Anti-Aging Effect of Chitosan Oligosaccharide on d-Galactose-Induced Subacute Aging in Mice
Abstract
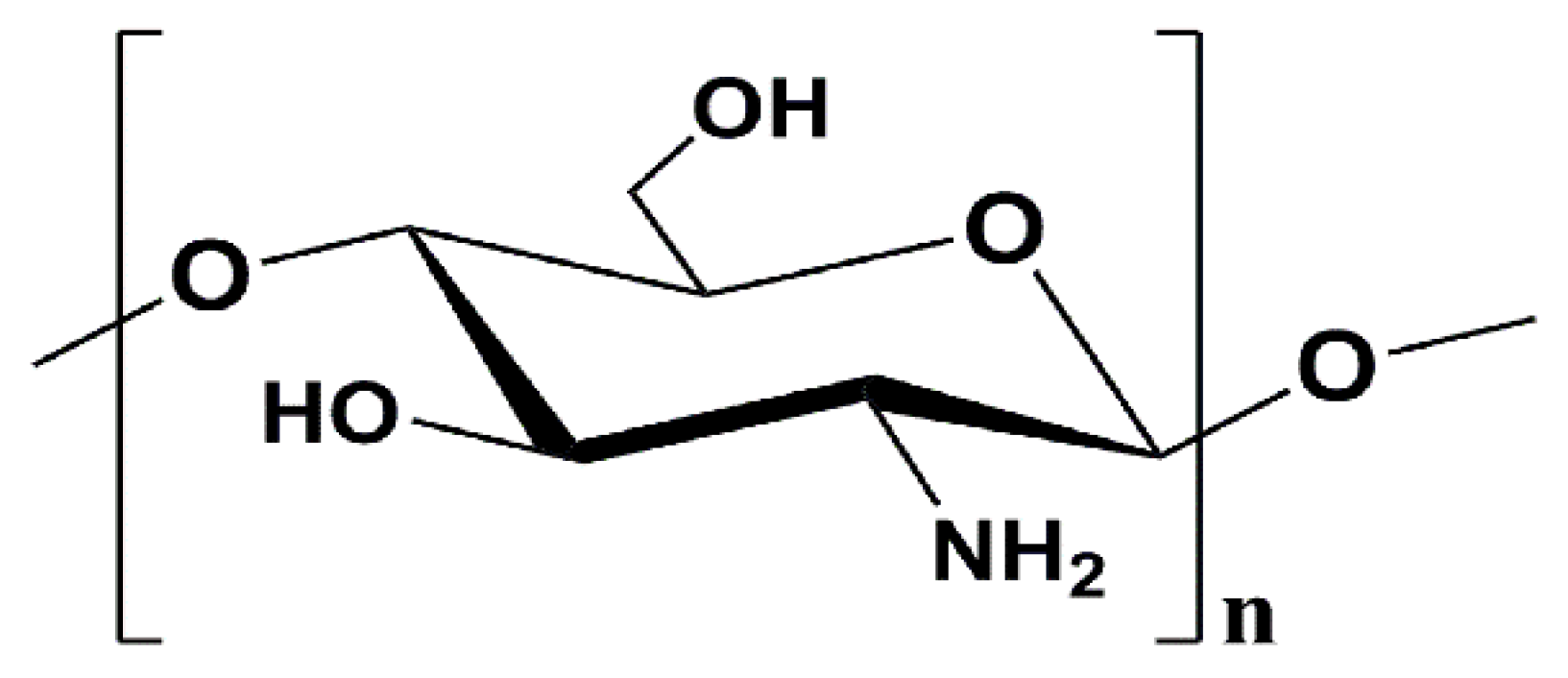
:1. Introduction
2. Results
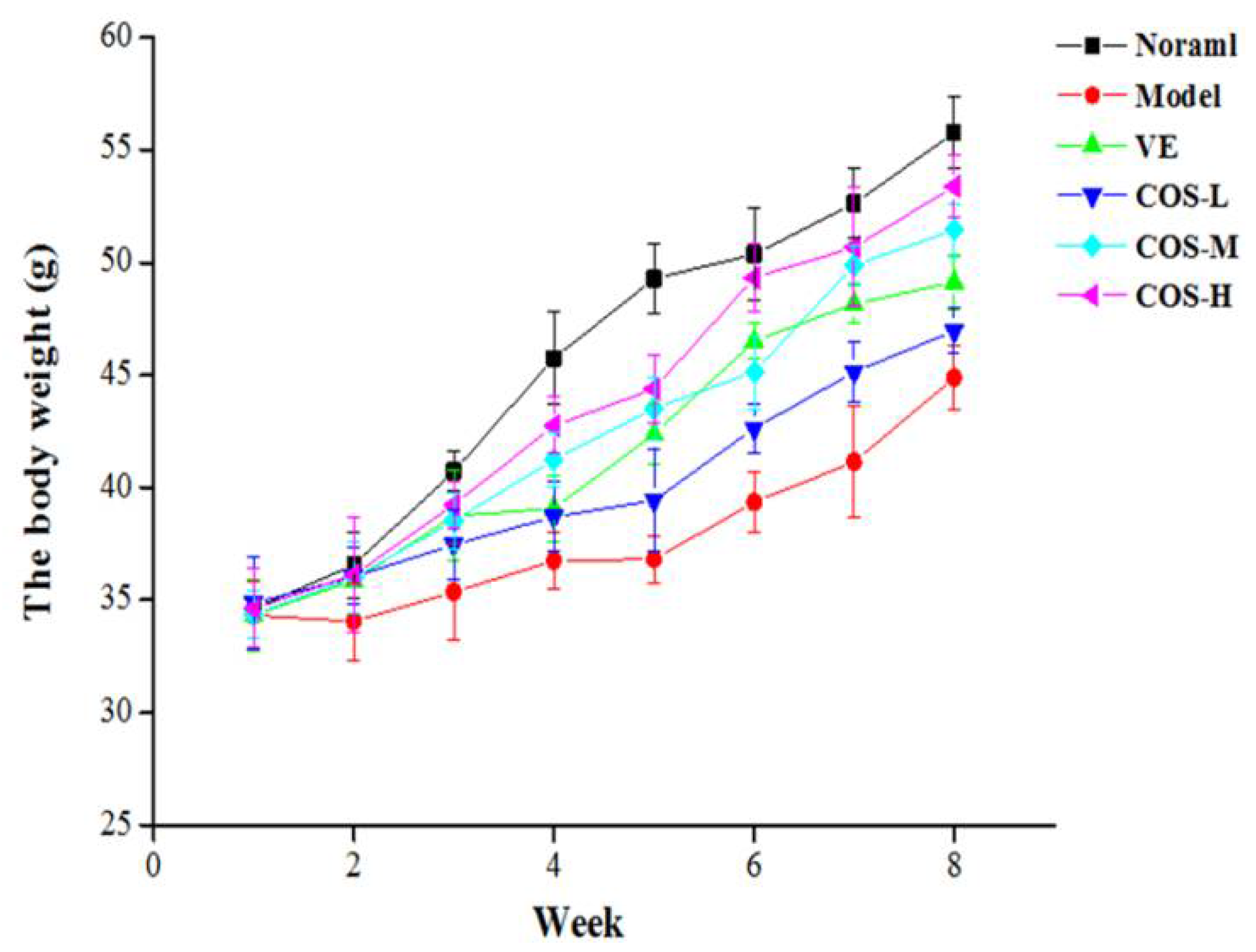
2.1. Daily Behavior Observation
2.2. Effect of COS on Immune Function
2.3. Effect of COS on Liver and Kidney Function
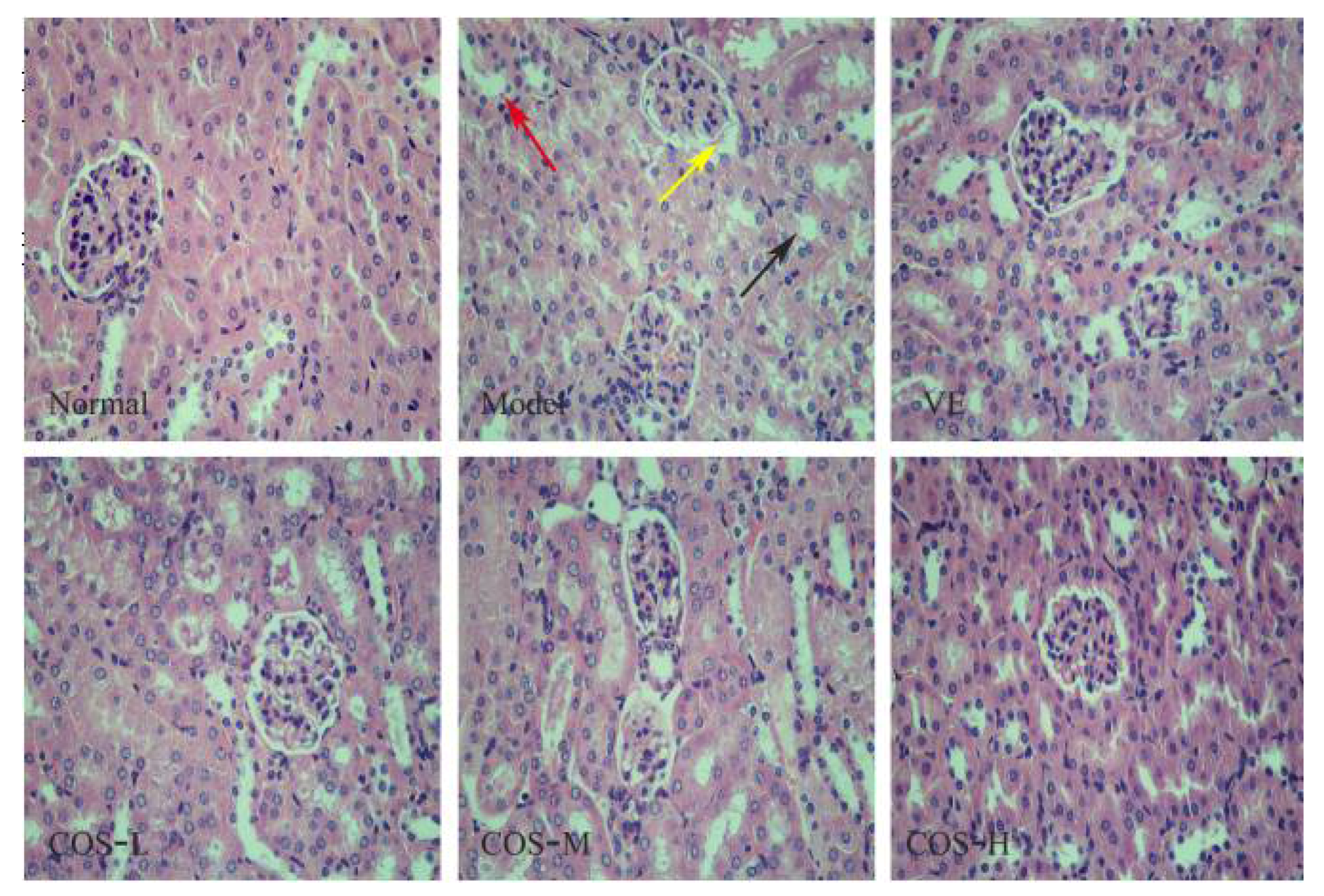
2.4. Effect of COS on d-Gal-Induced Histochemical Damages of the Liver and Kidney
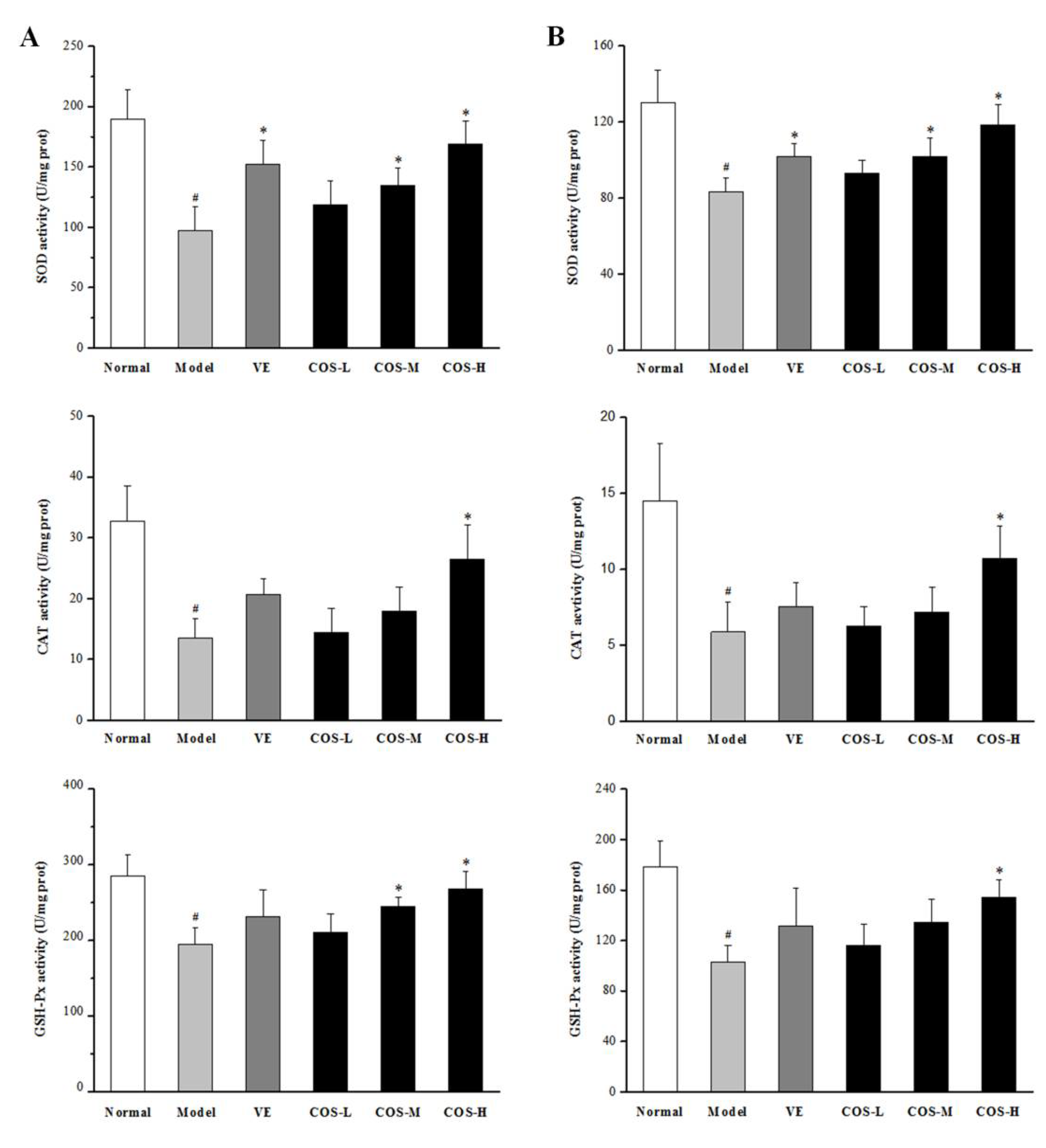
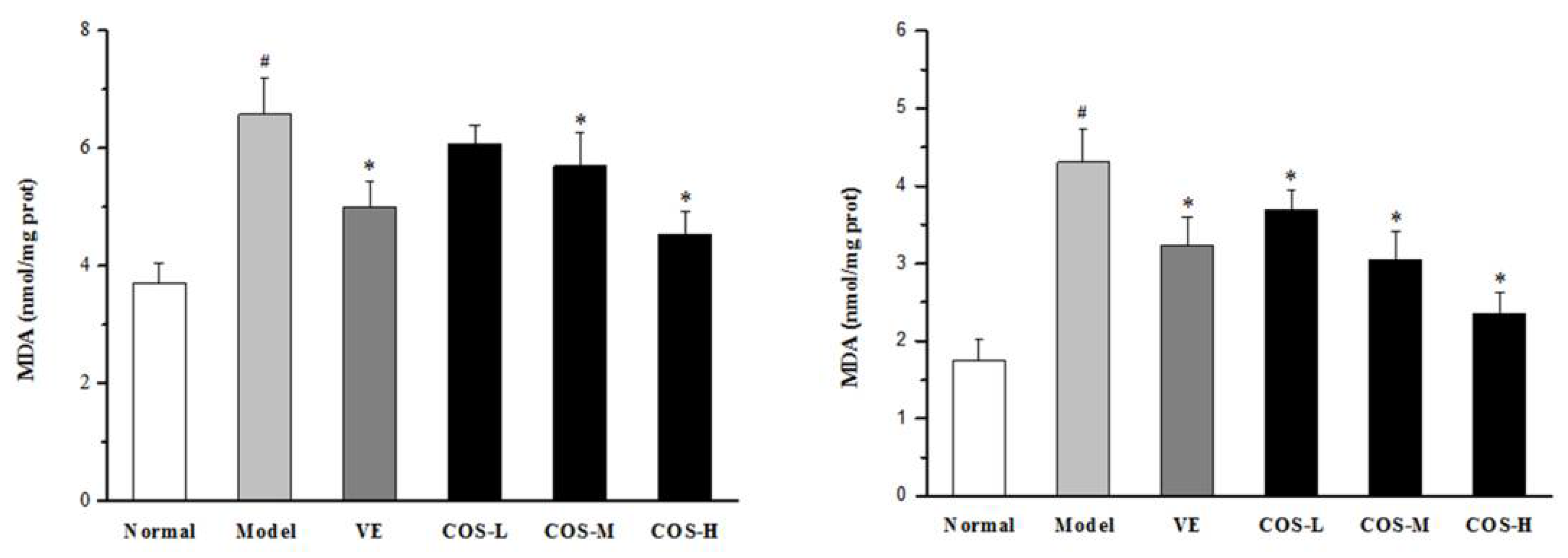
2.5. Effect of COS on Anti-Oxidation Indices of Liver and Kidney
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Animals
4.3. Preparation of Subacute Aging Mouse Model and COS Treatment
4.4. Daily Observation of Mice
4.5. Preparation of Mice and Sample Collection
4.6. Determination of Serum Indices
4.7. Measurement of the Anti-Oxidation Activity in Mice Liver and Kidney
4.8. Histological Examination of Liver and Kidney
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sergiev, P.V.; Dontsova, O.A.; Berezkin, G.V. Theories of aging: An Ever-Evolving Field. Acta Nat. 2015, 7, 9–18. [Google Scholar]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Liu, M.; Zhang, C.; Tian, C.Y.; Wang, X.X.; Song, X.L.; Jing, H.J.; Gao, Z.; Ren, Z.Z.; Liu, W.R.; et al. Purification, in vitro antioxidant and in vivo anti-aging activities of soluble polysaccharides by enzyme-assisted extraction from Agaricus bisporus. Int. J. Biol. Macromol. 2018, 109, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Li, J.; Zhang, J.J.; Wang, X.X.; Hao, L.; Jia, L. Purification, in vitro antioxidant and in vivo anti-aging activities of exopolysaccharides by Agrocybe cylindracea. Int. J. Biol. Macromol. 2017, 102, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Ginter, E.; Simko, V.; Panakova, V. Antioxidants in health and disease. Bratisl. Lek. Listy 2014, 115, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Jasiulionis, M.G. Abnormal epigenetic regulation of immune system during aging. Front. Immunol. 2018, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.F.; Yu, H.L.; Zhang, Y.X.; Liu, G.Y. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharm. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Kunanusornchai, W.; Witoonpanith, B.; Tawonsawatruk, T.; Pichyangkura, R.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide suppresses synovial inflammation via AMPK activation: An in vitro and in vivo study. Pharmacol. Res. 2016, 113, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.Z.; Li, D.D.; Luo, H.; Li, W.J.; Huang, Y.M.; Li, J.C.; Hu, Z.; Huang, N.; Guo, M.H.; Chen, Y.; et al. Anti-photoaging effects of chitosan oligosaccharide in ultraviolet-irradiated hairless mouse skin. Exp. Gerontol. 2018, 103, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Fang, I.M.; Yang, C.M.; Yang, C.H. Chitosan oligosaccharides prevented retinal ischemia and reperfusion injury via reduced oxidative stress and inflammation in rats. Exp. Eye Res. 2015, 130, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xiao, D.F.; Tan, B.; Xiao, H.; Wang, J.; Yin, J.; Duan, J.L.; Huang, R.L.; Yang, C.B.; Yin, Y.L. Chitosan oligosaccharide reduces intestinal inflammation that involves Calcium-sensing receptor (CaSR) activation in Lipopolysaccharide (LPS)-challenged piglets. J. Agric. Food Chem. 2016, 64, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Jiang, F.; Xu, Q.S.; Chen, D.W.; Yu, B.; Huang, Z.Q.; Mao, X.B.; Yu, J.; He, J. New insights into the role of chitosan oligosaccharide in enhancing growth performance, antioxidant capacity, immunity and intestinal development of weaned pigs. RSC Adv. 2017, 7, 9669–9679. [Google Scholar] [CrossRef]
- Fang, I.M.; Yang, C.H.; Yang, C.M.; Chen, M.S. Chitosan oligosaccharides attenuates oxidative-stress related retinal degeneration in rats. PLoS ONE 2013, 8, e77323. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Izzo, V.; Corbi, G.; Russomanno, G.; Manzo, V.; De Lise, F.; Di Donato, A.; Filippelli, A. Antioxidant supplementation in the treatment of aging-associated diseases. Front. Pharmacol. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.Y.; Zhang, Y.Y.; Li, J.; Xia, J.Y.; Chen, X.B.; Jing, P.W.; Song, X.Y.; Wang, L.; Wang, Y.P. Angelica sinensis polysaccharide prevents hematopoietic stem cells senescence in d-galactose-induced aging mouse model. Stem Cells Int. 2017, 2017, 3508907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.Q.; Yang, L.; Zu, Y.G.; Lu, Q. Effects of rosmarinic acid on liver and kidney antioxidant enzymes, lipid peroxidation and tissue ultrastructure in aging mice. Food Funct. 2015, 6, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Kubo, E.; Chhunchha, B.; Singh, P.; Sasaki, H.; Singh, D.P. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci. Rep. 2017, 7, 14130. [Google Scholar] [CrossRef] [PubMed]
- Clements, S.J.; Carding, S.R. Diet, the intestinal microbiota, and immune health in aging. Crit. Rev. Food Sci. Nutr. 2018, 58, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Research on effect of ginkgo aglucone flavone to Human body organs and immune function. Pak. J. Pharm. Sci. 2014, 27, 1099–1102. [Google Scholar] [PubMed]
- Chen, N.Y.; Liu, C.W.; Lin, W.; Ding, Y.; Bian, Z.Y.; Huang, L.; Huang, H.; Yu, K. H.; Chen, S.B.; Sun, Y.; et al. Extract of fructus cannabis ameliorates learning and memory impairment induced by d-galactose in an aging rats model. Evid-Based Compl Altern. Med. 2017, 2017, 4757520. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, H.P.; Luo, Y.M. Anti-aging implications of astragalus membranaceus (Huangqi): A well-known Chinese tonic. Aging Dis. 2017, 8, 868–886. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, Z. The protective effect of aminobutyric acid on the development of immune function in chickens under heat stress. J. Anim. Physiol. Anim. Nutr. 2016, 100, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.Y.; He, X.L.; Huang, H.; Yin, Z.J. Effects of feeding Chinese herbal medicine additives to newly weaned piglets on mucosal immune function of the small intestine. J. Anim. Vet. Adv. 2012, 11, 2817–2823. [Google Scholar]
- Ladomenou, F.; Gaspar, B. How to use immunoglobulin levels in investigating immune deficiencies. Arch. Dis. Child. Educ. Pract. Ed. 2016, 101, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Delwing-Dal Magro, D.; Roecker, R.; Junges, G.M.; Rodrigues, A.F.; Delwing-de Lima, D.; da Cruz, J.G.; Wyse, A.T.S.; Pitz, H.S.; Zeni, A.L.B. Protective effect of green tea extract against proline-induced oxidative damage in the rat kidney. Biomed. Pharmacother. 2016, 83, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.B.; Shin, G.H.; Kim, J.M.; Kim, Y.H.; Lee, J.H.; Lee, J.S.; Song, H.J.; Choe, S.Y.; Park, I.J.; Cho, J.H.; et al. Antioxidant and anti-ageing activities of citrus-based juice mixture. Food Chem. 2016, 194, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.L.; Ma, Q.Y.; Guo, Y.; Sun, L.P. Protective effects of rambutan (Nephelium lappaceum) peel phenolics on H2O2-induced oxidative damages in HepG2 cells and d-galactose induced aging mice. Food Chem. Toxicol. 2017, 108, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Li, H.; Zhou, R.; Liu, M.J.; Yu, H.; Wu, D.F. Pioglitazone attenuates aging-related disorders in aged apolipoprotein E deficient mice. Exp. Gerontol. 2018, 102, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.Z.; Lin, Y.J.; Li, K.J.; Zhang, G.L.; Zhao, Y.F.; Wang, M.M.; Wei, J.B.; Wei, J.; Hu, G. Effects of rhein lysinate on d-galactose-induced aging mice. Exp. Ther. Med. 2016, 11, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Mo, Z.Z.; Xie, J.H.; Xu, L.Q.; Tan, L.H.; Luo, D.D.; Chen, H.B.; Yang, H.M.; Li, Y.C.; Su, Z.R.; Su, Z.Q. Chongcao-Shencha attenuates liver and kidney injury through attenuating oxidative stress and inflammatory response in d-galactose-treated mice. Evid.-Based Complement. Altern. Med. 2016, 2016, 3878740. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.Z.; Liu, Y.H.; Li, C.L.; Xu, L.Q.; Wen, L.L.; Xian, Y.F.; Lin, Z.X.; Zhan, J.Y.X.; Chen, J.N.; Xu, F.F.; Su, Z.R. Protective effect of SFE-CO2 of ligusticum chuanxiong hort against d-galactose-induced injury in the mouse liver and kidney. Rejuvenation Res. 2017, 20, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Long, Y.Y.; Guo, L. Antiaging effect of inula britannica on aging mouse model induced by d-Galactose. Evid.-Based Complement. Altern. Med. 2016, 2016, 6049083. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Jia, R.R.; Tang, L.; Chen, F. In vivo antioxidant and anti-skin-aging activities of ethyl acetate extraction from Idesia polycarpa defatted fruit residue in aging mice induced by d-galactose. Evid.-Based Complement. Altern. Med. 2014, 2014, 185716. [Google Scholar]

| Groups | Organ Index (mg/g) | Immune Function | ||
|---|---|---|---|---|
| Thymus | Spleen | IgG (g/L) | IgM (g/L) | |
| Normal | 0.22 ± 0.05 | 0.58 ± 0.28 | 0.80 ± 0.03 | 0.16 ± 0.03 |
| Model | 0.14 ± 0.05 # | 0.34 ± 0.09 # | 0.57 ± 0.04 # | 0.07 ± 0.02 # |
| VE | 0.19 ± 0.04 | 0.43 ± 0.16 | 0.61 ± 0.05 | 0.10 ± 0.03 |
| COS-L | 0.14 ± 0.04 | 0.42 ± 0.07 | 0.70 ± 0.04 * | 0.08 ± 0.02 |
| COS-M | 0.16 ± 0.06 | 0.49 ± 0.23 * | 0.72 ± 0.07 * | 0.11 ± 0.02 * |
| COS-H | 0.19 ± 0.05 | 0.53 ± 0.14 * | 0.77 ± 0.08 * | 0.12 ± 0.02 * |
| Groups | Liver Function | Kidney Function | |||
|---|---|---|---|---|---|
| ALP (U/L) | ALP (U/L) | AST (U/L) | CREA (μmol/L) | UA (μmol/L) | |
| Normal | 50.08 ± 5.46 | 20.88 ± 3.17 | 53.79 ± 4.40 | 51.46 ± 3.08 | 125.24 ± 9.52 |
| Model | 82.26 ± 3.09 # | 28.20 ± 4.39 # | 78.51 ± 3.77 # | 61.49 ± 5.53 # | 162.55 ± 8.93 # |
| VE | 69.26 ± 6.05 * | 22.05 ± 4.02 * | 68.61 ± 5.24 * | 58.36 ± 6.28 | 142.83 ± 7.76 * |
| COS-L | 68.28 ± 4.57 * | 25.59 ± 3.63 | 65.15 ± 4.21 * | 55.51 ± 3.36 | 160.33 ± 5.55 |
| COS-M | 64.07 ± 3.29 * | 20.45 ± 2.05 * | 60.08 ± 2.26 * | 53.14 ± 3.70 * | 139.13 ± 11.03 * |
| COS-H | 58.85 ± 4.83 * | 19.10 ± 1.52 * | 57.01 ± 3.67 * | 51.99 ± 1.09 * | 130.39 ± 8.65 * |
| Groups | d-Gal (250 mg/kg/day) | COS (mg/kg/day) | ||
|---|---|---|---|---|
| 300 | 600 | 1200 | ||
| Normal | − | − | − | − |
| Model | + | − | − | − |
| VE | + | − | − | − |
| COS-L | + | + | − | − |
| COS-M | + | − | + | − |
| COS-H | + | − | − | + |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, S.-Z.; Li, J.-C.; Li, S.-D.; Liao, M.-N.; Li, C.-P.; Zheng, P.-J.; Guo, M.-H.; Tan, W.-X.; Zheng, Z.-H.; Hu, Z. Anti-Aging Effect of Chitosan Oligosaccharide on d-Galactose-Induced Subacute Aging in Mice. Mar. Drugs 2018, 16, 181. https://doi.org/10.3390/md16060181
Kong S-Z, Li J-C, Li S-D, Liao M-N, Li C-P, Zheng P-J, Guo M-H, Tan W-X, Zheng Z-H, Hu Z. Anti-Aging Effect of Chitosan Oligosaccharide on d-Galactose-Induced Subacute Aging in Mice. Marine Drugs. 2018; 16(6):181. https://doi.org/10.3390/md16060181
Chicago/Turabian StyleKong, Song-Zhi, Ji-Cheng Li, Si-Dong Li, Ming-Neng Liao, Cheng-Peng Li, Pin-Jin Zheng, Min-Hui Guo, Wei-Xiang Tan, Zhao-Hui Zheng, and Zhang Hu. 2018. "Anti-Aging Effect of Chitosan Oligosaccharide on d-Galactose-Induced Subacute Aging in Mice" Marine Drugs 16, no. 6: 181. https://doi.org/10.3390/md16060181
APA StyleKong, S.-Z., Li, J.-C., Li, S.-D., Liao, M.-N., Li, C.-P., Zheng, P.-J., Guo, M.-H., Tan, W.-X., Zheng, Z.-H., & Hu, Z. (2018). Anti-Aging Effect of Chitosan Oligosaccharide on d-Galactose-Induced Subacute Aging in Mice. Marine Drugs, 16(6), 181. https://doi.org/10.3390/md16060181




