Raistrickiones A−E from a Highly Productive Strain of Penicillium raistrickii Generated through Thermo Change
Abstract
1. Introduction
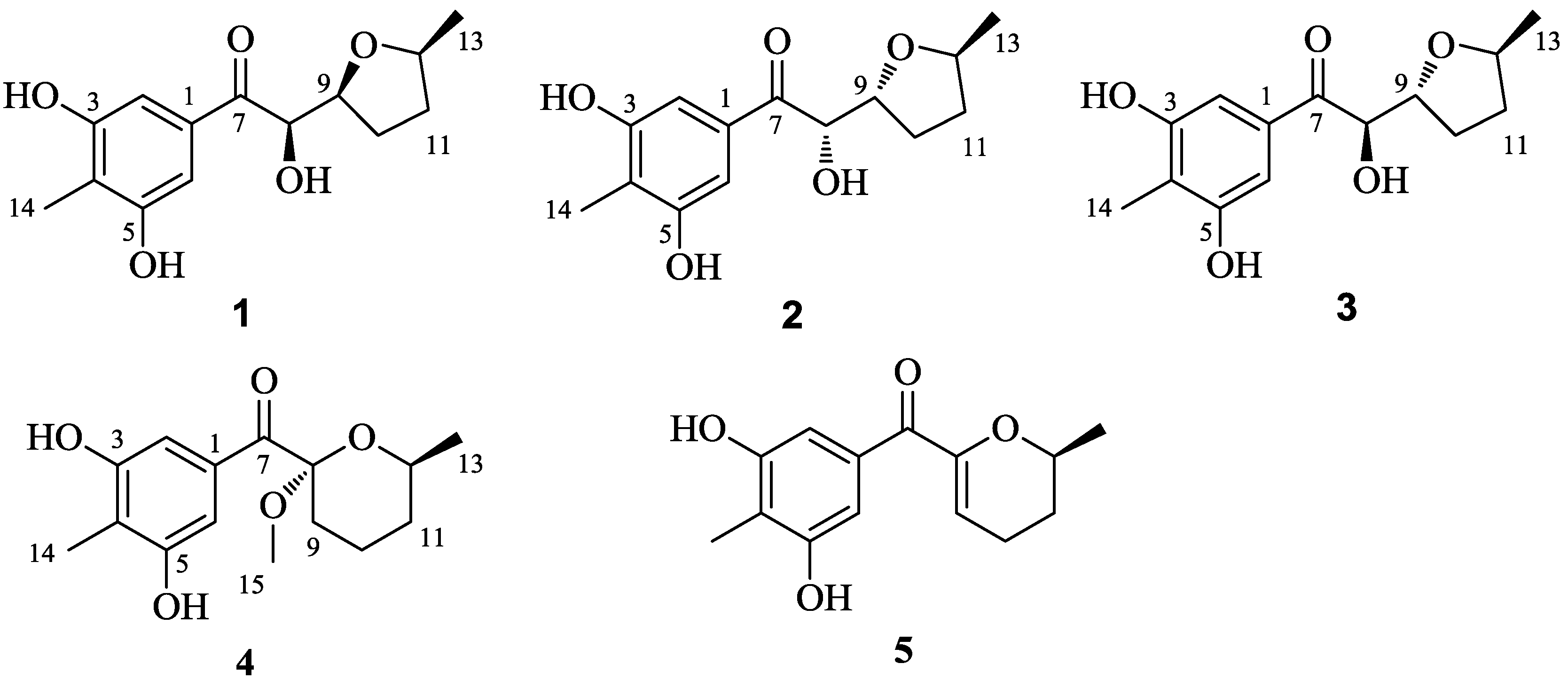
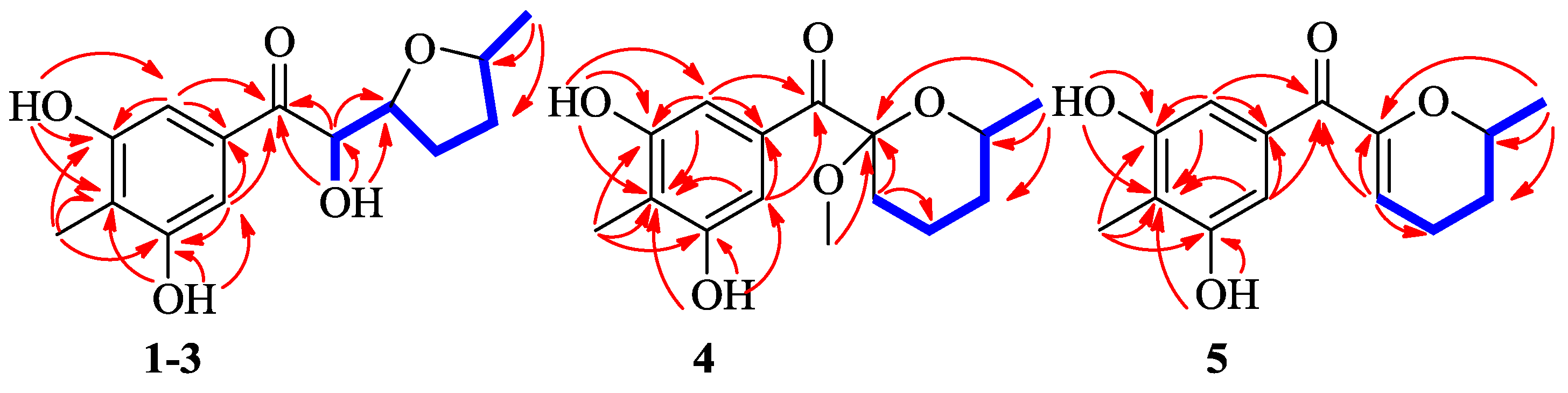
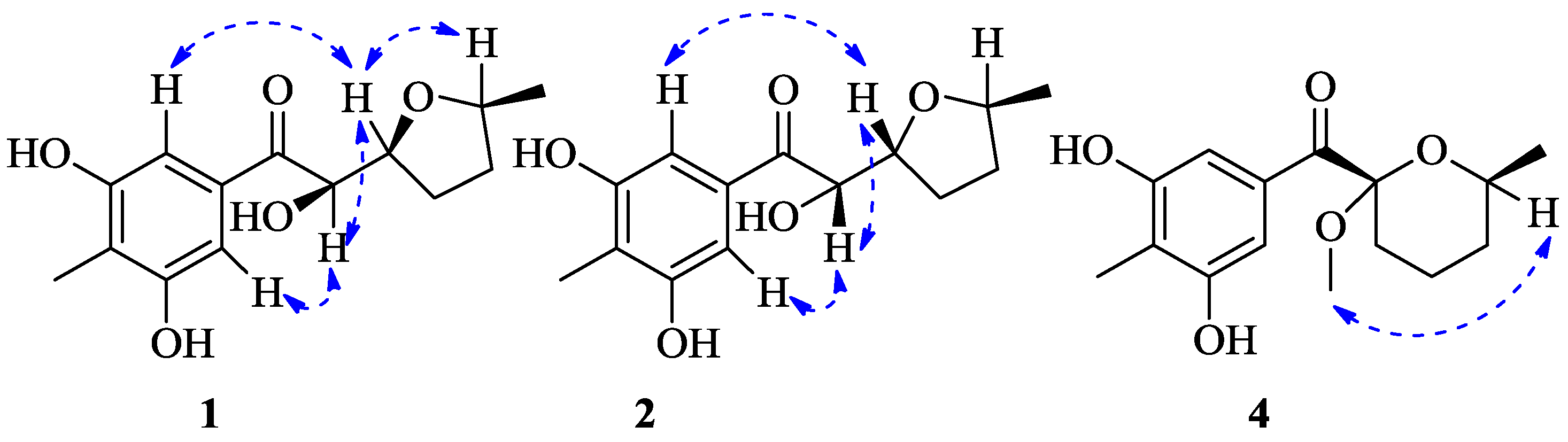
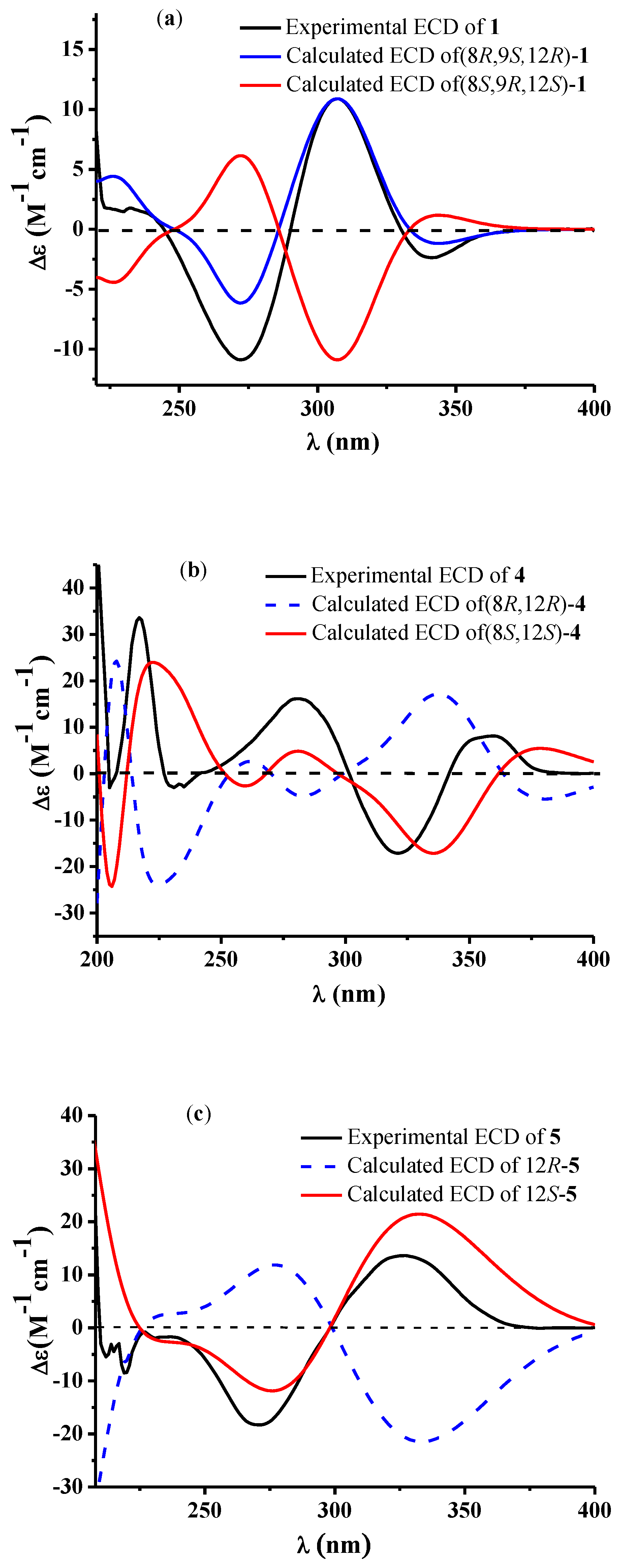
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungal Material
4.3. Fermentation, Extraction, and Isolation
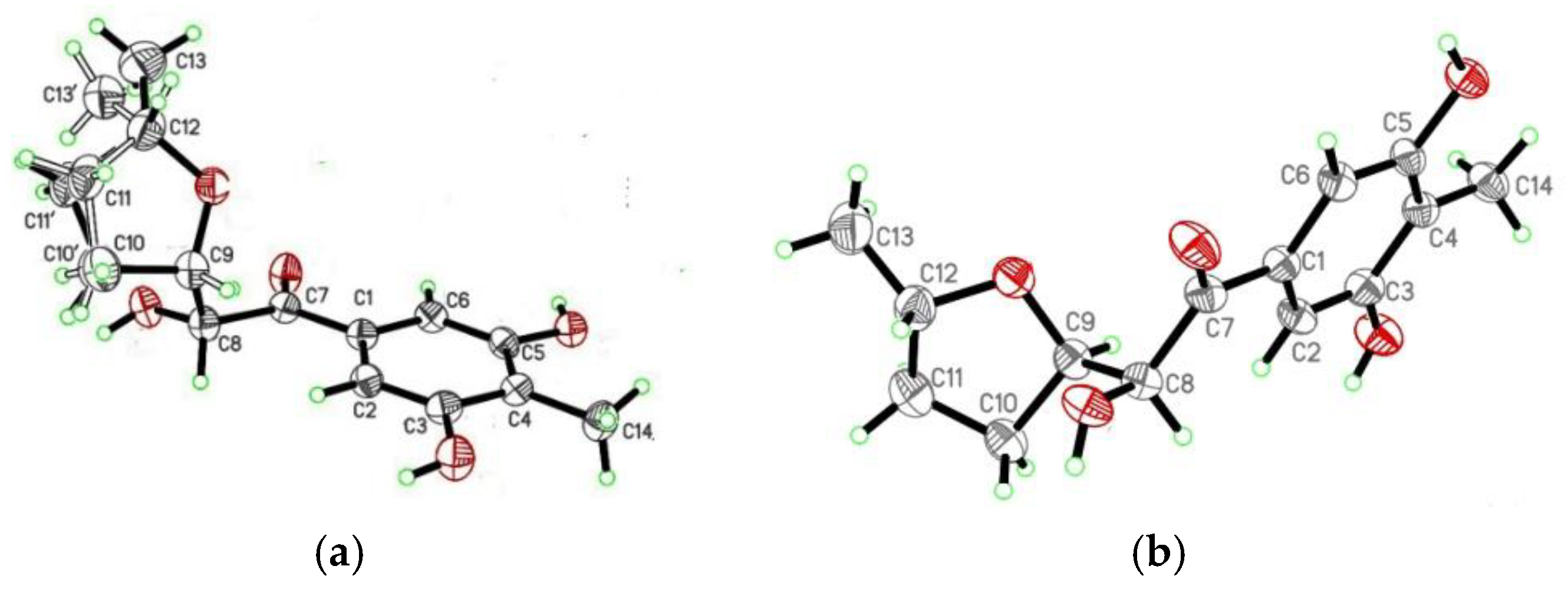
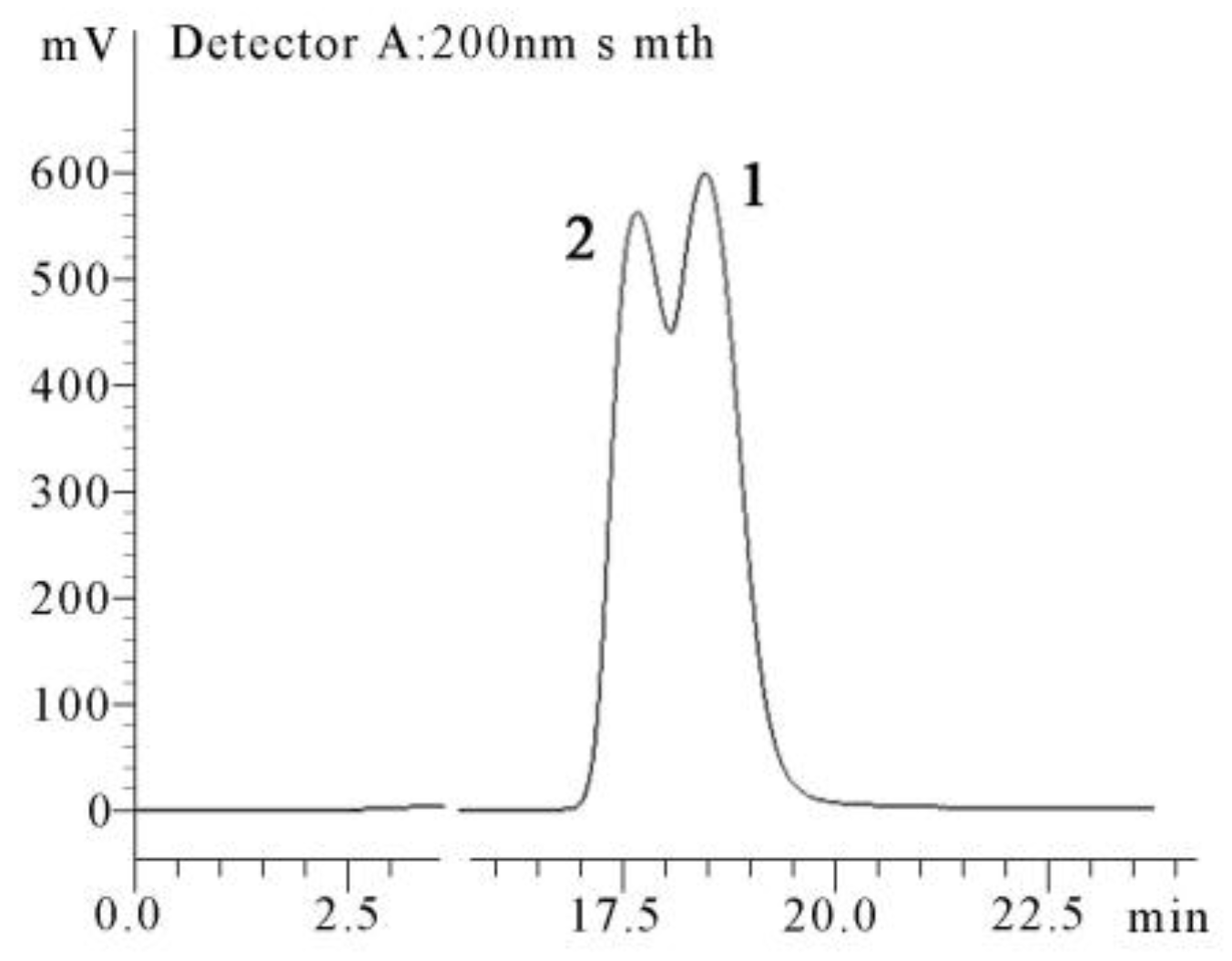
4.4. X-ray Crystallographic Analysis of the Diastereomeric Mixture of 1 and 2
4.5. Antioxidant Activity Assay
4.6. Cytotoxicity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.S.; Yan, L.; Ma, L.Y.; Huang, Y.L.; Pan, X.H.; Liu, W.Z.; Lv, Z.H. Diphenyl derivatives from coastal saline soil fungus Aspergillus iizukae. Arch. Pharm. Res. 2015, 38, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.J.; Koch, M.; Abdel Aziz, M.H.; Galindo-Murillo, R.; Tianero, M.D.; Cheatham, T.E.; Barrows, L.R.; Reilly, C.A.; Schmidt, E.W. Oxazinin A, a pseudodimeric natural product of mixed biosynthetic origin from a filamentous fungus. Org. Lett. 2014, 16, 4774–4777. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Gunasekara, N.W.; Ratnaweera, P.B.; Zheng, Z.; Ellis, S.; Dada, S.; Patrick, B.O.; Wijesundera, R.L.C.; Nanayakkara, C.M.; Jefferies, W.A.; et al. Serpulanines A to C, N-oxidized tyrosine derivatives isolated from the Sri Lankan fungus Serpula sp.: Structure elucidation, synthesis, and histone deacetylase unhibition. J. Nat. Prod. 2018, 81, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.W.; Niu, S.B.; Li, L.; Geng, Z.F.; Liu, X.Z.; Che, Y.S. Identification of oxaphenalenone ketals from the Ascomycete fungus Neonectria sp. J. Nat. Prod. 2015, 78, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.C.; Grijseels, S.; Prigent, S.; Ji, B.; Dainat, J.; Nielsen, K.F.; Frisvad, J.C.; Workman, M.; Nielsen, J. Global analysis of biosynthetic gene clusters reveals vast potential of secondary metabolite production in Penicillium species. Nat. Microbiol. 2017, 2, 17044. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Tsai, K.J.S.; Harvey, C.J.B.; Li, J.J.; Ary, B.E.; Berlew, E.E.; Boehman, B.L.; Findley, D.M.; Friant, A.G.; Gardner, C.A.; et al. Comprehensive curation and analysis of fungal biosynthetic gene clusters of published natural products. Fungal Genet. Biol. 2016, 89, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, M.; Li, L.; Si, J.; Song, B.; Zhou, C.; Yu, M.; Wang, X.; Zhang, Y.; Ding, G.; et al. Overexpression of the global regulator Laea in Chaetomium globosum leads to the biosynthesis of chaetoglobosin Z. J. Nat. Prod. 2016, 79, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Enghiad, B.; Zhao, H. New tools for reconstruction and heterologous expression of natural product biosynthetic gene clusters. Nat. Prod. Rep. 2016, 33, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Reen, F.J.; Romano, S.; Dobson, A.D.; O’Gara, F. The sound of silence: Activating silent biosynthetic gene clusters in marine microorganisms. Mar. Drugs 2015, 13, 4754–4783. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Liu, Y.; Li, X.M.; Xu, G.M.; Ji, N.Y.; Wang, B.G. Citrifelins A and B, citrinin adducts with a tetracyclic framework from cocultures of marine-derived isolates of Penicillium citrinum and Beauveria felina. J. Nat. Prod. 2015, 78, 2301–2305. [Google Scholar] [CrossRef] [PubMed]
- He, X.Q.; Zhang, Z.Z.; Che, Q.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Varilactones and wortmannilactones produced by Penicillium variabile cultured with histone deacetylase inhibitor. Arch. Pharm. Res. 2018, 41, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Doull, J.L.; Singh, A.K.; Hoare, M.; Ayer, S.W. Conditions for the production of jadomycin B by Streptomyces venezuelae ISP5230: Effects of heat shock, ethanol treatment and phage infection. J. Ind. Microbiol. 1994, 13, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.H.; Wu, H.; Bai, L.Q.; Deng, Z.X.; Zhong, J.J. Temperature shift-induced reactive oxygen species enhanced validamycin A production in fermentation of Streptomyces hygroscopicus 5008. Bioprocess Biosyst. Eng. 2012, 35, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Ingebrigtsen, R.A.; Hansen, E.; Andersen, J.H.; Eilertsen, H.C. Light and temperature effects on bioactivity in diatoms. J. Appl. Phycol. 2016, 28, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Liu, D.S.; Li, D.G.; Huang, Y.L.; Kang, H.H.; Wang, C.H.; Liu, W.Z. Pyran rings containing polyketides from Penicillium raistrickii. Mar. Drugs 2017, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Ma, L.Y.; Liu, D.S.; Huang, Y.L.; Wang, C.H.; Shi, S.S.; Pan, X.H.; Song, X.D.; Zhu, R.X. Peniciketals A–C, new spiroketals from saline soil derived Penicillium raistrichii. Org. Lett. 2014, 16, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Liu, W.Z.; Shen, L.; Huang, Y.L.; Rong, X.G.; Xu, Y.Y.; Gao, X.D. Spiroketals, isocoumarin and indoleformic acid derivatives from saline soil derived fungus Penicillium raistrickii. Tetrahedron 2012, 68, 2276–2282. [Google Scholar] [CrossRef]
- Belofsky, G.N.; Gloer, K.B.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. New p-terphenyl and polyketide metabolites from the sclerotia of Penicillium raistrickii. J. Nat. Prod. 1998, 61, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.H.; Liu, D.S.; Wang, J.; Zhang, X.L.; Yan, M.M.; Zhang, D.H.; Zhang, J.J.; Liu, W.Z. Peneciraistin C induces caspase-independent autophagic cell death through mitochondrial-derived reactive oxygen species production in lung cancer cells. Cancer Sci. 2013, 104, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. Chembiochem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Karplus, M. Vicinal proton coupling in nuclear magnetic resonance. J. Am. Chem. Soc. 1963, 85, 2870–2871. [Google Scholar] [CrossRef]
- Afifi, A.H.; Kagiyama, I.; El-Desoky, A.H.; Kato, H.; Mangindaan, R.E.P.; de Voogd, N.J.; Ammar, N.M.; Hifnawy, M.S.; Tsukamoto, S. Sulawesins A–C, furanosesterterpene tetronic acids that inhibit USP7, from a Psammocinia sp. marine sponge. J. Nat. Prod. 2017, 80, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Grkovic, T.; Pearce, A.N.; Munro, M.H.; Blunt, J.W.; Davies-Coleman, M.T.; Copp, B.R. Isolation and characterization of diastereomers of discorhabdins H and K and assignment of absolute configuration to discorhabdins D, N, Q, S, T, and U. J. Nat. Prod. 2010, 73, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; de Luna, R.D.; Cruz, W.C., Jr.; Rideout, J.A. Monoterpene lactones from the seeds of Nephelium lappaceum. J. Nat. Prod. 2005, 68, 1394–1396. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Andolfi, A.; Fiore, M.; Spanu, E.; Maddau, L.; Franceschini, A.; Marras, F.; Motta, A. Diplobifuranylones A and B, 5′-monosubstituted tetrahydro-2H-bifuranyl-5-ones produced by Diplodia corticola, a fungus pathogen of cork oak. J. Nat. Prod. 2006, 69, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.B.; Wang, S. Stereochemistry of the roridins. diastereomers of roridin E. J. Nat. Prod. 1999, 62, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Presley, C.C.; Valenciano, A.L.; Fernández-Murga, M.L.; Du, Y.; Shanaiah, N.; Cassera, M.B.; Goetz, M.; Clement, J.A.; Kingston, D.G.I. Antiplasmodial chromanes and chromenes from the monotypic plant species Koeberlinia spinosa. J. Nat. Prod. 2018, 81, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Thoison, O.; Fahy, J.; Dumontet, V.; Chiaroni, A.; Riche, C.; Tri, M.V.; Sévenet, T. Cytotoxic prenylxanthones from Garcinia bracteata. J. Nat. Prod. 2000, 63, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Boonnak, N.; Chantrapromma, S.; Fun, H.K.; Yuenyongsawad, S.; Patrick, B.O.; Maneerat, W.; Williams, D.E.; Andersen, R.J. Three types of cytotoxic natural caged-scaffolds: Pure enantiomers or partial racemates. J. Nat. Prod. 2014, 77, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Liu, W.Z.; Huang, Y.L.; Rong, X.G. Two acid sorbicillin analogues from saline lands-derived fungus Trichoderma sp. J. Antibiot. 2011, 64, 645–647. [Google Scholar] [CrossRef] [PubMed]
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 133.0, C | 132.9, C | 133.5, C | |||
| 2, 6 | 106.2, CH | 6.90, s | 106.2, CH | 6.89, s | 106.2, CH | 6.94, s |
| 3, 5 | 156.2, C | 156.2, C | 156.3, C | |||
| 4 | 116.6, C | 116.6, C | 116.7, C | |||
| 7 | 199.0, C | 199.2, C | 199.4, C | |||
| 8 | 74.9, CH | 4.74, dd (7.3, 3.5) | 75.4, CH | 4.69, dd (7.3, 3.3) | 74.8, CH | 4.73, t (5.8) |
| 9 | 80.1, CH | 4.13, m | 79.5, CH | 4.27, td (7.0, 3.3) | 79.3, CH | 4.20, q (5.8) |
| 10 | 27.3, CH2 | 1.84, a m | 27.8, CH2 | 1.93, a m | 26.5, CH2 | 1.84, m; 1.77, m |
| 11 | 32.5, CH2 | 1.84, a m; 1.34, m | 33.4, CH2 | 1.93, a m; 1.31, m | 33.1, CH2 | 1.99, m; 1.34, m |
| 12 | 75.2, CH | 3.77, m | 75.4, CH | 3.99, m | 75.0, CH | 4.04, m |
| 13 | 20.6, CH3 | 1.06, d (6.0) | 21.0, CH3 | 1.02, d (6.1) | 21.0, CH3 | 1.04, d (6.0) |
| 14 | 8.9, CH3 | 1.99, s | 8.9, CH3 | 1.99, s | 8.9, CH3 | 1.99, s |
| 15 | ||||||
| OH-3, 5 | 9.51, s | 9.52, s | 9.49, s | |||
| OH-8 | 4.84, d (7.3) | 4.96, d (7.3) | 5.31, d (5.8) | |||
| Position | 4 a | 5 a | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 133.9, C | 136.3, C | ||
| 2, 6 | 109.2, CH | 7.36, s | 108.7, CH | 6.94, s |
| 3, 5 | 156.8, C | 156.8, C | ||
| 4 | 117.6, C | 116.8, C | ||
| 7 | 196.5, C | 190.1, C | ||
| 8 | 102.6, C | 152.7, C | ||
| 9 | 32.3, CH2 | 1.80, m; 1.65, b m | 112.2, CH | 5.71, t (3.8) |
| 10 | 19.7, CH2 | 1.91, m; 1.65, b m | 21.6, CH2 | 2.30, m; 2.20, m |
| 11 | 32.7, CH2 | 1.65, b m; 1.40, m | 29.3, CH2 | 1.94, m; 1.55, m |
| 12 | 68.0, CH | 3.88, m | 73.0, CH | 4.04, m |
| 13 | 22.0, CH3 | 1.22, d (6.3) | 21.2, CH3 | 1.32, d (6.2) |
| 14 | 9.0, CH3 | 2.13, s | 9.0, CH3 | 2.15, s |
| 15 | 50.5, CH3 | 3.17, s | ||
| OH-3, 5 | 8.43, s | 8.45, s | ||
| OH-8 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.-S.; Rong, X.-G.; Kang, H.-H.; Ma, L.-Y.; Hamann, M.T.; Liu, W.-Z. Raistrickiones A−E from a Highly Productive Strain of Penicillium raistrickii Generated through Thermo Change. Mar. Drugs 2018, 16, 213. https://doi.org/10.3390/md16060213
Liu D-S, Rong X-G, Kang H-H, Ma L-Y, Hamann MT, Liu W-Z. Raistrickiones A−E from a Highly Productive Strain of Penicillium raistrickii Generated through Thermo Change. Marine Drugs. 2018; 16(6):213. https://doi.org/10.3390/md16060213
Chicago/Turabian StyleLiu, De-Sheng, Xian-Guo Rong, Hui-Hui Kang, Li-Ying Ma, Mark T. Hamann, and Wei-Zhong Liu. 2018. "Raistrickiones A−E from a Highly Productive Strain of Penicillium raistrickii Generated through Thermo Change" Marine Drugs 16, no. 6: 213. https://doi.org/10.3390/md16060213
APA StyleLiu, D.-S., Rong, X.-G., Kang, H.-H., Ma, L.-Y., Hamann, M. T., & Liu, W.-Z. (2018). Raistrickiones A−E from a Highly Productive Strain of Penicillium raistrickii Generated through Thermo Change. Marine Drugs, 16(6), 213. https://doi.org/10.3390/md16060213




