Effect of Fish Collagen Hydrolysates on Type I Collagen mRNA Levels of Human Dermal Fibroblast Culture
Abstract
1. Introduction
2. Results
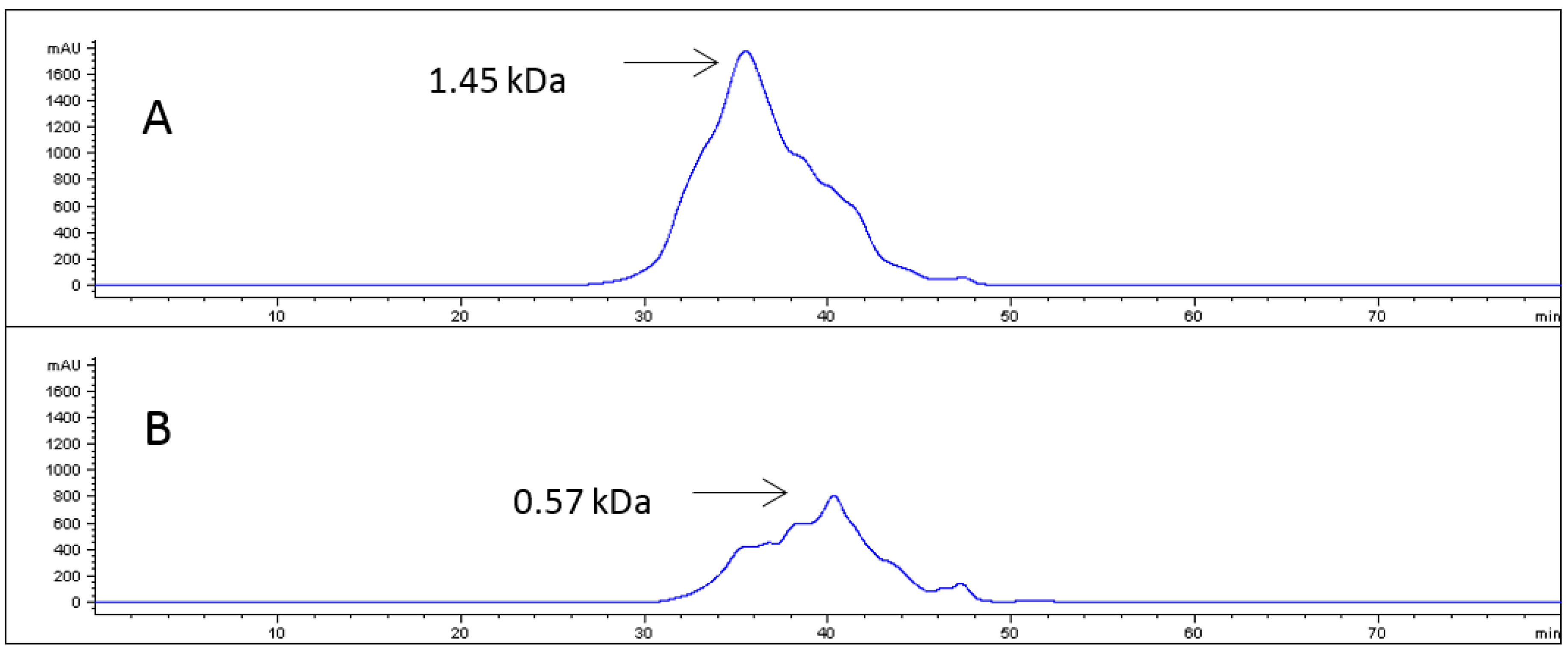
2.1. Collagen Hydrolysates
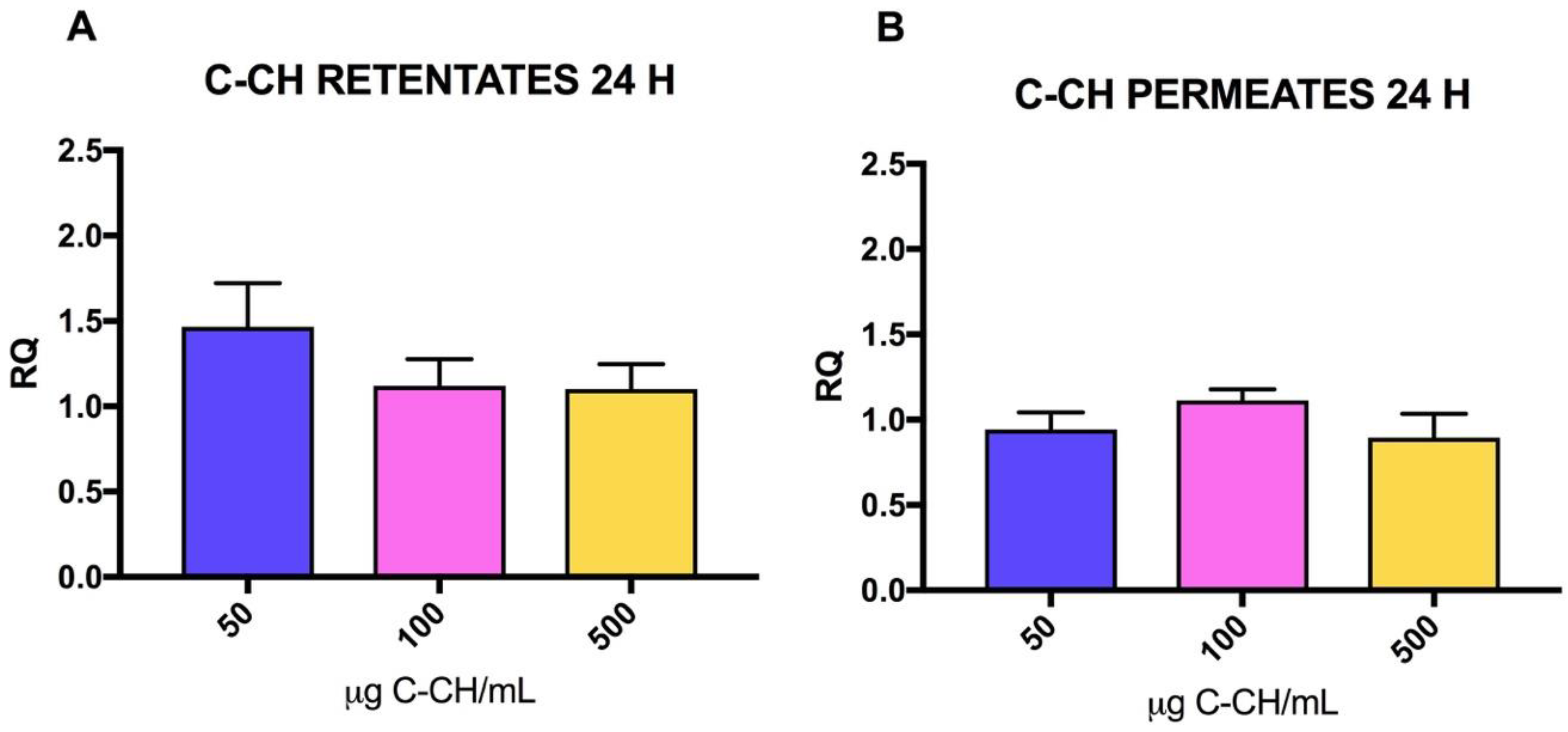
2.2. Effect of CH on mRNA Collagen Synthesis of Fibroblast Culture
3. Discussion
4. Materials and Methods
4.1. Raw Material
4.2. Extraction of Pepsin Soluble Collagen (PSC) from Skin
4.3. Enzymatic Hydrolysis of Collagen and Characterization of PSC Hydrolyzate
4.3.1. Fractionation of the Hydrolysate
4.3.2. Size Exclusion Chromatography
4.4. Cell Culture and Treatment with PSC Hydrolyzates
4.5. RT-PCR Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Nalinanon, S.; Shahidi, F. Fish Collagen. In Food Biochemistry and Food Processing; Simpson, B.K., Nollet, L.M.L., Toldra, F., Benjakul, S., Paliyath, G., Hui, Y.H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 365–387. [Google Scholar]
- Lindner, D.; Zietsch, C.; Becher, P.M.; Schulze, K.; Schultheiss, H.P.; Tschöpe, C.; Westermann, D. Differential expression of matrix metalloproteases in human fibroblasts with different origins. Biochem. Res. Int. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Koyama, Y.; Nomura, Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci. Bioechnol. Biochem. 2009, 73, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Marques, A.; Martins, E.; Silva, T.; Reis, R. Cosmetic Potential of Marine Fish Skin Collagen. Cosmetics 2017, 4, 3. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Nakyinsige, K.; Man, Y.B.C.; Sazili, A.Q. Halal authenticity issues in meat and meat products. Meat Sci. 2012, 91, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lupi, O. Doenças priônicas: Avaliaçao dos riscos envolvidos na utilizaçao de produtos de origem bovina. An. Bras. Dermatol. 2003, 78, 7–16. [Google Scholar] [CrossRef]
- Scott, M.R.; Will, R.; Ironside, J.; Nguyen, H.-O.B.; Tremblay, P.; DeArmond, S.J.; Prusiner, S.B. Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc. Natl. Acad. Sci. USA 1999, 96, 15137–15142. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Sotelo, C.G.; Chapela, M.J.; Pérez-Martín, R.I. Towards Sustainable and Efficient Use of Fishery Resources: Present and Future Trends. Trends Food Sci Tech. 2007, 18, 29–36. [Google Scholar] [CrossRef]
- Vigo, A.P. De Puerto de Vigo. Annual Report 2016; Puerto de Vigo: Galicia, Spain, 2016. [Google Scholar]
- Pratt, H.L.; Carrier, J.C. A review of elasmobranch reproductive behavior with a case study on the nurse shark, Ginglymostoma cirratum. Environ. Biol. Fishes 2001, 60, 157–188. [Google Scholar] [CrossRef]
- Limpisophon, K.; Tanaka, M.; Weng, W.Y.; Abe, S.; Osako, K. Characterization of gelatin films prepared from under-utilized blue shark (Prionace glauca) skin. Food Hydrocoll. 2009, 23, 1993–2000. [Google Scholar] [CrossRef]
- Weng, W.; Tang, L.; Wang, B.; Chen, J.; Su, W.; Osako, K.; Tanaka, M. Antioxidant properties of fractions isolated from blue shark (Prionace glauca) skin gelatin hydrolysates. J. Funct. Foods 2014, 11, 342–351. [Google Scholar] [CrossRef]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of fish skin collagen: An opportunity for valorizing fish industry byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine collagen peptides from the skin of Nile Tilapia (Oreochromis niloticus): Characterization and wound healing evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Oliveira de Sousa, R.A. Valorization of Cod (Gadus morhua) by-Products by Extraction of Collagen with Potential Application in Cosmetic and Biomedical Field. Master Thesis, Escola Superior de Turismo e Tecnologia do Mar, Intituto Politecnico de Leiria, Leiria, Portugal, 2017. [Google Scholar]
- Koizumi, S.; Inoue, N.; Shimizu, M.; Kwon, C.J.; Kim, H.Y.; Park, K.S. Effects of Dietary Supplementation with Fish Scales-Derived Collagen Peptides on Skin Parameters and Condition: A Randomized, Placebo-Controlled, Double-Blind Study. Int. J. Pept. Res. Ther. 2017, 1–6. [Google Scholar] [CrossRef]
- Schadow, S.; Siebert, H.C.; Lochnit, G.; Kordelle, J.; Rickert, M.; Steinmeyer, J. Collagen Metabolism of Human Osteoarthritic Articular Cartilage as Modulated by Bovine Collagen Hydrolysates. PLoS ONE 2013, 8, e53955. [Google Scholar] [CrossRef] [PubMed]
- Noma, T.; Takasugi, S.; Shioyama, M.; Yamaji, T.; Itou, H.; Suzuki, Y.; Sakuraba, K.; Sawaki, K. Effects of dietary gelatin hydrolysates on bone mineral density in magnesium-deficient rats. BMC Musculoskelet. Disord. 2017, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Postlethwaite, A.E.; Seyer, J.M.; Kang, A.H. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc. Natl. Acad. Sci. USA 1978, 75, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Pérez-Martín, R.I.; Sotelo, C.G. Identification of shark species in seafood products by forensically informative nucleotide sequencing (FINS). J. Agric. Food Chem. 2008, 56, 9868–9874. [Google Scholar] [CrossRef] [PubMed]
- Medhurst, A.D.; Harrison, D.C.; Read, S.J.; Campbell, C.A.; Robbins, M.J.; Pangalos, M.N. The use of TaqMan RT-PCR assays for semiquantitative analysis of gene expression in CNS tissues and disease models. J. Neurosci. Methods 2000, 98, 9–20. [Google Scholar] [CrossRef]
- Marlovits, S.; Hombauer, M.; Truppe, M.; Vècsei, V.; Schlegel, W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J. Bone Jt. Surg. 2004, 86, 286–295. [Google Scholar] [CrossRef]


| Amino Acid | PGLA-CH RF | PGLA-CH PF | C-CH RF | C-CH PF |
|---|---|---|---|---|
| Aspartic acid | 4.39 ± 0.02 | 3.43 ± 0.03 | 4.98 ± 0.01 | 3.37 ± 0.05 |
| Threonine | 2.19 ± 0.02 | 2.31 ± 0.02 | 2.18 ± 0.01 | 2.44 ± 0.03 |
| Serine | 4.09 ± 0.15 | 4.35 ± 0.13 | 3.57 ± 0.10 | 3.71 ± 0.01 |
| Glutamic acid | 7.18 ± 0.02 | 6.92 ± 0.04 | 7.61 ± 0.05 | 5.99 ± 0.19 |
| Glycine | 33.97 ± 0.06 | 34.20 ± 0.16 | 32.56 ± 0.02 | 38.90 ± 0.21 |
| Alanine | 11.40 ± 0.10 | 13.55 ± 0.10 | 11.57 ± 0.01 | 11.46 ± 0.01 |
| Cysteine | 0.20 ± 0.03 | 0.29 ± 0.04 | 0.23 ± 0.07 | 0.28 ± 0.01 |
| Valine | 1.81 ± 0.05 | 2.48 ± 0.05 | 2.53 ± 0.03 | 3.15 ± 0.02 |
| Methionine | 1.38 ± 0.01 | 1.87 ± 0.11 | 0.86 ± 0.00 | 1.13 ± 0.03 |
| Isoleucine | 1.92 ± 0.02 | 1.49 ± 0.03 | 1.11 ± 0.01 | 1.58 ± 0.02 |
| Leucine | 2.07 ± 0.04 | 2.63 ± 0.01 | 3.21 ± 0.01 | 4.44 ± 0.01 |
| Tyrosine | 0.00 ± 0.00 | 0.09 ± 0.12 | 0.65 ± 0.01 | 1.22 ± 0.13 |
| Phenylalanine | 1.50 ± 0.01 | 1.49 ± 0.03 | 1.73 ± 0.02 | 2.79 ± 0.07 |
| Histidine | 0.49 ± 0.00 | 0.57 ± 0.01 | 0.42 ± 0.01 | 0.53 ± 0.02 |
| Lysine | 2.61 ± 0.02 | 2.50 ± 0.06 | 3.05 ± 0.01 | 2.76 ± 0.04 |
| Arginine | 4.95± 0.01 | 5.31± 0.01 | 4.65 ± 0.04 | 4.73 ± 0.22 |
| Hydroxyproline | 8.29 ± 0.04 | 6.07 ± 0.11 | 8.62 ± 0.07 | 5.07 ± 0.40 |
| Proline | 11.56 ± 0.02 | 10.46 ± 0.02 | 10.49 ± 0.06 | 6.45 ± 0.09 |
| Concentration | ||||
|---|---|---|---|---|
| Treatment Components | 500 µg/mL | 100 µg/mL | 50 µg/mL | Control |
| CH PF and RF | 250 µL | 50 µL | 25 µL | - |
| FM | 250 µL | 450 µL | 475 µL | 500 µL |
| Primer/Probe | Sequence 5′→3′ |
|---|---|
| GAPDH-Forward | GGAAGCTCACTGGCATGGC |
| GAPDH-Reverse | TAGACGGCAGGTCAGGTCCA |
| GAPDH-Probe | VIC-CCCCACTGCCAACGTGTCAGTG-MGB |
| COL_I-Forward | ATGCCTGGTGAACGTGGT |
| COL_I-Reverse | AGGAGAGCCATCAGCACCT |
| COL_I-Probe | 6-FAM-ACCAGCATCACCTCTGTC-MGB |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, A.; Blanco, M.; Correa, B.; Perez-Martin, R.I.; Sotelo, C.G. Effect of Fish Collagen Hydrolysates on Type I Collagen mRNA Levels of Human Dermal Fibroblast Culture. Mar. Drugs 2018, 16, 144. https://doi.org/10.3390/md16050144
Sanchez A, Blanco M, Correa B, Perez-Martin RI, Sotelo CG. Effect of Fish Collagen Hydrolysates on Type I Collagen mRNA Levels of Human Dermal Fibroblast Culture. Marine Drugs. 2018; 16(5):144. https://doi.org/10.3390/md16050144
Chicago/Turabian StyleSanchez, Ana, Maria Blanco, Begoña Correa, Ricardo I. Perez-Martin, and Carmen G. Sotelo. 2018. "Effect of Fish Collagen Hydrolysates on Type I Collagen mRNA Levels of Human Dermal Fibroblast Culture" Marine Drugs 16, no. 5: 144. https://doi.org/10.3390/md16050144
APA StyleSanchez, A., Blanco, M., Correa, B., Perez-Martin, R. I., & Sotelo, C. G. (2018). Effect of Fish Collagen Hydrolysates on Type I Collagen mRNA Levels of Human Dermal Fibroblast Culture. Marine Drugs, 16(5), 144. https://doi.org/10.3390/md16050144