A Hetero-Photoautotrophic Two-Stage Cultivation Process for Production of Fucoxanthin by the Marine Diatom Nitzschia laevis
Abstract
:1. Introduction
2. Results
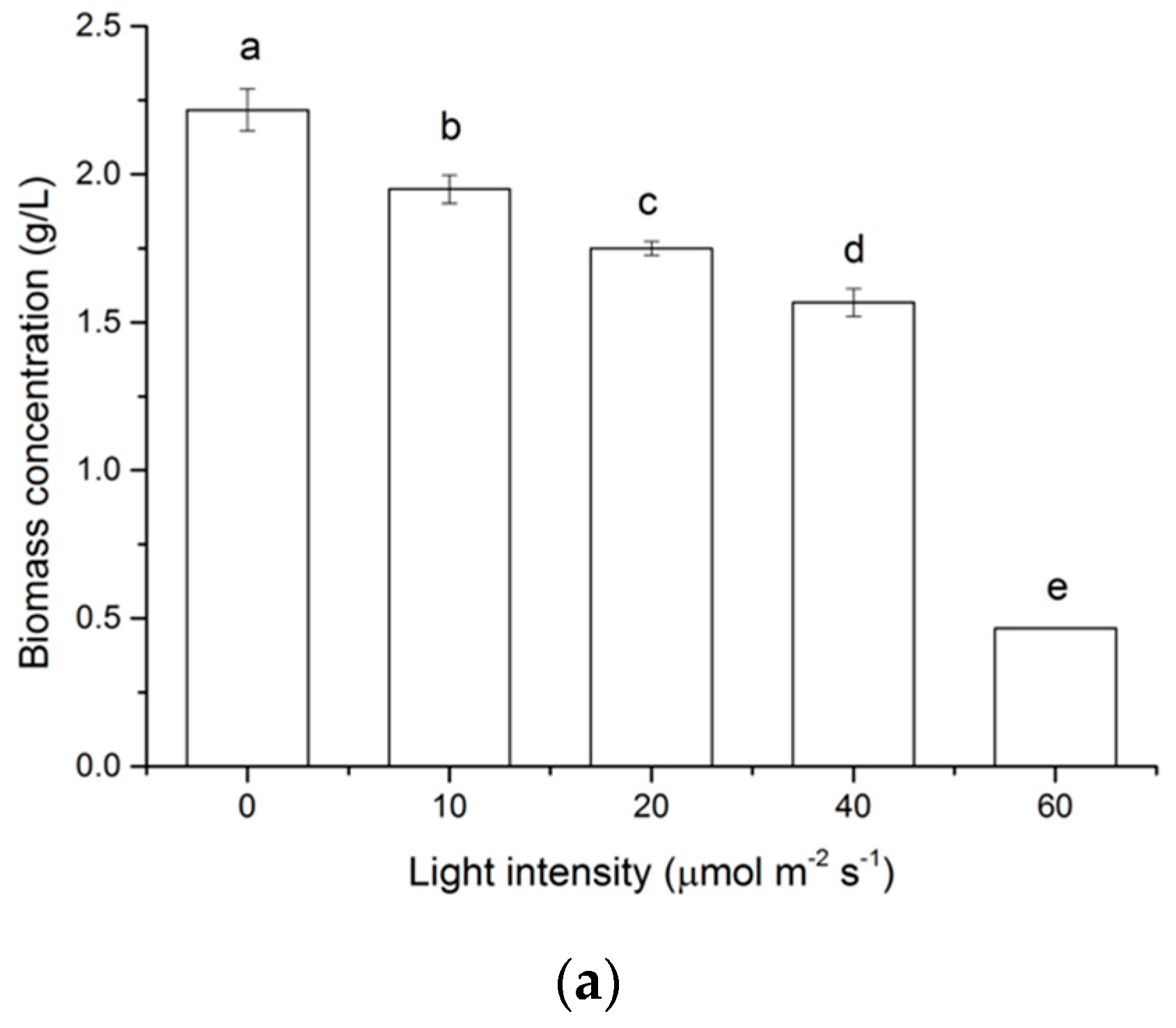
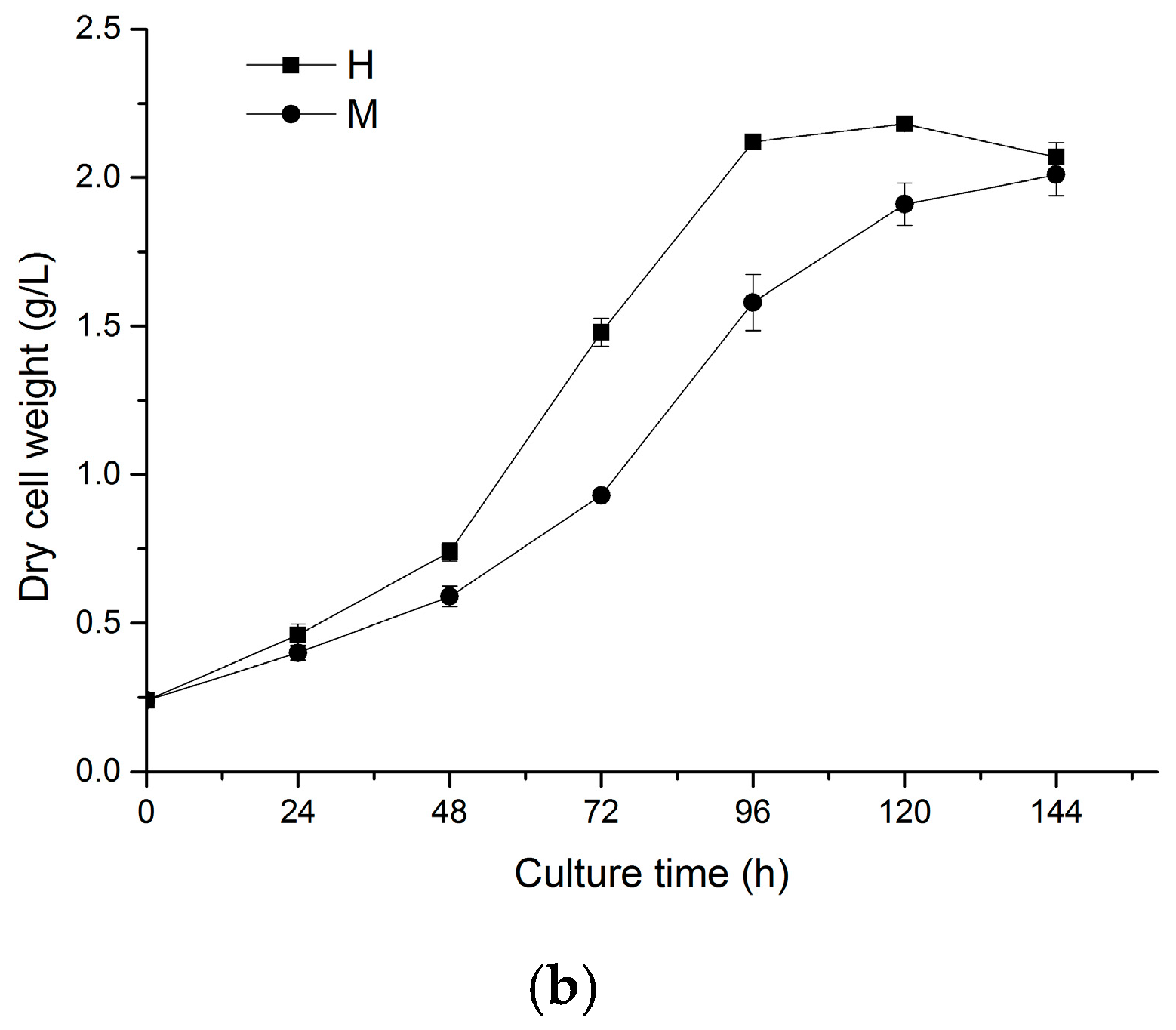
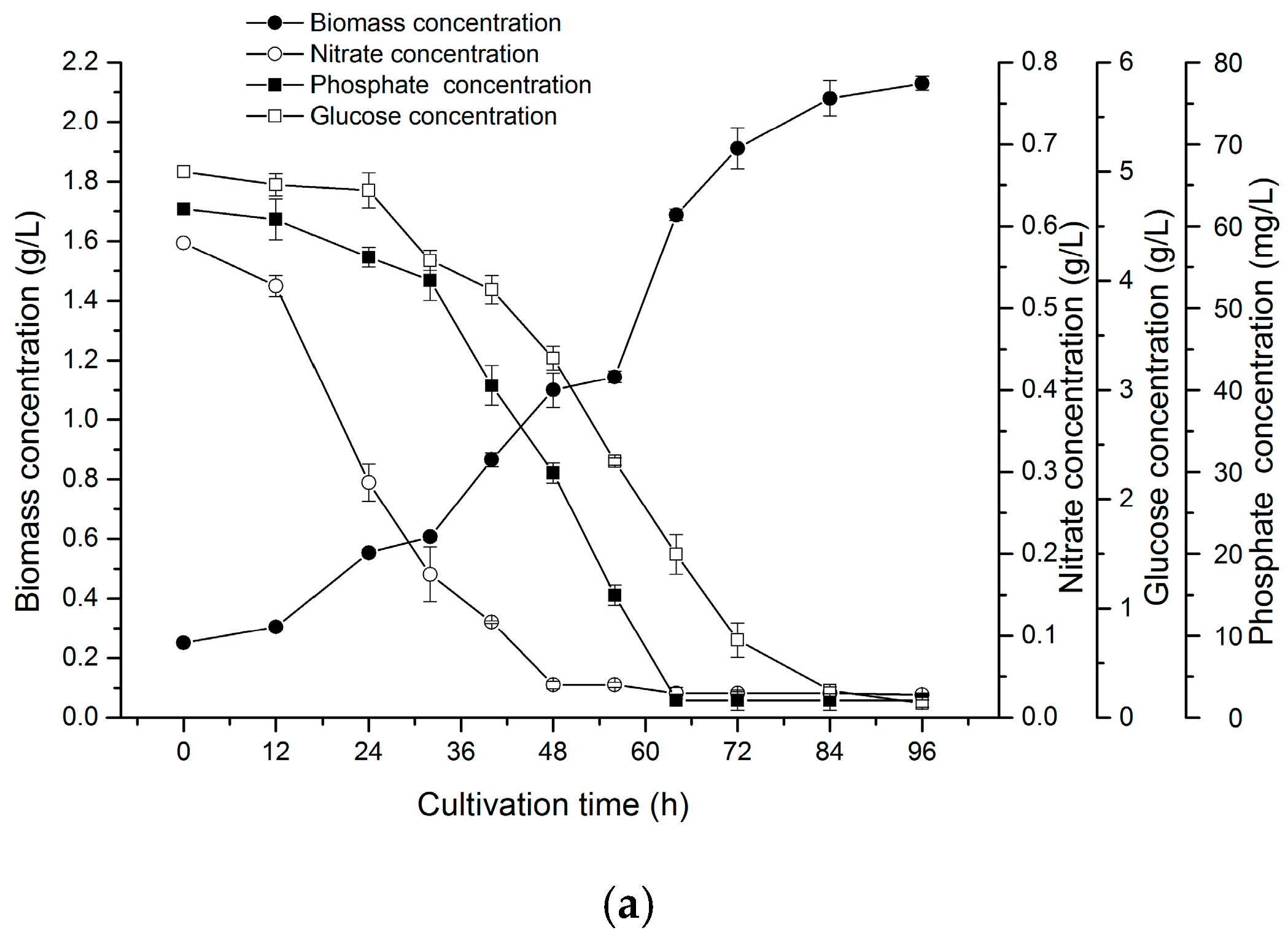
2.1. Effects of Light on Cell Growth of N. laevis
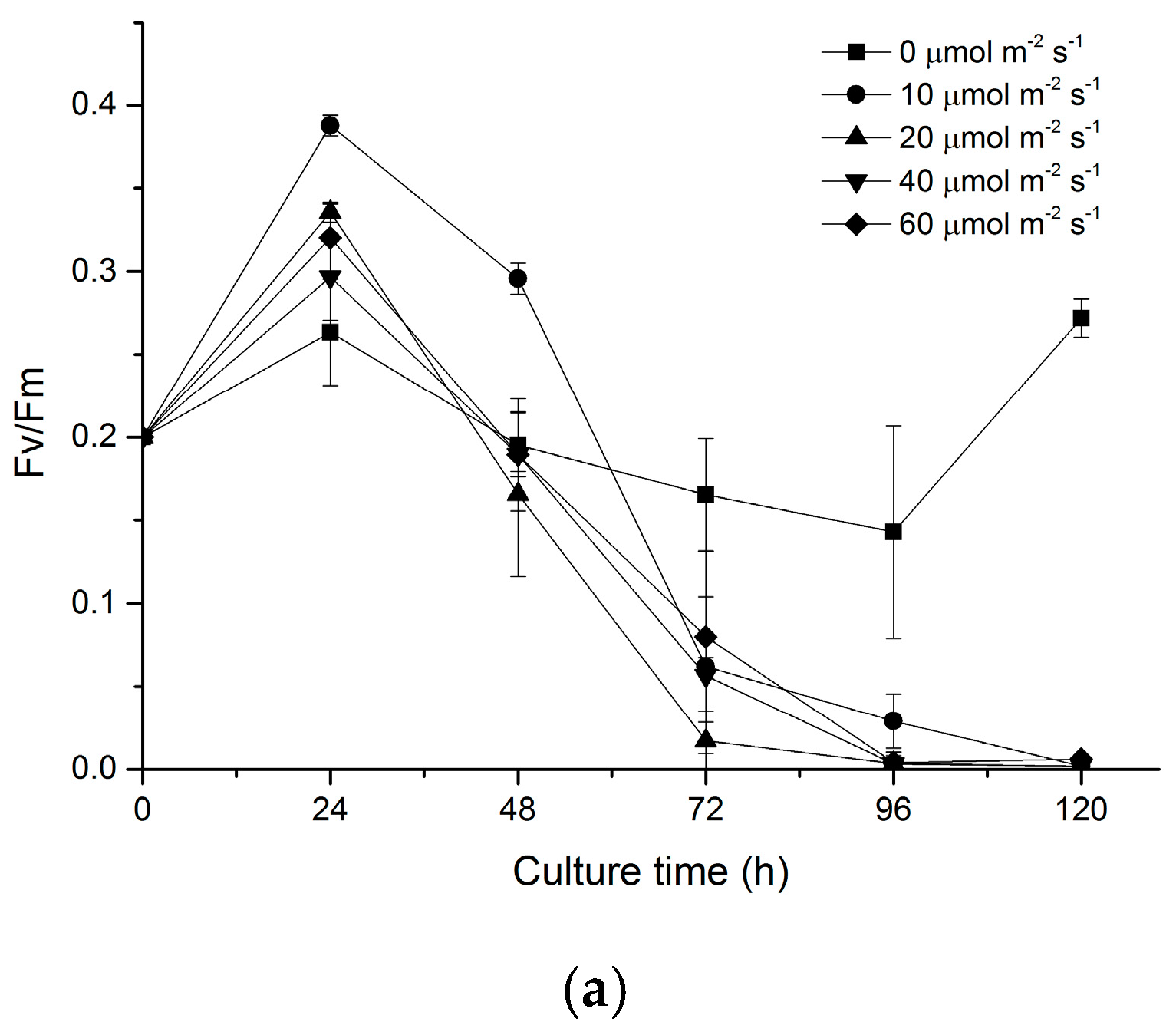
2.2. Effects of Light on the Photosystem II of N. laevis
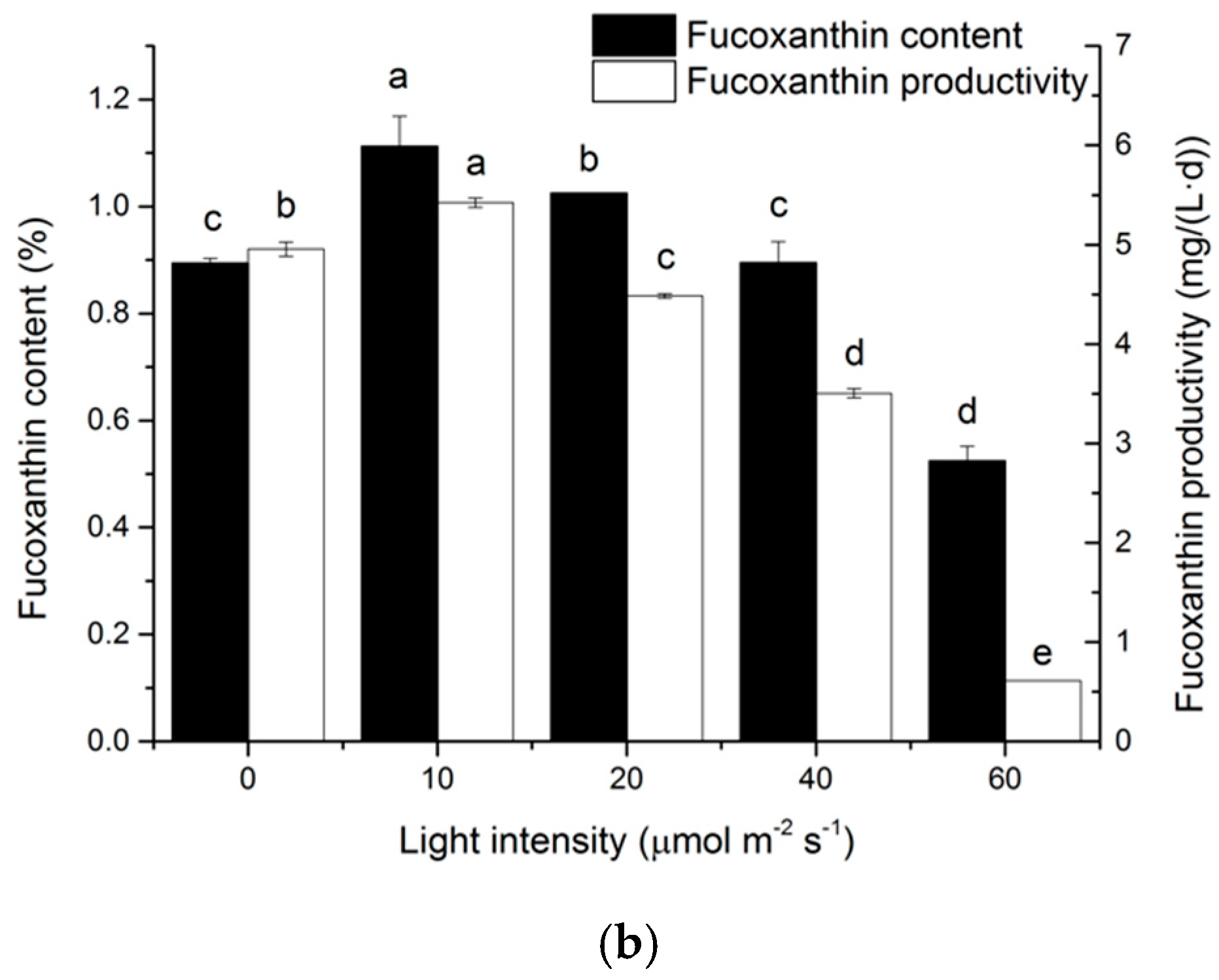
2.3. Effects of Light Intensity on Fucoxanthin Accumulation of N. laevis
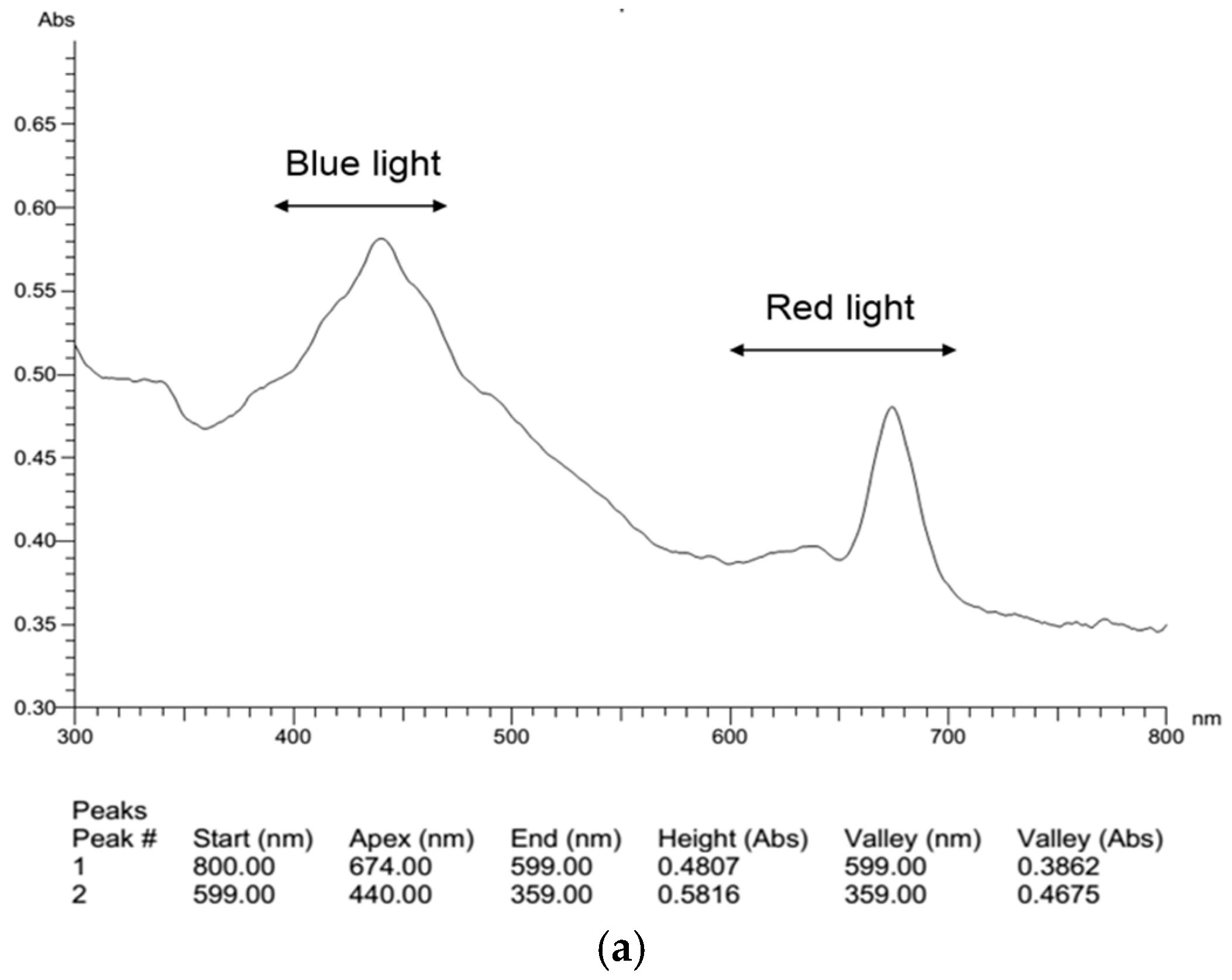
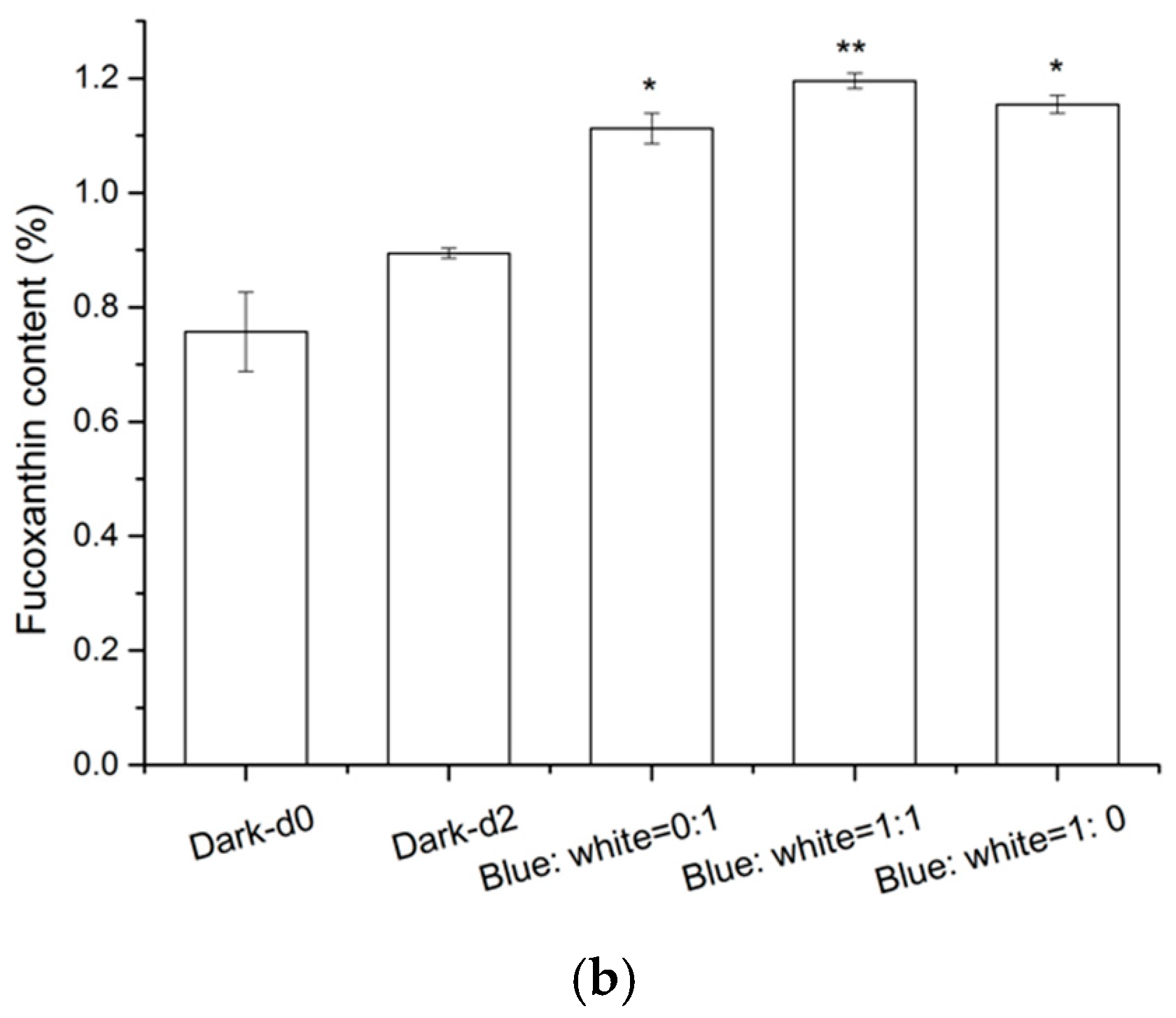
2.4. Effects of Light Composition on Fucoxanthin Accumulation in N. laevis
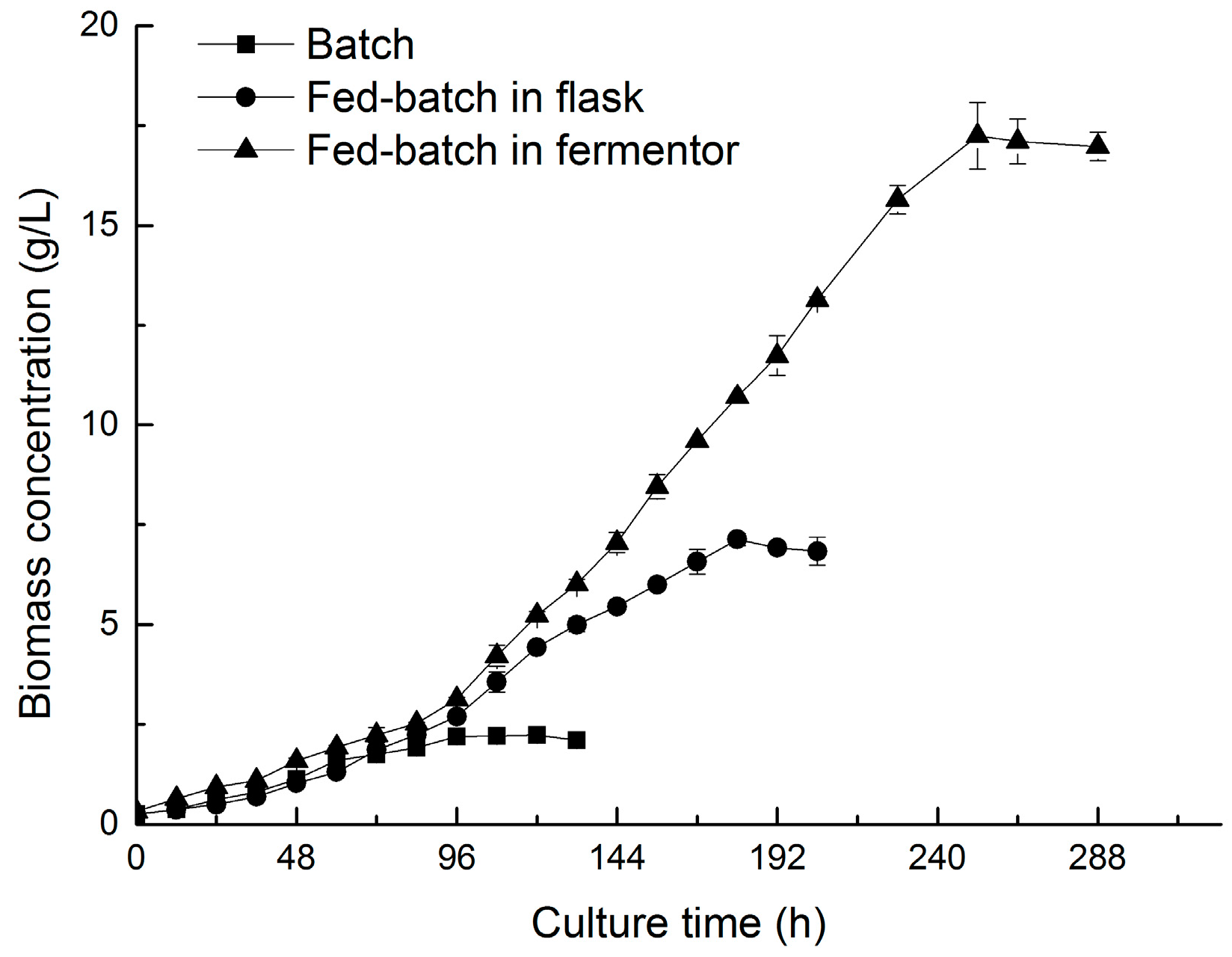
2.5. Two-Stage Cultivation Strategy for Fucoxanthin Production by N. laevis
3. Discussion
4. Materials and Methods
4.1. Algal Strain and Culture Conditions
4.2. Feeding Model of Fed-Batch Culture
4.3. Determination of Biomass Concentration
4.4. Determination of Glucose, Nitrate and Phosphate Concentration
4.5. Measurement of Quantum Yield of Photosystem II (PSII)
4.6. Measurement of Reactive Oxygen Species (ROS)
4.7. Carotenoid Analysis
4.8. Fucoxanthin Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic pigments in diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Xu, M.; Magnusdottir, M.; Zhang, Y.; Brynjolfsson, S.; Fu, W. Photo-oxidative stress-driven mutagenesis and adaptive evolution on the marine diatom Phaeodactylum tricornutum for enhanced carotenoid accumulation. Mar. Drugs 2015, 13, 6138–6151. [Google Scholar] [CrossRef] [PubMed]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Characterization of dietary fucoxanthin from Himanthalia elongata brown seaweed. Food Res. Int. 2017, 99, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Mok, I.K.; Lee, J.K.; Kim, J.H.; Pan, C.H.; Kim, S.M. Fucoxanthin bioavailability from fucoxanthin-fortified milk: In vivo and in vitro study. Food Chem. 2018, 258, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Cheng, K.-W.; He, Y.; Liu, B.; Mao, X.; Chen, F. Screening and identification of inhibitors of advanced glycation endproduct formation from microalgal extracts. Food Funct. 2018, 9, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liu, B.; Yang, B.; Sun, P.; Lu, X.; Liu, J.; Chen, F. Screening of diatom strains and characterization of Cyclotella cryptica as a potential fucoxanthin producer. Mar. Drugs 2016, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Ishika, T.; Moheimani, N.R.; Bahri, P.A.; Laird, D.W.; Blair, S.; Parlevliet, D. Halo-adapted microalgae for fucoxanthin production: Effect of incremental increase in salinity. Algal Res. 2017, 28, 66–73. [Google Scholar] [CrossRef]
- Endo, H.; Okumura, Y.; Sato, Y.; Agatsuma, Y. Interactive effects of nutrient availability, temperature, and irradiance on photosynthetic pigments and color of the brown alga Undaria pinnatifida. J. Appl. Phycol. 2017, 29, 1683–1693. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese seaweed, wakame (Undaria pinnatifida) as an ingredient in pasta: Chemical, functional and structural evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Chen, L.; Cheng, W.; Liu, T. Combined production of fucoxanthin and EPA from two diatom strains Phaeodactylum tricornutum and Cylindrotheca fusiformis cultures. Bioprocess Biosyst. Eng. 2018, 41, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Jung, Y.J.; Kwon, O.N.; Cha, K.H.; Um, B.H.; Chung, D.; Pan, C.H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Jiang, J.G. Lipid accumulation mechanisms in auto-and heterotrophic microalgae. J. Agric. Food Chem. 2017, 65, 8099–8110. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.Y.; Chen, F. Production potential of eicosapentaenoic acid by the diatom Nitzschia laevis. Biotechnol. Lett. 2000, 22, 727–733. [Google Scholar] [CrossRef]
- McClure, D.D.; Luiz, A.; Gerber, B.; Barton, G.W.; Kavanagh, J.M. An investigation into the effect of culture conditions on fucoxanthin production using the marine microalgae Phaeodactylum tricornutum. Algal Res. 2018, 29, 41–48. [Google Scholar] [CrossRef]
- Mercado, J.M.; Sanchez-Saavedra, M.D.; Correa-Reyes, G.; Lubian, L.; Montero, O.; Figueroa, F.L. Blue light effect on growth, light absorption characteristics and photosynthesis of five benthic diatom strains. Aquat. Bot. 2004, 78, 265–277. [Google Scholar] [CrossRef]
- Chen, G.Q.; Jiang, Y.; Chen, F. Salt-induced alterations in lipid composition of diatom Nitzschia laevis (Bacillariophyceae) under heterotrophic culture condition. J. Phycol. 2008, 44, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Chen, Z.; Xue, S.; Zhang, W. Increased lipid production of the marine oleaginous microalgae Isochrysis zhangjiangensis (Chrysophyta) by nitrogen supplement. Bioresour. Technol. 2011, 102, 6710–6716. [Google Scholar] [CrossRef] [PubMed]
- Tokushim, H.; Inoue-Kashino, N.; Nakazato, Y.; Masuda, A.; Ifuku, K.; Kashino, Y. Advantageous characteristics of the diatom Chaetoceros gracilis as a sustainable biofuel producer. Biotechnol. Biofuels 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. H.; Du, L.; Hosokawa, M.; Miyashita, K.; Kokubun, Y.; Arai, H.; Taroda, H. Fatty acid and lipid class composition of the microalga Phaeodactylum tricornutum. J. Oleo. Sci. 2017, 66, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Chen, A.; Zhang, W.; Li, A.; Zhang, C. Co-production of lipids, eicosapentaenoic acid, fucoxanthin, and chrysolaminarin by Phaeodactylum tricornutum cultured in a flat-plate photobioreactor under varying nitrogen conditions. J. Ocean U. China 2017, 16, 916–924. [Google Scholar] [CrossRef]
- Chojnacka, K.; Noworyta, A. Evaluation of Spirulina sp. growth in photoautotrophic, heterotrophic and mixotrophic cultures. Enzym. Microb. Technol. 2004, 34, 461–465. [Google Scholar] [CrossRef]
- Heredia-Arroyo, T.; Wei, W.; Hu, B. Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Appl. Biochem. Biotechnol. 2010, 162, 1978–1995. [Google Scholar] [CrossRef] [PubMed]
- Radchenko, I.G.; Il’yash, L.V.; Fedorov, V.D. Effect of exogenous glucose on photosynthesis in the diatom Thalassiosira weissflogii depending on nitrate nitrogen supply and illumination. Biol. Bull. 2004, 31, 67–74. [Google Scholar] [CrossRef]
- Gomez-Loredo, A.; Benavides, J.; Rito-Palomares, M. Growth kinetics and fucoxanthin production of Phaeodactylum tricornutum and Isochrysis galbana cultures at different light and agitation conditions. J. Appl. Phycol. 2016, 28, 849–860. [Google Scholar] [CrossRef]
- Fu, W.; Guomundsson, O.; Paglia, G.; Herjolfsson, G.; Andresson, O.S.; Palsson, B.O.; Brynjolfsson, S. Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Appl. Microbiol. Biotechnol. 2013, 97, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, B.; Lu, X.; Cheng, K.W.; Chen, F. Staged cultivation enhances biomass accumulation in the green growth phase of Haematococcus pluvialis. Bioresour. Technol. 2017, 233, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Ruizvazquez, R.; Fontes, M.; Murillo, F.J. Clustering and co-ordinated activation of carotenoid genes in Myxococcus xanthus by blue light. Mol. Microbiol. 1993, 10, 25–34. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Jiang, Y.; Chen, F. High cell density culture of the diatom Nitzschia laevis for eicosapentaenoic acid production: Fed-batch development. Process Biochem. 2002, 37, 1447–1453. [Google Scholar] [CrossRef]
- Chen, F. High cell density culture of microalgae in heterotrophic growth. Trends Biotechnol. 1996, 14, 421–426. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Sun, Z.; Zhong, Y.; Jiang, Y.; Chen, F. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel production. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.X.; Zhang, Z.; Wang, J.; Huang, J.K.; Fan, J.H.; Yu, A.Q.; Wang, W.L.; Li, Y.G. Sequential Heterotrophy–Dilution–Photoinduction Cultivation of Haematococcus pluvialis for efficient production of astaxanthin. Bioresour. Technol. 2015, 198, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J.; Sun, P.; Ma, X.; Jiang, Y.; Chen, F. Sesamol enhances cell growth and the biosynthesis and accumulation of Docosahexaenoic Acid in the microalga Crypthecodinium cohnii. J. Agric. Food Chem. 2015, 63, 5640–5645. [Google Scholar] [CrossRef] [PubMed]
- Collos, Y.; Mornet, F.; Sciandra, A.; Waser, N.; Larson, A.; Harrison, P.J. An optical method for the rapid measurement of micromolar concentrations of nitrate in marine phytoplankton cultures. J. Appl. Phycol. 1999, 11, 179–184. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Hu, G.; Sommerfeld, M.; Hu, Q. Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2010, 107, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Chan, K.W.; Khong, N.M.H.; Yau, S.K. Production of fucoxanthin-rich fraction (FxRF) from a diatom, Chaetoceros calcitrans (Paulsen) Takano 1968. Algal Res. 2015, 12, 26–32. [Google Scholar] [CrossRef]

| Species | Biomass Concentration, g/L | Fucoxanthin Content, % | Fucoxanthin Productivity, mg/(L·d) | References | |
|---|---|---|---|---|---|
| Mallomonas sp. | 3.75 | 2.66 | 7.13 | [19] | |
| Cyclotella cryptica | 1.72 | 1.29 | 3.38 | [9] | |
| Chaetoceros calcitrans | 0.53 | [20] | |||
| Chaetoceros gracilis | 4.63 | [21] | |||
| Chaetoceros gracilis | 0.22 | [10] | |||
| Phaeodactylum tricornutum | 2.4 | 1.02 | 1.75 | [19] | |
| Phaeodactylum tricornutum | 0.41 | 0.45 | 0.18 | [22] | |
| Phaeodactylum tricornutum | 4.1 | 0.69 | 4.73 | [23] | |
| Isochrysis aff. galbana | 1.82 | [20] | |||
| Odontella aurita | 6.36 | 2.17 | 7.96 | [11] | |
| Nitzschia sp. | 0.49 | [11] | |||
| Nitzschi laevis | 0.2 | 0.5 | 0.17 | [11] | |
| Nitzschi laevis (batch in flask) | 2.22 | 1.20 | 4.44 | This study | |
| Nitzschi laevis (fed-batch in flask) | 7.13 | 1.20 | 9.01 | This study | |
| Nitzschi laevis (fed-batch in fermentor) | 17.25 | 1.20 | 16.5 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Sun, H.; Zhao, W.; Cheng, K.-W.; Chen, F.; Liu, B. A Hetero-Photoautotrophic Two-Stage Cultivation Process for Production of Fucoxanthin by the Marine Diatom Nitzschia laevis. Mar. Drugs 2018, 16, 219. https://doi.org/10.3390/md16070219
Lu X, Sun H, Zhao W, Cheng K-W, Chen F, Liu B. A Hetero-Photoautotrophic Two-Stage Cultivation Process for Production of Fucoxanthin by the Marine Diatom Nitzschia laevis. Marine Drugs. 2018; 16(7):219. https://doi.org/10.3390/md16070219
Chicago/Turabian StyleLu, Xue, Han Sun, Weiyang Zhao, Ka-Wing Cheng, Feng Chen, and Bin Liu. 2018. "A Hetero-Photoautotrophic Two-Stage Cultivation Process for Production of Fucoxanthin by the Marine Diatom Nitzschia laevis" Marine Drugs 16, no. 7: 219. https://doi.org/10.3390/md16070219
APA StyleLu, X., Sun, H., Zhao, W., Cheng, K.-W., Chen, F., & Liu, B. (2018). A Hetero-Photoautotrophic Two-Stage Cultivation Process for Production of Fucoxanthin by the Marine Diatom Nitzschia laevis. Marine Drugs, 16(7), 219. https://doi.org/10.3390/md16070219







