonicus). Fish. Shellfish Immunol. 2011, 31, 540–547. [Google Scholar] [CrossRef] [PubMed]
| Metabolite | FC | p Value | Related Pathway | Metabolite | FC | p Value | Related Pathway |
|---|---|---|---|---|---|---|---|
| Tryptamine | 7.80 | <0.0001 | Tryptophan metabolism | Linoleyl linolenate | 0.28 | <0.0001 | |
| 4-Hydroxy-l-proline | 1.26 | <0.0001 | Arginine and proline metabolism | Uridine | 0.19 | <0.0001 | Pyrimidine metabolism |
| Citrulline | 1.17 | <0.0005 | Arginine biosynthesis | l-Proline | 0.13 | <0.0001 | Arginine and proline metabolism |
| l-Leucine | 1.14 | <0.0001 | Valine, leucine and isoleucine metabolism | Inosine | 0.12 | <0.0001 | Purine metabolism |
| 3-(Phosphoacetylamido)-l-alanine | 1.09 | <0.0001 | l-asparagine biosynthesis | Hypoxanthine | 0.12 | <0.0001 | Purine metabolism |
| l-Threonine | 0.88 | 0.0007 | Valine, leucine and isoleucine biosynthesis | l-Valine | 0.07 | <0.0001 | Valine, leucine and isoleucine metabolism |
| l-Glutamate | 0.54 | <0.0001 | Arginine and proline metabolism | Guanosine | 0.05 | <0.0001 | Purine metabolism |
| Succinylacetone | 0.44 | <0.0001 | Tyrosine metabolism |
| Accession | Swissprot Annotation | Description | Matched Species | Identify (%) |
|---|---|---|---|---|
| Metalloproteases | ||||
| TRINITY_DN45838_c0_g1|m.27904 | sp|Q20191 | Zinc metalloproteinase nas-13 | Caenorhabditis elegans | 42.6 |
| TRINITY_DN44124_c13_g11|m.23323 | sp|Q20191 | Zinc metalloproteinase nas-13 | Caenorhabditis elegans | 34.9 |
| TRINITY_DN35212_c0_g1|m.10040 | sp|P55115 | Zinc metalloproteinase nas-15 | Caenorhabditis elegans | 30.8 |
| TRINITY_DN45621_c1_g1|m.27292 | sp|Q8N119 | Matrix metalloproteinase-21 | Mus musculus | 34.9 |
| TRINITY_DN42325_c7_g8|m.19392 | sp|P51511 | Matrix metalloproteinase-15 | Homo sapiens | 33.6 |
| TRINITY_DN47471_c0_g1|m.30857 | sp|Q9UKF2 | ADAM30 | Homo sapiens | 45.1 |
| TRINITY_DN45145_c0_g4|m.26001 | sp|Q05910 | ADAM8 | Mus musculus | 34.8 |
| TRINITY_DN55675_c0_g2|m.32080 | sp|O75077 | ADAM23 | Homo sapiens | 31.1 |
| TRINITY_DN46375_c2_g1|m.29588 | sp|Q9UKP4 | ADMTMS7 | Homo sapiens | 41.5 |
| TRINITY_DN45686_c0_g1|m.27481 | sp|Q9P2N4 | ADMTMS9 | Homo sapiens | 33.2 |
| TRINITY_DN44955_c0_g2|m.25457 | sp|Q69Z28 | ADMTMS16 | Mus musculus | 31.4 |
| TRINITY_DN44955_c0_g1|m.25456 | sp|Q9UKP5 | ADMTMS6 | Homo sapiens | 30.6 |
| Serine protease inhibitors | ||||
| TRINITY_DN43329_c0_g6|m.21485 | sp|Q6J201 | serine protease inhibitor | Cyanea capillata | 56.4 |
| TRINITY_DN45322_c11_g3|m.26476 | sp|Q60854 | Serpin B6 | Mus musculus | 37.5 |
| TRINITY_DN9397_c0_g3|m.37796 | sp|Q4R3G2 | Serpin B6 | Macaca fascicularis | 38.8 |
| TRINITY_DN29892_c0_g1|m.6537 | sp|Q4R3G2 | Serpin B6 | Macaca fascicularis | 36.6 |
| Superoxide dismutase | ||||
| TRINITY_DN37669_c0_g1|m.12406 | sp|Q8HXP2 | Superoxide dismutase [Mn], mitochondrial | Macaca mulatta | 75.3 |
| TRINITY_DN36380_c0_g1|m.11103 | sp|P11428 | Superoxide dismutase [Cu-Zn] 2 | Zea mays | 65.8 |
| TRINITY_DN37490_c0_g1|m.12223 | sp|P24706 | Superoxide dismutase [Cu-Zn] | Onchocerca volvulus | 51.8 |
| Complements | ||||
| TRINITY_DN45426_c0_g1|m.26742 | sp|Q00685 | Complement C3 | Lethenteron camtschaticum | 30.7 |
| TRINITY_DN45758_c0_g1|m.27712 | sp|Q00685 | Complement C3 | Lethenteron camtschaticum | 25.9 |
| TRINITY_DN43257_c1_g1|m.21313 | sp|P81187 | Complement factor B | Bos taurus | 23.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Mo, F.; Jiang, G.; Liang, H.; Ma, C.; Li, T.; Zhang, L.; Xiong, L.; Mariottini, G.L.; Zhang, J.; et al. Stress-Induced Mucus Secretion and Its Composition by a Combination of Proteomics and Metabolomics of the Jellyfish Aurelia coerulea. Mar. Drugs 2018, 16, 341. https://doi.org/10.3390/md16090341
Liu W, Mo F, Jiang G, Liang H, Ma C, Li T, Zhang L, Xiong L, Mariottini GL, Zhang J, et al. Stress-Induced Mucus Secretion and Its Composition by a Combination of Proteomics and Metabolomics of the Jellyfish Aurelia coerulea. Marine Drugs. 2018; 16(9):341. https://doi.org/10.3390/md16090341
Chicago/Turabian StyleLiu, Wenwen, Fengfeng Mo, Guixian Jiang, Hongyu Liang, Chaoqun Ma, Tong Li, Lulu Zhang, Liyan Xiong, Gian Luigi Mariottini, Jing Zhang, and et al. 2018. "Stress-Induced Mucus Secretion and Its Composition by a Combination of Proteomics and Metabolomics of the Jellyfish Aurelia coerulea" Marine Drugs 16, no. 9: 341. https://doi.org/10.3390/md16090341
APA StyleLiu, W., Mo, F., Jiang, G., Liang, H., Ma, C., Li, T., Zhang, L., Xiong, L., Mariottini, G. L., Zhang, J., & Xiao, L. (2018). Stress-Induced Mucus Secretion and Its Composition by a Combination of Proteomics and Metabolomics of the Jellyfish Aurelia coerulea. Marine Drugs, 16(9), 341. https://doi.org/10.3390/md16090341





