Structural Characterization and Interaction with RCA120 of a Highly Sulfated Keratan Sulfate from Blue Shark (Prionace glauca) Cartilage
Abstract
1. Introduction
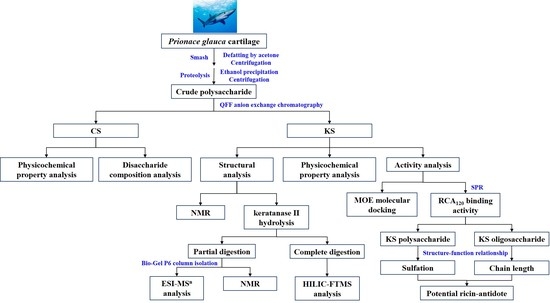
2. Results and Discussion
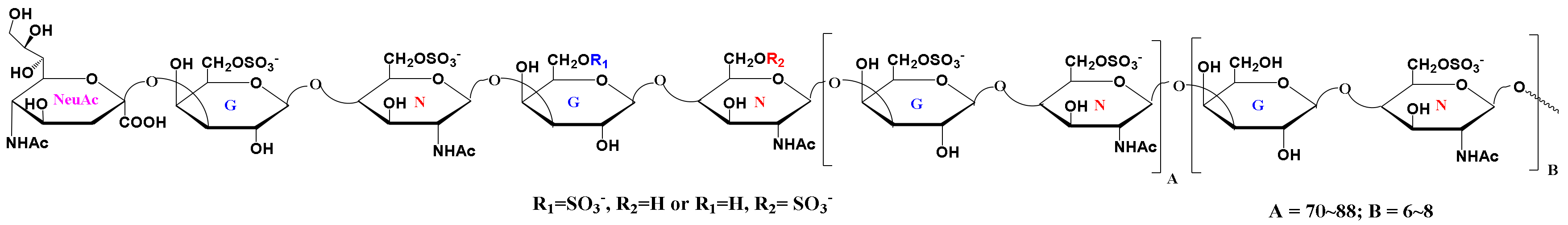
2.1. Isolation and Chemical Composition Analysis
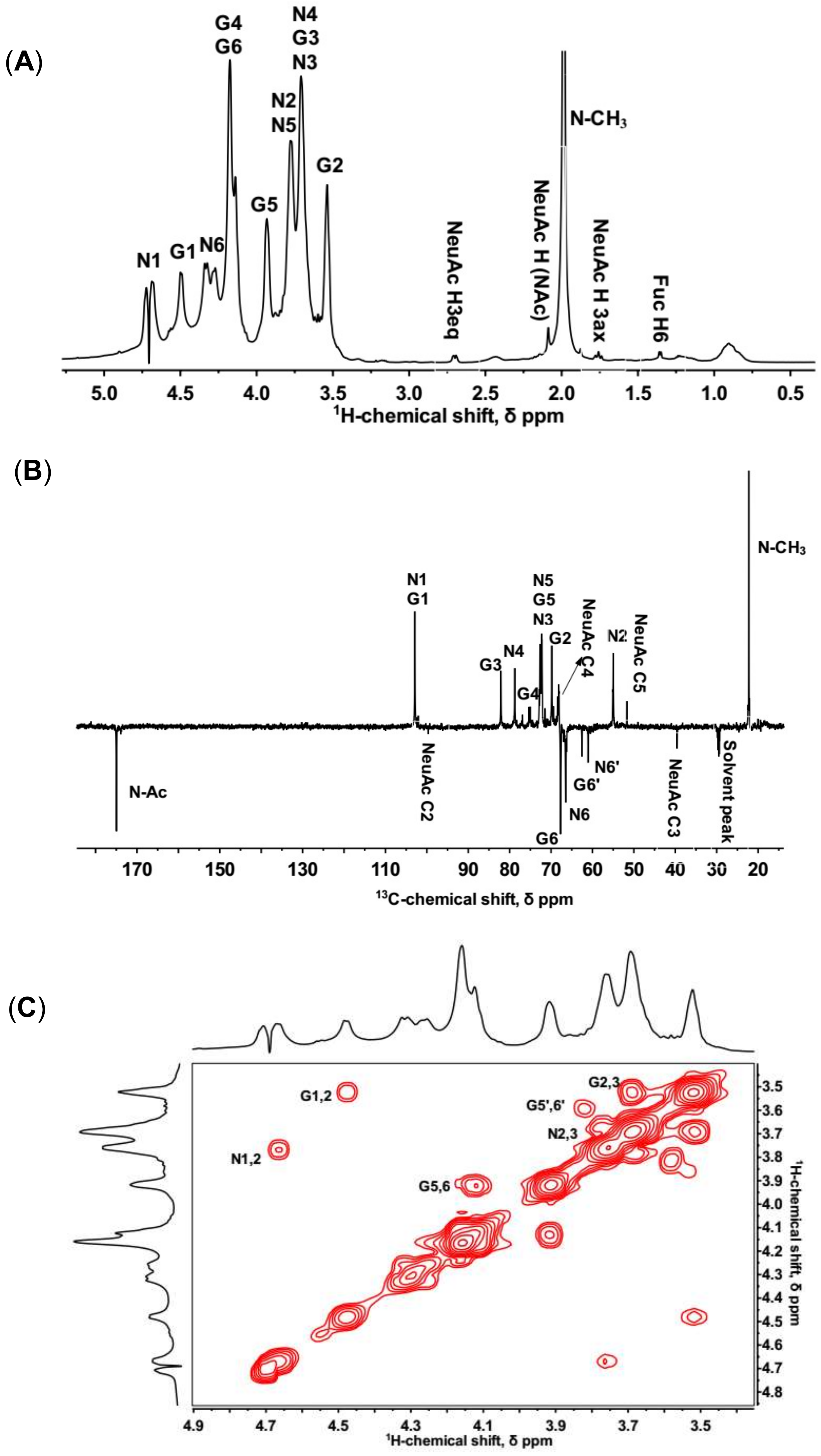
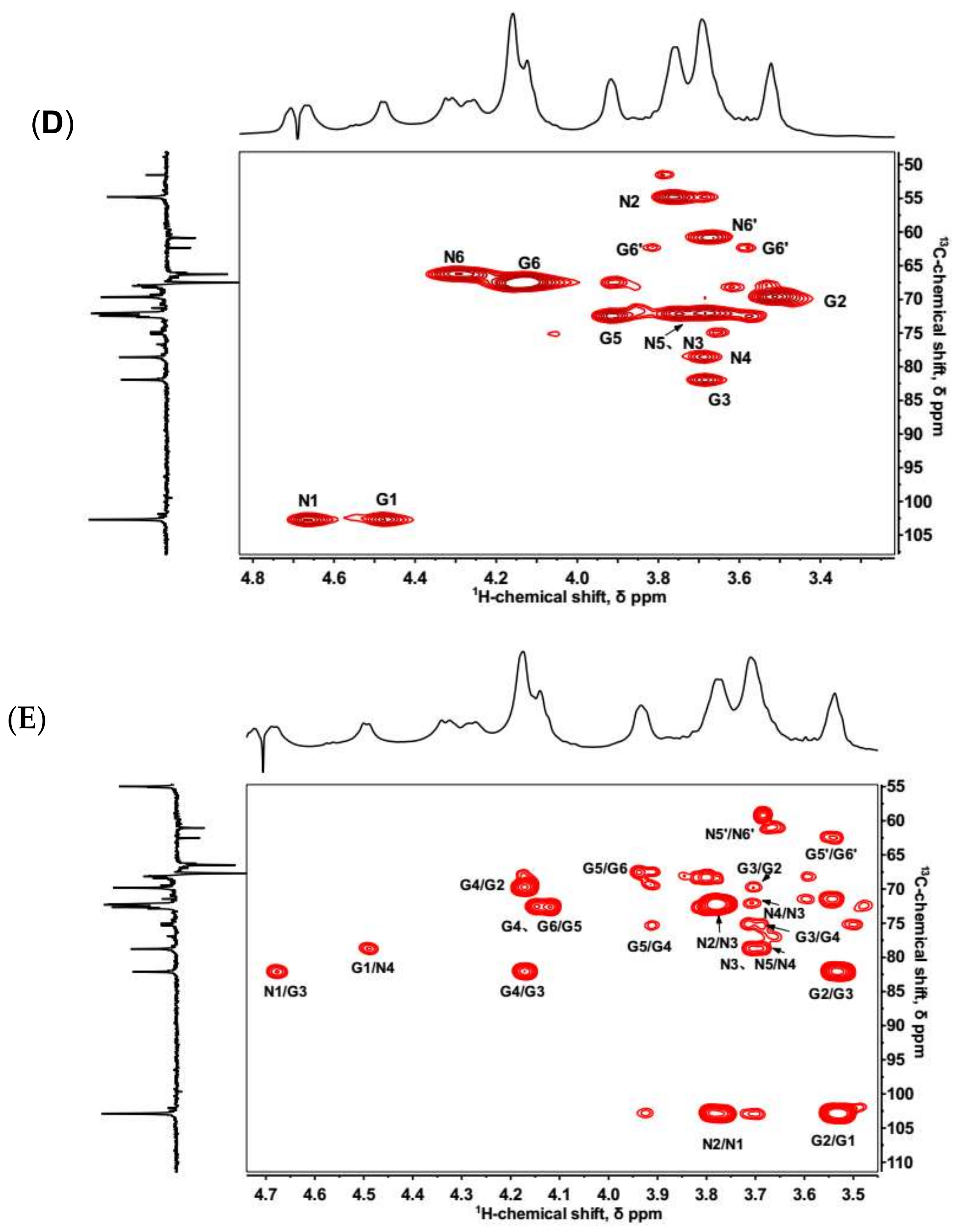
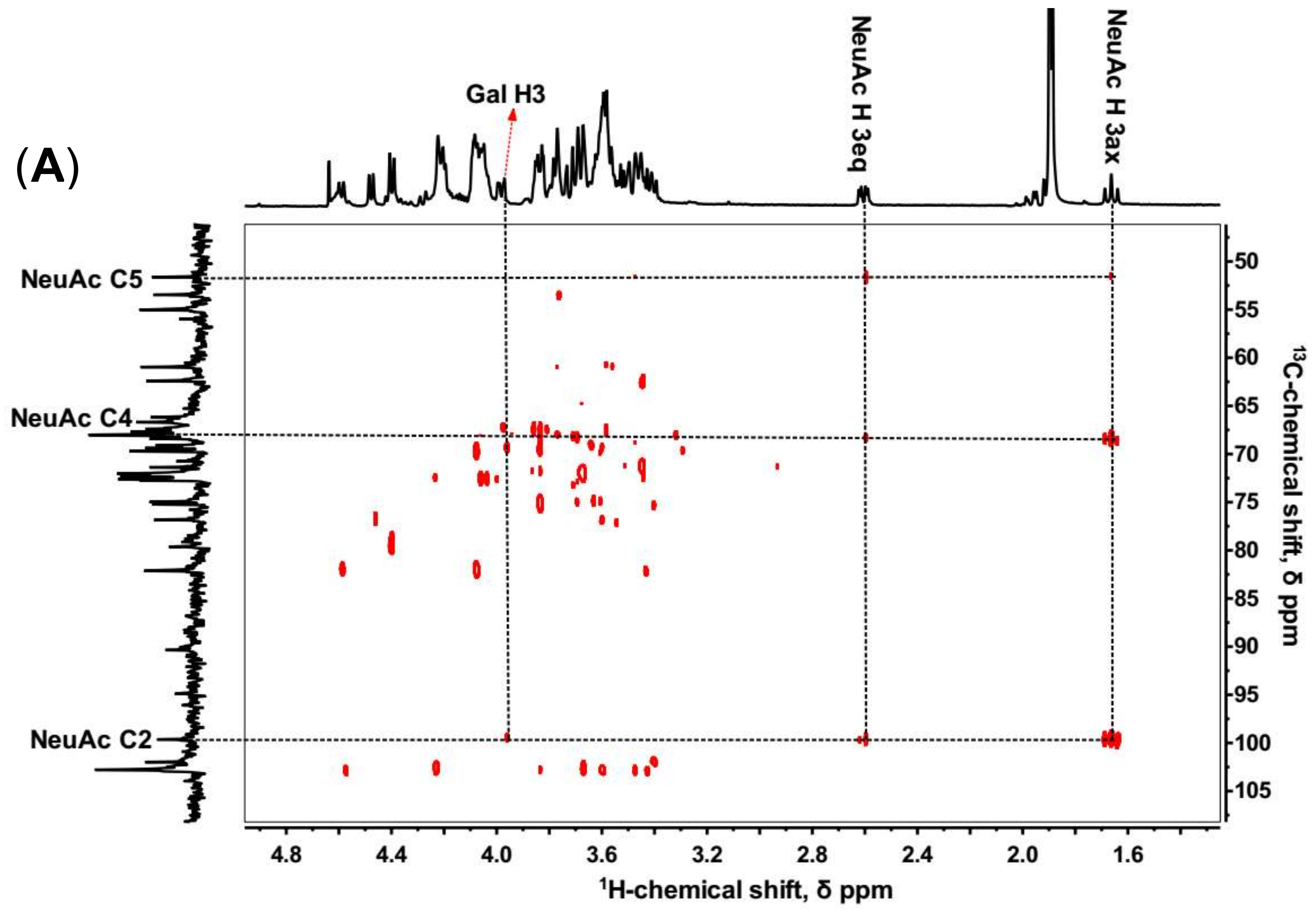
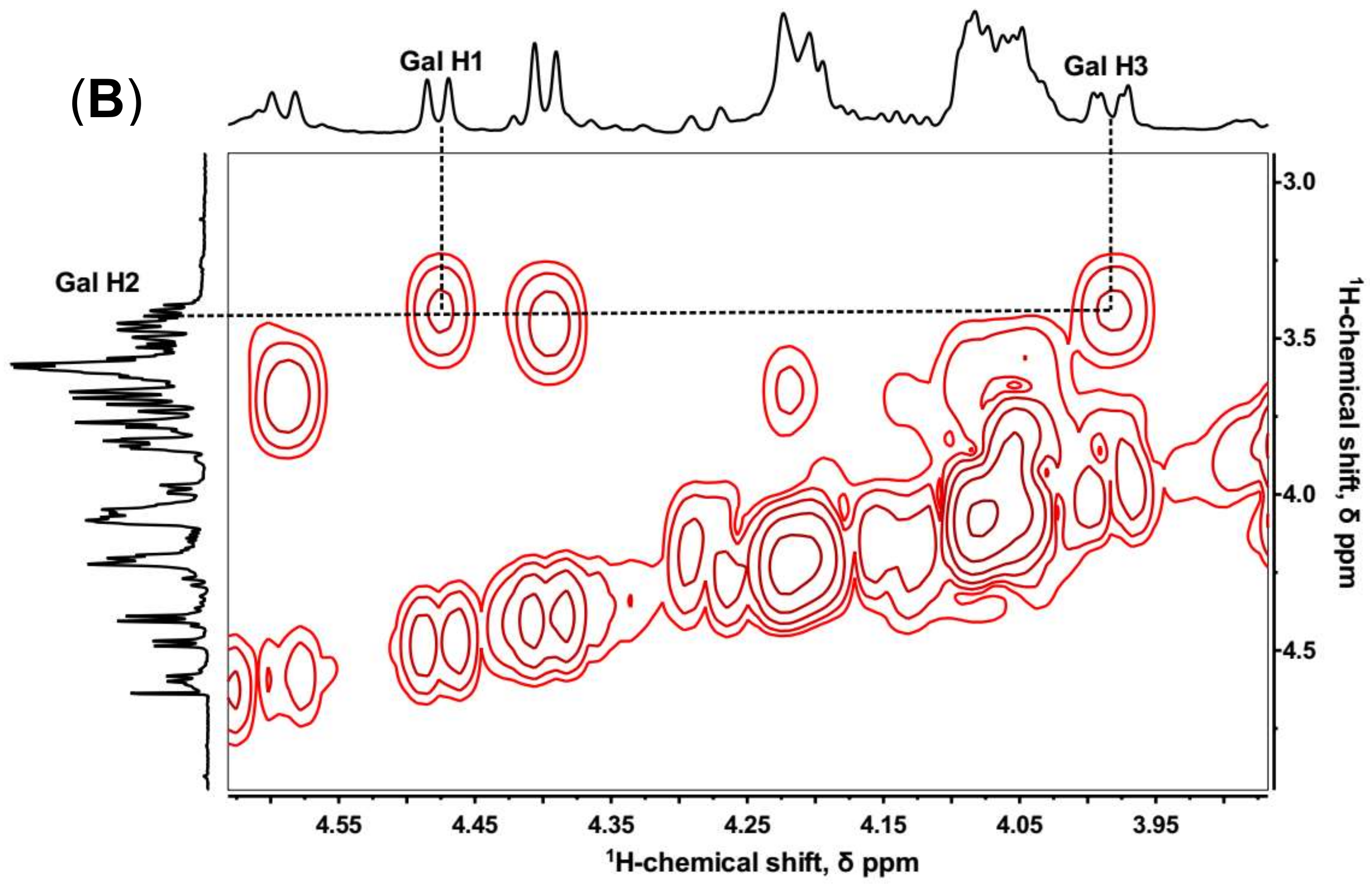
2.2. NMR Spectroscopy
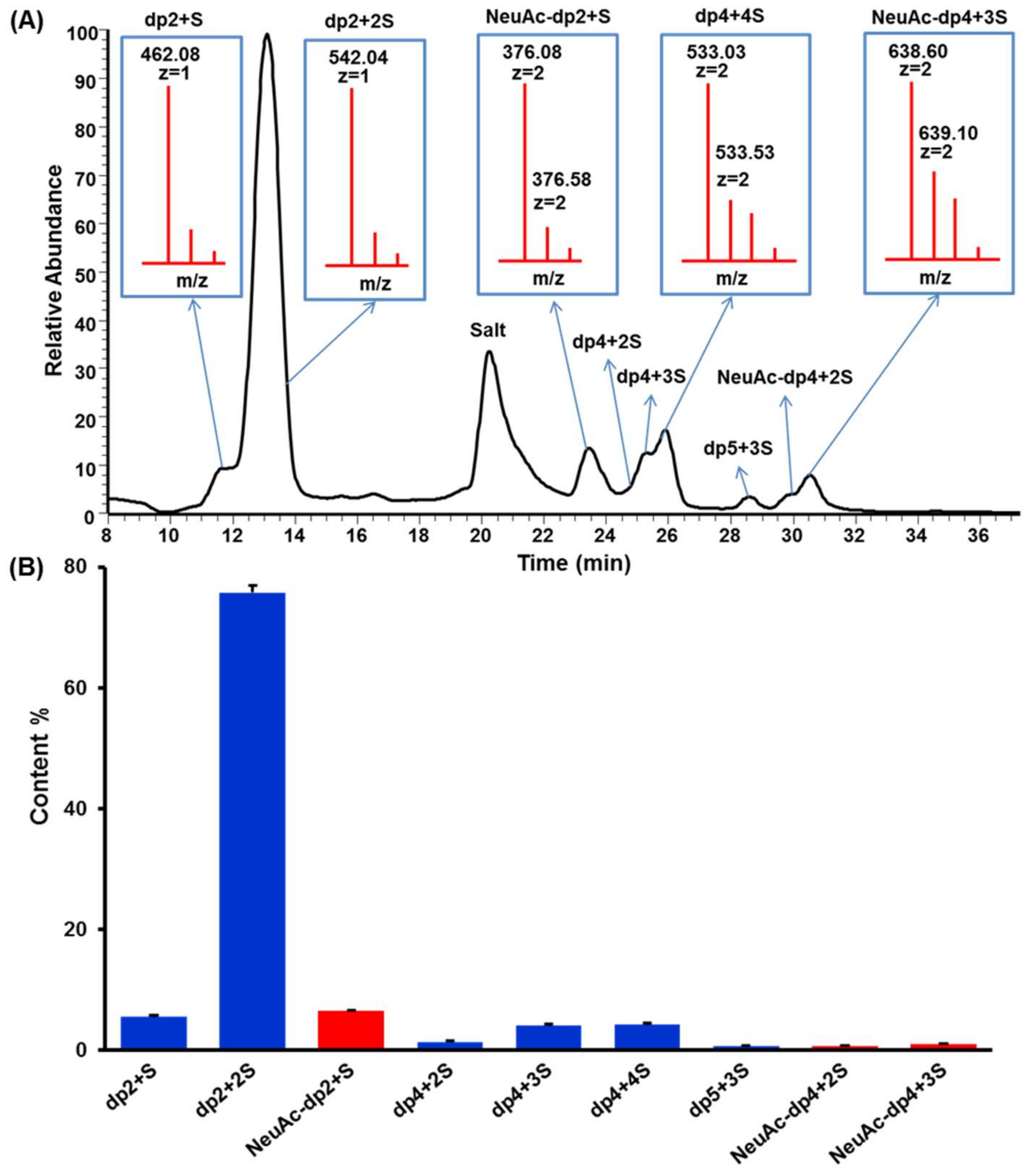
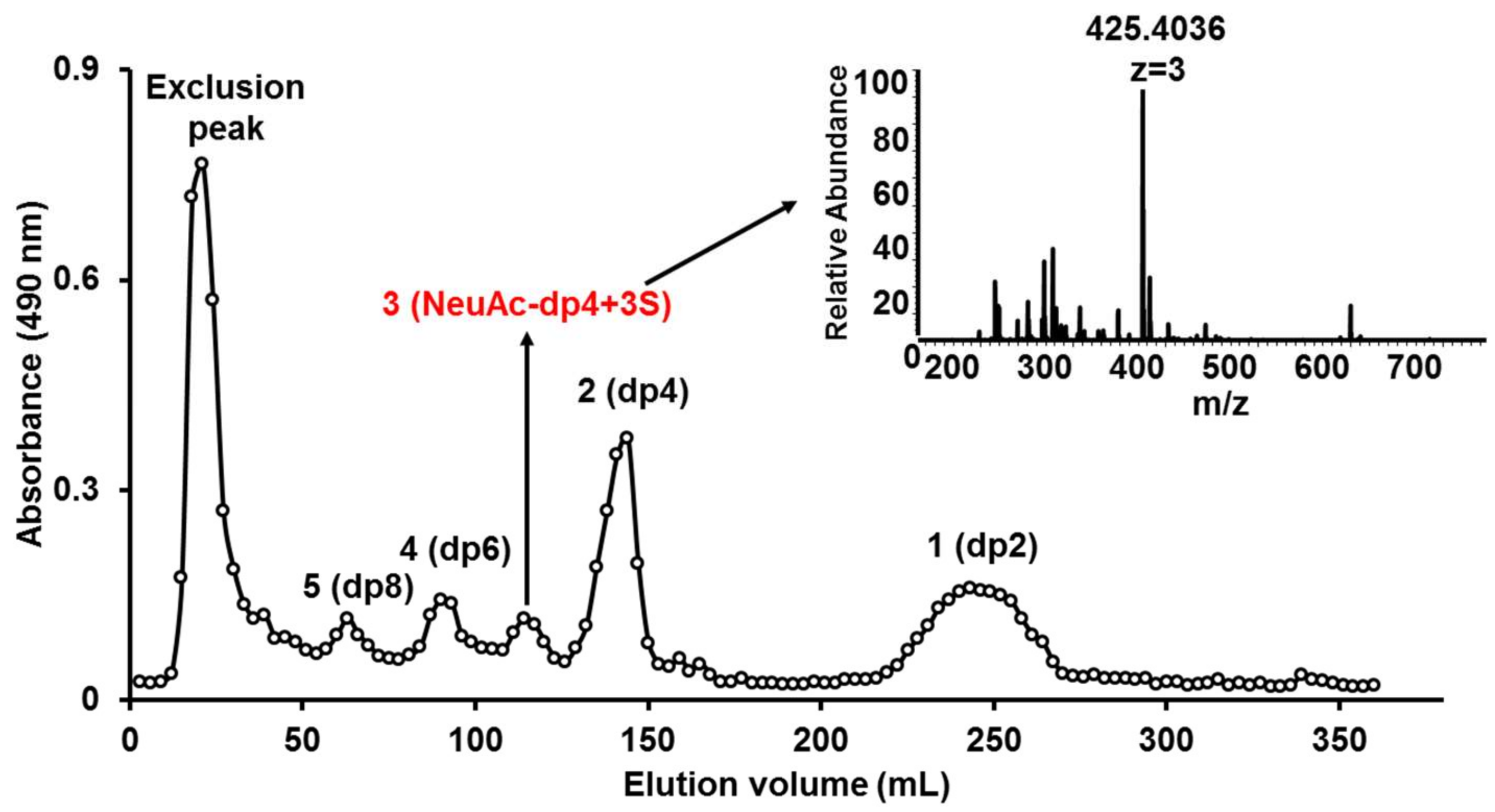
2.3. HILIC-FTMS Analysis of KS
2.4. Preparation and Mapping of KSO
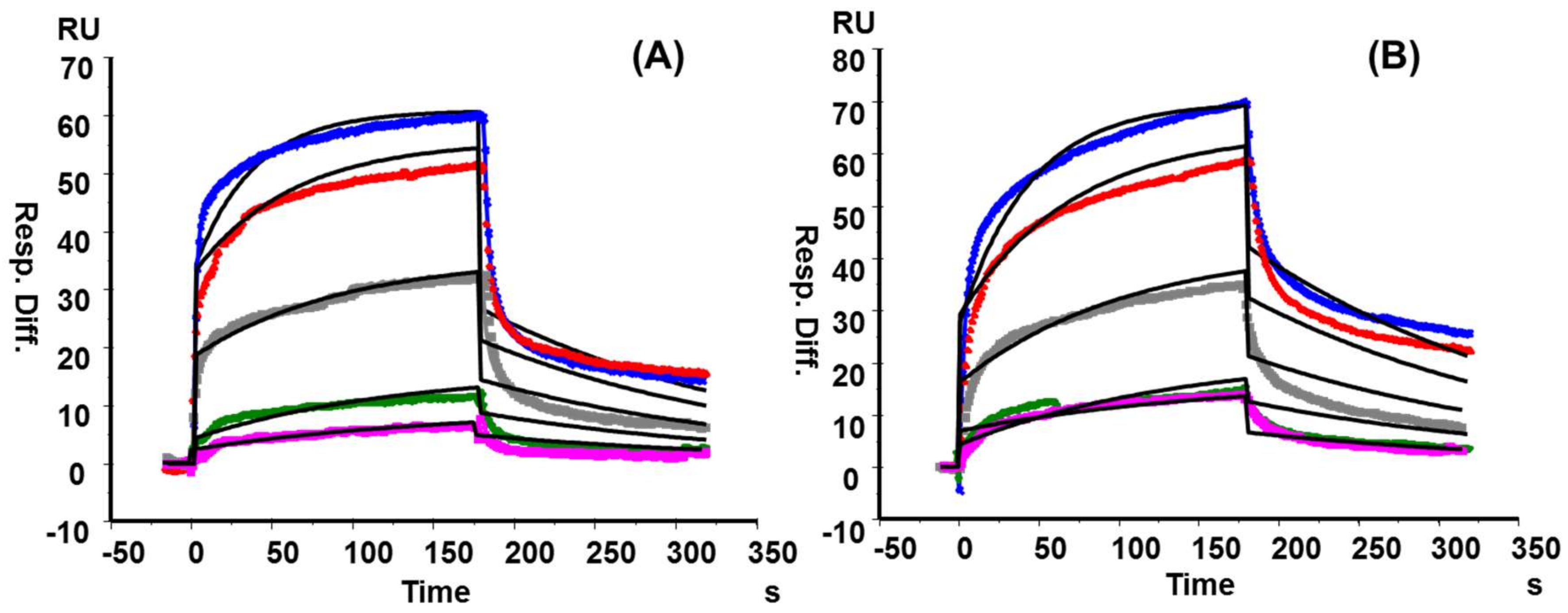
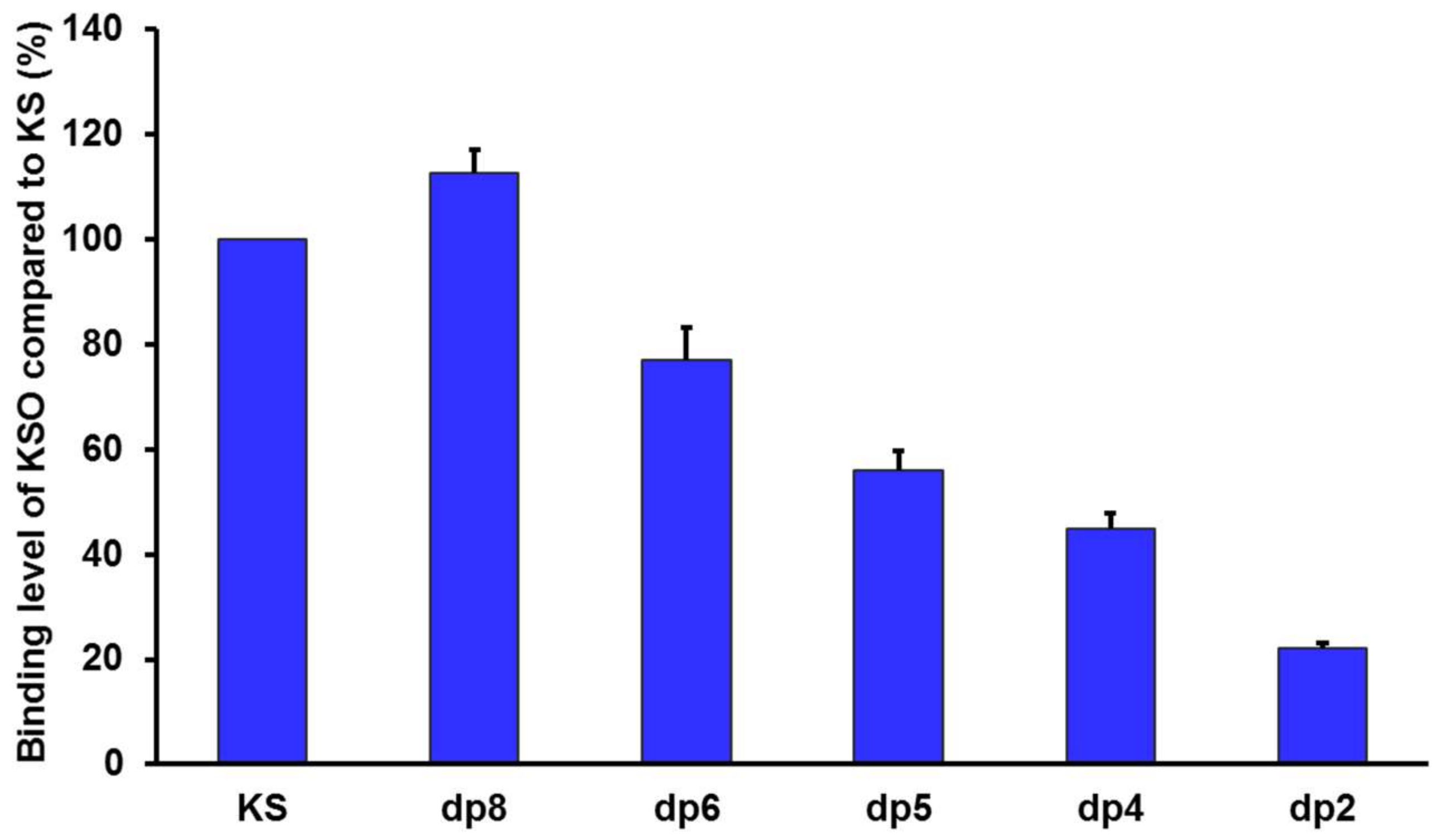
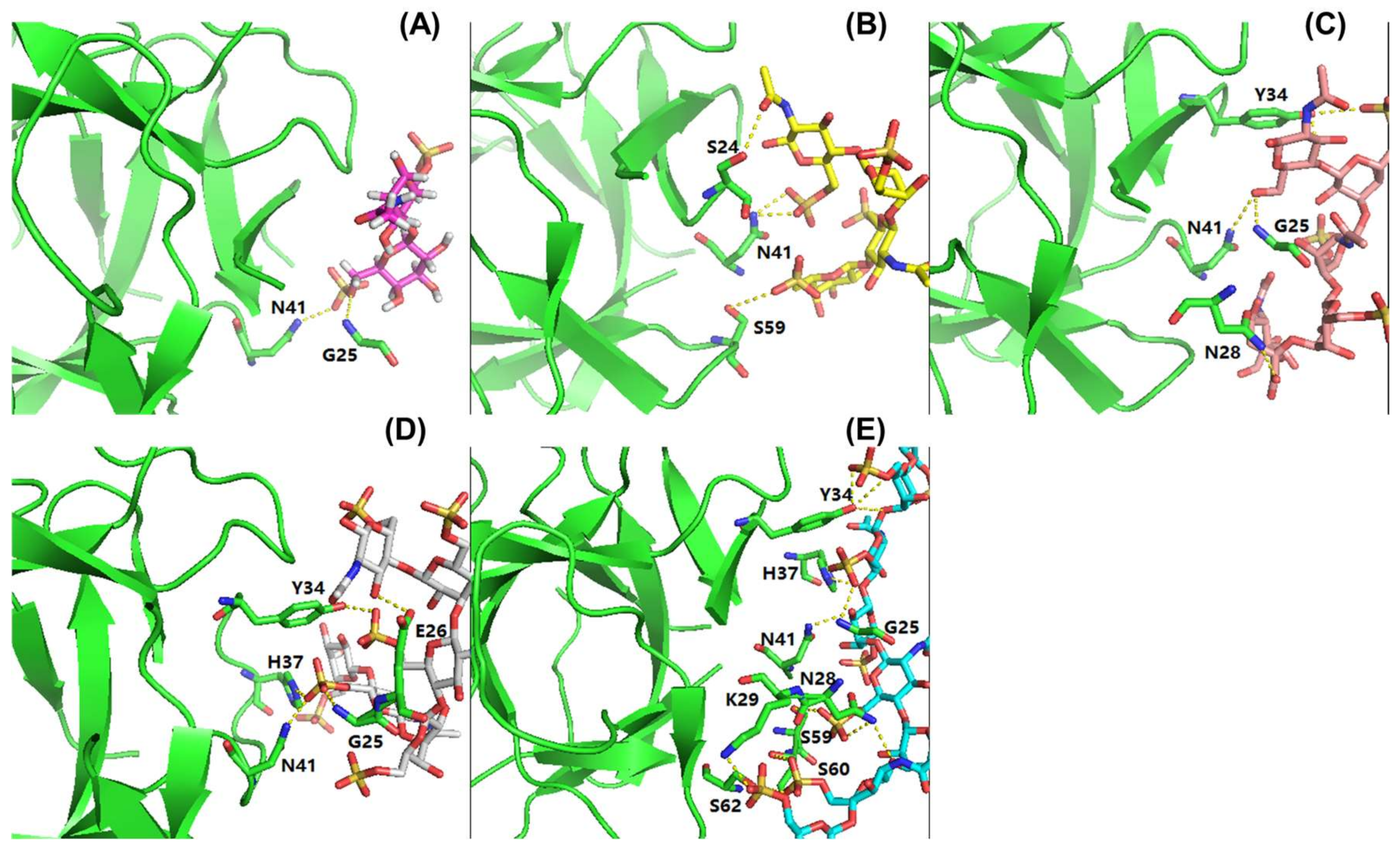
2.5. RCA120 Binding Activities of KS and KSO
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Isolation and Purification of KS
3.3. Molecular Weight and Chemical Composition Analysis
3.4. Profiling of KSO Generated by Keratanase II Digestion Through HILIC-FTMS
3.5. Preparation and Sequence Analysis of KSO
3.6. NMR Spectroscopy Analysis
3.7. Surface Plasmon Resonance (SPR) Binding Kinetics of RCA120-KS/KSO Interactions
3.8. MOE Binding Affinity Calculation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, L.; Ly, M.; Linhardt, R.J. Proteoglycan sequence. Mol. Biosyst. 2012, 8, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, M.F.; Lacerda, L.; Alves, S. Glycosaminoglycan storage disorders: A review. Biochem. Res. Int. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, J.L. MINI REVIEW Keratan sulfate: Structure, biosynthesis, and function. Glycobiology 2000, 10, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, J.L. Keratan Sulfate Biosynthesis. IUBMB. Life 2002, 54, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Linker, A.; Davidson, E.A.; Weissmann, B. The mucopolysaccharides of bovine cornea. J. Biol. Chem. 1953, 205, 611–616. [Google Scholar] [PubMed]
- Funderburgh, J.L.; Caterson, B.; Conrad, G.W. Distribution of proteoglycans antigenically related to corneal keratan sulfate proteoglycan. J. Biol. Chem. 1987, 262, 11634–11640. [Google Scholar] [PubMed]
- Lauder, R.M.; Huckerby, T.N.; Nieduszynski, I.A.; Plaas, A.H. Age-related changes in the structure of the keratan sulphate chains attached to fibromodulin isolated from articular cartilage. Biochem. J. 1998, 330, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Krusius, T.; Finne, J.; Margolis, R.K.; Margolis, R.U. Identification of an O-glycosidic mannose-linked sialylated tetrasaccharide and keratan sulfate oligosaccharides in the chondroitin sulfate proteoglycan of brain. J. Biol. Chem. 1986, 261, 8237–8242. [Google Scholar] [PubMed]
- Gardell, S. Separation of mucopolysaccharides on a cellulose column. Acta Chem. Scand. 1957, 11, 668. [Google Scholar] [CrossRef]
- Seno, N.; Meyer, K.; Anderson, B.; Hoffman, P. Variations in keratosulfates. J. Biol. Chem. 1965, 240, 1005–1010. [Google Scholar] [PubMed]
- Tai, G.H.; Huckerby, T.N.; Nieduszynski, I.A. 600 MHz 1H NMR study of a fucose-containing heptasaccharide derived from a keratanase digestion of bovine articular cartilage keratan sulphate. Carbohydr. Res. 1994, 255, 303–309. [Google Scholar] [CrossRef]
- Ashwell, G.; Morell, A.G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv. Enzymol. Relat. Areas Mol. Biol. 1974, 41, 99–128. [Google Scholar] [PubMed]
- Brown, G.M.; Huckerby, T.N.; Morris, H.G.; Nieduszynski, I.A. Degradation of articular cartilage keratan sulphates using hydrazinolysis and nitrous acid. Environment of fucose residues. Biochem. J. 1992, 286, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Quantock, A.J.; Young, R.D.; Akama, T.O. Structural and biochemical aspects of keratan sulphate in the cornea. Cell. Mol. Life Sci. 2010, 67, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Sommarin, Y.; Wendel, M.; Shen, Z.; Hellman, U.; Heinegard, D. Osteoadherin, a cell-binding keratan sulfate proteoglycan in bone, belongs to the family of leucine-rich repeat proteins of the extracellular matrix. J. Biol. Chem. 1998, 273, 16723–16729. [Google Scholar] [CrossRef] [PubMed]
- Takeda-Uchimura, Y.; Uchimura, K.; Sugimura, T.; Yanagawa, Y.; Kawasaki, T.; Komatsu, Y.; Kadomatsu, K. Requirement of keratan sulfate proteoglycan phosphacan with a specific sulfation pattern for critical period plasticity in the visual cortex. Exp. Neurol. 2015, 274, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Ohgomori, T.; Natori, T.; Miyamoto, K.; Kusunoki, S.; Sakamoto, K.; Ishiguro, N.; Imagama, S.; Kadomatsu, K. Keratan sulfate expression in microglia is diminished in the spinal cord in experimental autoimmune neuritis. Cell Death Dis. 2013, 4, e946. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Ishino, Y.; Jiang, W.; Yoshimura, T.; Takeda-Uchimura, Y.; Uchimura, K.; Kadomatsu, K.; Ikenaka, K. Keratan sulfate regulates the switch from motor neuron to oligodendrocyte generation during Development of the mouse spinal cord. Neurochem. Res. 2016, 41, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Ishii, K.; Asaka, R.; Suzuki, A.; Takatsu, A.; Kashima, H.; Shiozawa, T. Immunohistochemical expression of keratan sulfate: A possible diagnostic marker for carcinomas of the female genital tract. J. Clin. Pathol. 2011, 64, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Kurihara, H.; Ito, T.; Kikuchi, H.; Yoshida, K.; Yamanokuchi, H.; Asari, A. The keratan sulfate disaccharide Gal(6S03) 1,4-GlcNAc(6S03) modulates interleukin 12 production by macrophages in murine Thy-1 type autoimmune disease. J. Biol. Chem. 2005, 280, 20879–20886. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Gao, C.; Ota, F.; Angata, T.; Shogomori, H.; Ohtsubo, K.; Yoshida, K.; Lepenies, B.; Taniguchi, N. Flagellin/Toll-like receptor 5 response was specifically attenuated by keratan sulfate disaccharide via decreased EGFR phosphorylation in normal human bronchial epithelial cells. Biochem. Biophys. Res. Commun. 2013, 435, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Fujinawa, R.; Yoshida, T.; Ueno, M.; Ota, F.; Kizuka, Y.; Hirayama, T.; Korekane, H.; Kitazume, S.; Maeno, T.; et al. A keratan sulfate disaccharide prevents inflammation and the progression of emphysema in murine models. Am. J. Physiol.-Lung. Cell. Mol. Physiol. 2016, 312, L268–L276. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.M.; Roberts, L.M.; Robertus, J.D. Ricin: Structure, mode of action, and some current applications. FASEB J. 1994, 8, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Itakura, Y.; Nakamuratsuruta, S.; Kominami, J.; Sharon, N.; Kasai, K.; Hirabayashi, J. Systematic comparison of oligosaccharide specificity of Ricinus communis agglutinin I and Erythrina lectins: A search by frontal affinity chromatography. J. Biochem. 2007, 142, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Huckerby, T.N.; Lauder, R.M. Keratan sulfates from bovine tracheal cartilage. Eur. J. Biochem. 2000, 267, 3360–3369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kariya, Y.; Conrad, A.H.; Tasheva, E.S.; Conrad, G.W. Analysis of keratan sulfate oligosaccharides by electrospray ionization tandem mass spectrometry. Anal. Chem. 2005, 77, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Minamisawa, T.; Suzuki, K.; Hirabayashi, J. Multistage mass spectrometric sequencing of keratan sulfate-related oligosaccharides. Anal. Chem. 2006, 78, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Dickenson, J.M.; Huckerby, T.N.; Nieduszynski, I.A. Two linkage-region fragments isolated from skeletal keratan sulphate contain a sulphated N-acetylglucosamine residue. Biochem. J. 1990, 269, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Weyers, A.; Yang, B.; Solakyildirim, K.; Yee, V.; Li, L.; Zhang, F.; Linhardt, R.J. Isolation of bovine corneal keratan sulfate and its growth factor and morphogen binding. FEBS J. 2013, 280, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Sun, X.; He, W.; Cai, C.; Onishi, A.; Zhang, F.; Linhardt, R.J.; Liu, Z. Keratan sulfate glycosaminoglycan from chicken egg white. Glycobiology 2016, 26, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Piquet, A.A.; Pereira, M.S.; Mourão, P.A.S. Residual keratan sulfate in chondroitin sulfate formulations for oral administration. Carbohydr. Polym. 2012, 90, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Huckerby, T.N.; Nieduszynski, I.A.; Brown, G.M.; Cockin, G.H. A full assignment of proton resonances for an α (1-3)-linked fucose residue in keratan sulphate from bovine articular cartilage. Glycoconj. J. 1991, 8, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, W.; Jiang, P.; Yu, Y.; Lin, L.; Sun, X.; Koffas, M.; Zhang, F.; Linhardt, R.J. Construction and functional characterization of truncated versions of recombinant keratinase II from Bacillus circulans. Glycoconj. J. 2017, 34, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.M.; Huckerby, T.N.; Morris, H.G.; Abram, B.L.; Nieduszynski, I.A. Oligosaccharides derived from bovine articular cartilage keratan sulfates after keratanase II digestion: Implications for keratan sulfate structural fingerprinting. Biochemistry 1994, 33, 4836–4846. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.; Tan, Y.; Tan, Y.; Hu, H.; Benson, G.; Aizikov, K.; Conley, S.; Staples, G.O.; Slysz, G.W.; Smith, R.D.; et al. GlycReSoft: A software package for automated recognition of glycans from LC/MS data. PLoS ONE 2012, 7, e45474. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Singh, T.; Herp, A.; Wu, A.M. Carbohydrate recognition factors of the lectin domains present in the Ricinus communis toxic protein (ricin). Biochimie 2006, 88, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Fais, M.; Karamanska, R.; Allman, S.; Fairhurst, S.A.; Innocenti, P.; Fairbanks, A.J.; Donohoe, T.J.; Davis, B.G.; Russell, D.A.; Field, R.A. Surface plasmon resonance imaging of glycoarrays identifies novel and unnatural carbohydrate-based ligands for potential ricin sensor development. Chem. Sci. 2011, 2, 1952–1959. [Google Scholar] [CrossRef][Green Version]
- Baenziger, J.U.; Fiete, D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J. Biol. Chem. 1979, 254, 9795–9799. [Google Scholar] [PubMed]
- Wang, Y.; Yu, G.; Han, Z.; Yang, B.; Hu, Y.; Zhao, X.; Wu, J.; Lv, Y.; Chai, W. Specificities of Ricinus communis agglutinin 120 interaction with sulfated galactose. FEBS Lett. 2011, 585, 3927–3934. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Blain, F.; Musil, R.A.; Zimmermann, J.J.; Gu, K.; Bennett, D.C. Isolation and expression in Escherichia coli of hepB and hepC, genes coding for the glycosaminoglycan-degrading enzymes heparinase II and heparinase III, respectively, from Flavobacterium heparinum. Appl. Environ. Microb. 1996, 62, 2723–2734. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Selvendran, R.R.; March, J.F.; Ring, S.G. Determination of aldoses and uronic acid content of vegetable fiber. Anal. Biochem. 1979, 96, 282–292. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Viebke, C.; Borgström, J.; Piculell, L. Characterisation of kappa-and iota-carrageenan coils and helices by MALLS/GPC. Carbohydr. Polym. 1995, 27, 145–154. [Google Scholar] [CrossRef]
- Chen, S.; Xu, J.; Xue, C.; Dong, P.; Sheng, W.; Yu, G.; Chai, W. Sequence determination of a non-sulfated glycosaminoglycan-like polysaccharide from melanin-free ink of the squid Ommastrephes bartrami by negative-ion electrospray tandem mass spectrometry and NMR spectroscopy. Glycoconj. J. 2008, 25, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhao, X.; Yang, B.; Ren, S.; Guan, H.; Zhang, Y.; Lawson, A.M.; Chai, W. Sequence determination of sulfated carrageenan-derived oligosaccharides by high-sensitivity negative-ion electrospray tandem mass spectrometry. Anal. Chem. 2006, 78, 8499–8505. [Google Scholar] [CrossRef] [PubMed]
| Signal/ppm | Nucleus | 1 | 2 | 3 | 4 | 5 | Sulfated C6 | Unsulfated C6 | CH3 | C=O |
|---|---|---|---|---|---|---|---|---|---|---|
| 4-β-GlcNAc-1 (N) | 1H | 4.66 | 3.77 | 3.67 | 3.69 | 3.75 | 4.29 | 3.67 | 1.97 | - |
| 13C | 102.73 | 54.82 | 72.04 | 78.54 | 72.13 | 66.21 | 61.06 | 22.24 | 174.97 | |
| 3-β-Gal-1 (G) | 1H | 4.48 | 3.52 | 3.69 | 4.16 | 3.92 | 4.13 | 3.58 | - | - |
| 13C | 102.68 | 69.61 | 81.95 | 75.25 | 72.47 | 67.49 | 62.54 | - | - |
| Ligands | Dock Score * |
|---|---|
| dp2 | −8.70 |
| dp4 | −10.03 |
| dp5 | −10.58 |
| dp6 | −11.95 |
| dp8 | −14.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Li, G.; Zhao, X.; Shan, X.; Cai, C.; Zhao, J.; Zhang, F.; Linhardt, R.J.; Yu, G. Structural Characterization and Interaction with RCA120 of a Highly Sulfated Keratan Sulfate from Blue Shark (Prionace glauca) Cartilage. Mar. Drugs 2018, 16, 128. https://doi.org/10.3390/md16040128
Li Q, Li G, Zhao X, Shan X, Cai C, Zhao J, Zhang F, Linhardt RJ, Yu G. Structural Characterization and Interaction with RCA120 of a Highly Sulfated Keratan Sulfate from Blue Shark (Prionace glauca) Cartilage. Marine Drugs. 2018; 16(4):128. https://doi.org/10.3390/md16040128
Chicago/Turabian StyleLi, Qinying, Guoyun Li, Xiaoliang Zhao, Xindi Shan, Chao Cai, Jing Zhao, Fuming Zhang, Robert J. Linhardt, and Guangli Yu. 2018. "Structural Characterization and Interaction with RCA120 of a Highly Sulfated Keratan Sulfate from Blue Shark (Prionace glauca) Cartilage" Marine Drugs 16, no. 4: 128. https://doi.org/10.3390/md16040128
APA StyleLi, Q., Li, G., Zhao, X., Shan, X., Cai, C., Zhao, J., Zhang, F., Linhardt, R. J., & Yu, G. (2018). Structural Characterization and Interaction with RCA120 of a Highly Sulfated Keratan Sulfate from Blue Shark (Prionace glauca) Cartilage. Marine Drugs, 16(4), 128. https://doi.org/10.3390/md16040128