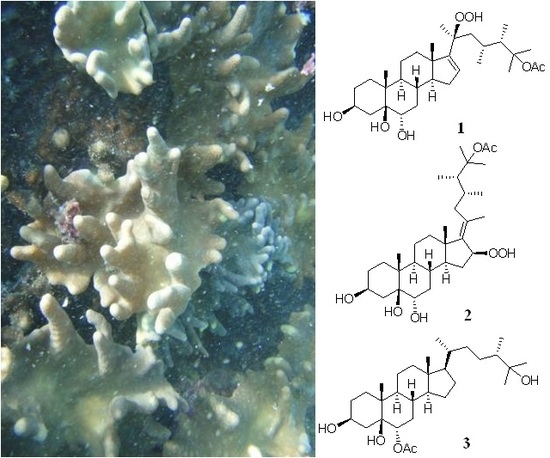
Anti-Inflammatory Polyoxygenated Steroids from the Soft Coral Lobophytum michaelae
Abstract
:1. Introduction
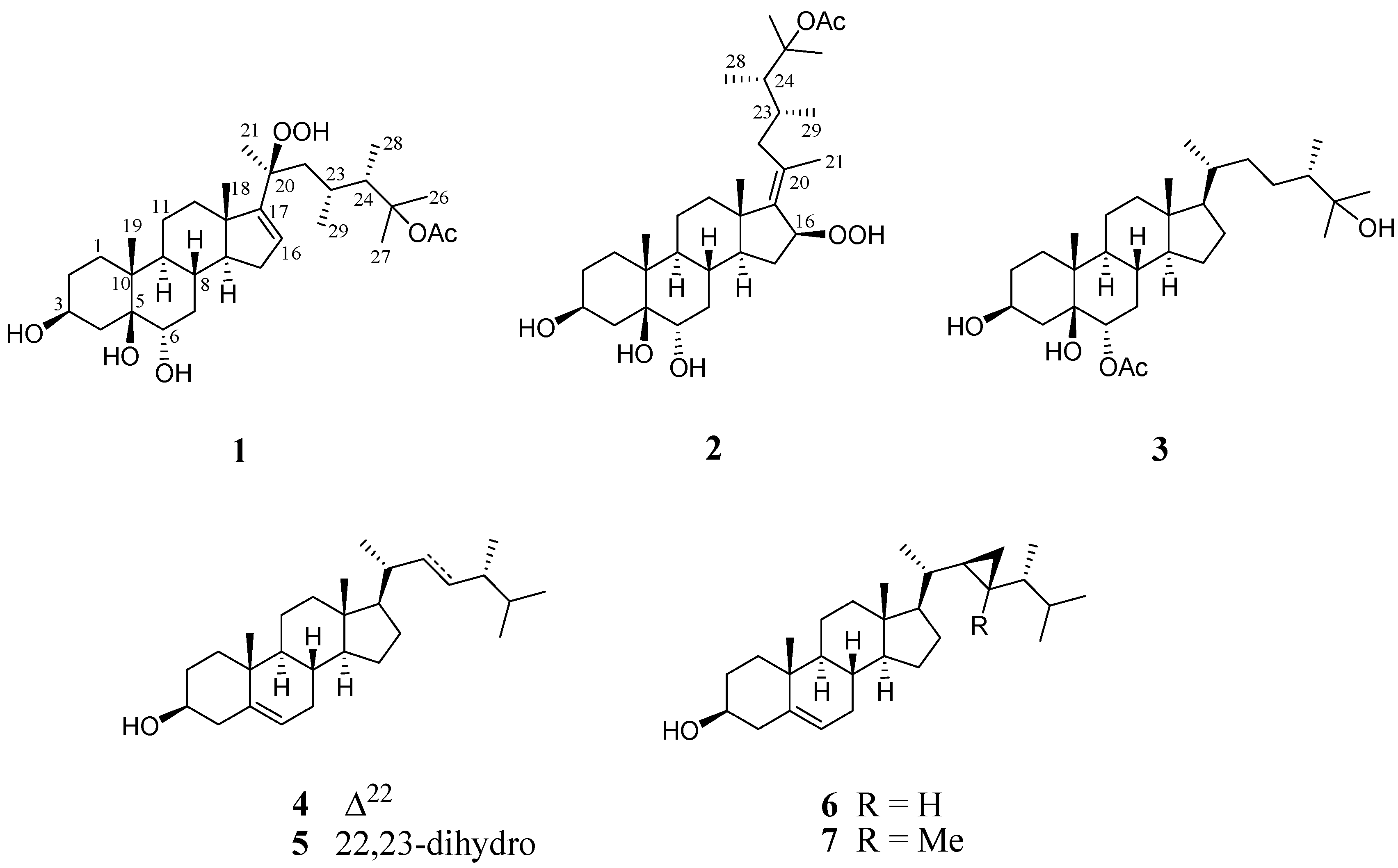
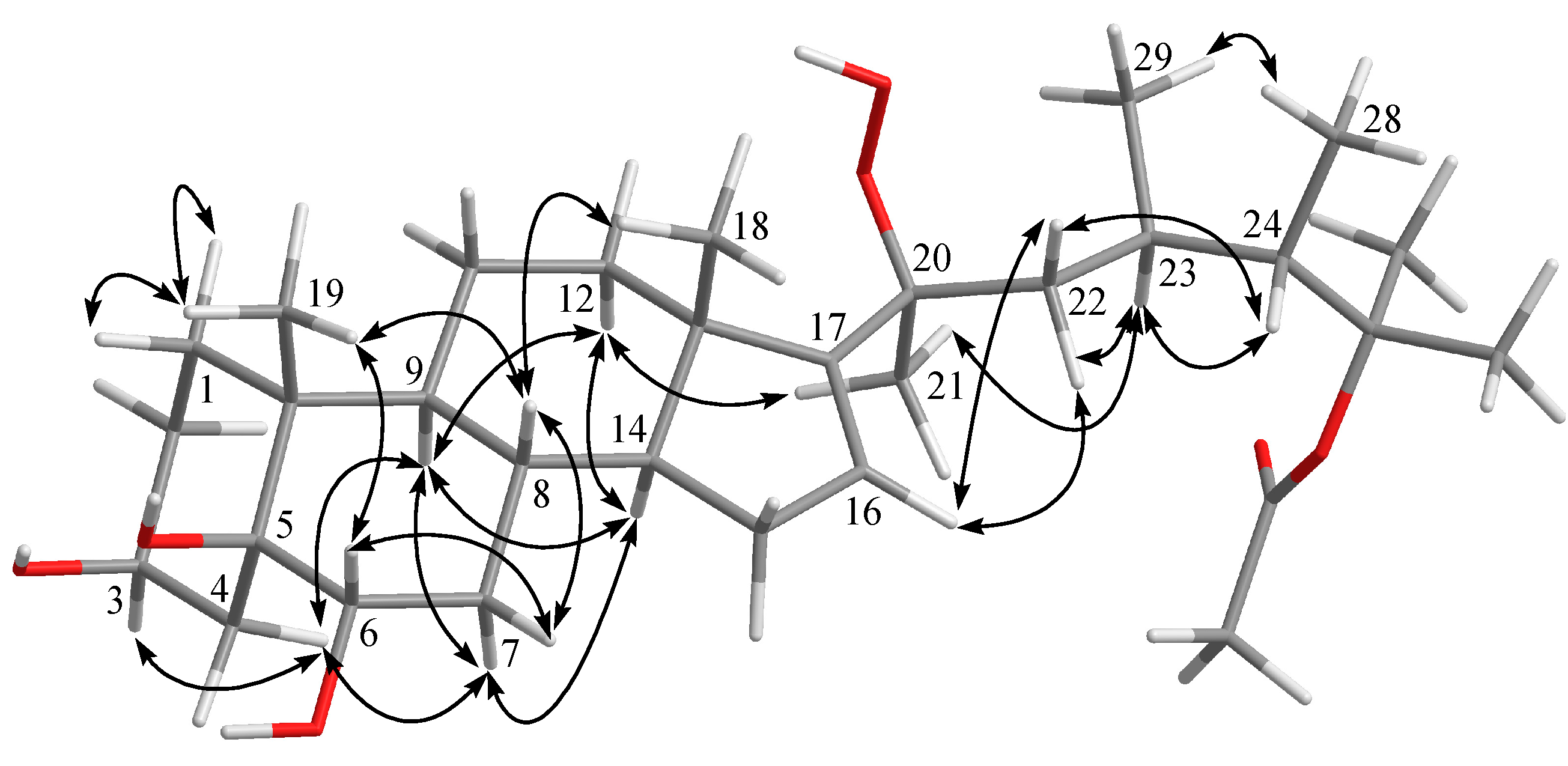
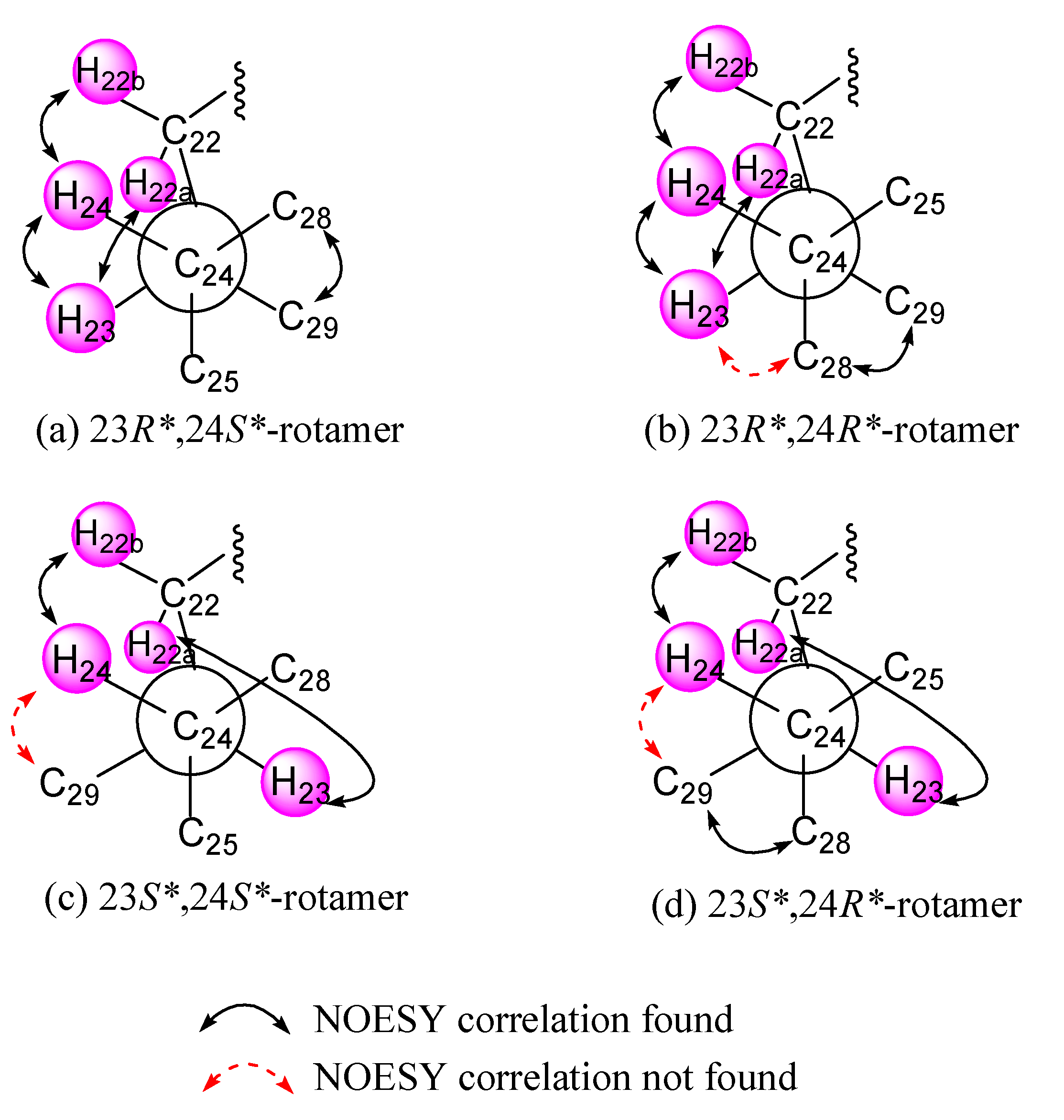
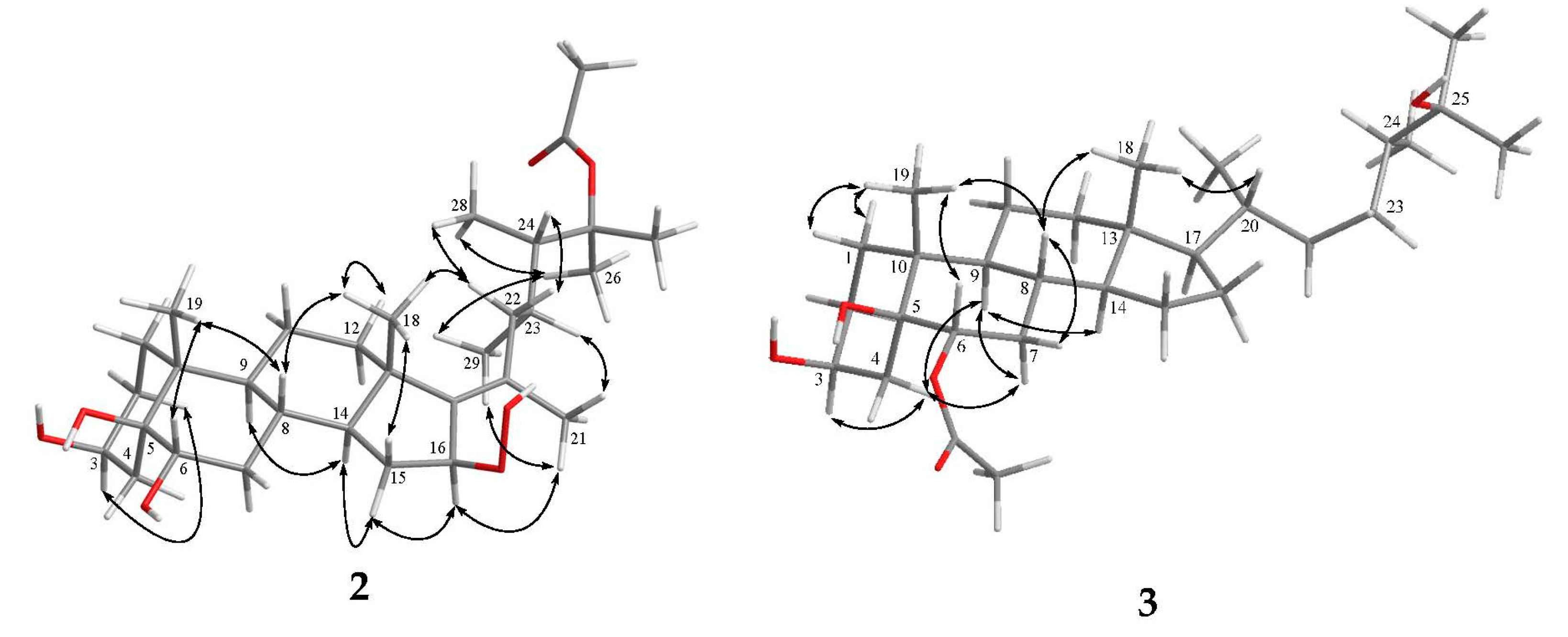
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Cytotoxicity Assay
3.5. Human Neutrophil Superoxide Anion Generation and Elastase Release
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rama Rao, M.; Venkatesham, U.; Rami Reddy, M.V.; Venkateswarlu, Y. An unusual novel C29 steroid from the soft coral Lobophytum crassum. J. Nat. Prod. 1999, 62, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lin, Y.C.; Wen, Z.H.; Su, J.H.; Sung, P.J.; Hsu, C.H.; Kuo, Y.H.; Chiang, M.Y.; Dai, C.F.; Sheu, J.H. Steroid and cembranoids from the Dongsha atoll soft coral Lobophytum sarcophytoides. Tetrahedron 2010, 66, 7129–7135. [Google Scholar] [CrossRef]
- Morris, L.A.; Christie, E.M.; Jaspars, M.; van Ofwegen, L.P. A bioactive secosterol with an unusual A- and B-ring oxygenation pattern isolated from an Indonesian soft coral Lobophytum sp. J. Nat. Prod. 1998, 61, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.; Mohamed, T.A.; Elshamy, A.I.; Hassanien, A.A.; Abdel-Azim, N.S.; Shreadah, M.A.; Abdelgawad, I.I.; Elkady, E.M.; Pare, P.W. A new steroid from the Red Sea soft coral Lobophytum lobophytum. Nat. Prod. Res. 2016, 30, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Quang, T.H.; Ngan, N.T.T.; Van Kiem, P.; Van Minh, C.; Kim, Y.H. A new sterol from the soft coral Lobophytum crassum. Bull. Korean Chem. Soc. 2013, 34, 249. [Google Scholar]
- Sheu, J.H.; Veh, T.H. Isolation of a bioactive sterol from the soft coral Lobophytum mirabile. J. Chin. Chem. Soc. 1991, 38, 397–399. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Teng, W.T.; Huang, C.Y.; Dai, C.F.; Hwang, T.L.; Sheu, J.H. Anti-inflammatory lobane and prenyleudesmane diterpenoids from the soft coral Lobophytum varium. Mar. Drugs 2017, 15, 300. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.A.; Elshamy, A.I.; Hussien, T.A.; Su, J.H.; Sheu, J.H.; Hegazy, M.E.F. Lobophylins F–H: Three new cembrene diterpenoids from soft coral Lobophytum crassum. J. Asian Nat. Prod. Res. 2017, 19, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.; Su, J.H.; Sung, P.J.; Sheu, J.H. Cembranoids with 3,14-ether linkage and a secocembrane with bistetrahydrofuran from the Dongsha Atoll soft coral Lobophytum sp. Mar. Drugs 2011, 9, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Wen, Z.H.; Wu, Y.C.; Yeh, H.C.; Sheu, J.H. Cytotoxic and anti-inflammatory cembranoids from the soft coral Lobophytum crassum. J. Nat. Prod. 2008, 71, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.J.; Wen, Z.H.; Hsu, C.H.; Dai, C.F.; Sheu, J.H. Bioactive cembranoids from the Dongsha atoll soft coral Lobophytum crassum. Bull. Chem. Soc. Jpn. 2011, 84, 1102–1106. [Google Scholar] [CrossRef]
- Wang, S.K.; Duh, C.Y. New cytotoxic cembranolides from the soft coral Lobophytum michaelae. Mar. Drugs 2012, 10, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; Wang, S.K.; Soong, K.; Duh, C.Y. New cytotoxic cembranolides from the soft coral Lobophytum michaelae. Chem. Phar. Bull. 2007, 55, 766–770. [Google Scholar] [CrossRef]
- Wang, S.K.; Duh, C.Y.; Wu, Y.C.; Wang, Y.; Cheng, M.C.; Soong, K.; Fang, L.S. Studies on Formosan soft corals. II. Cytotoxic cembranolides from the soft coral Lobophytum michaelae. J. Nat. Prod. 1992, 55, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Jinming, G.; Lin, H.; Jikai, L. A novel sterol from Chinese truffles Tuber indicum. Steroids 2001, 66, 771–775. [Google Scholar] [CrossRef]
- Rahelivao, M.P.; Lubken, T.; Gruner, M.; Kataeva, O.; Ralambondrahety, R.; Andriamanantoanina, H.; Checinski, M.P.; Bauer, I.; Knolker, H.J. Isolation and structure elucidation of natural products of three soft corals and a sponge from the coast of Madagascar. Org. Biomol. Chem. 2017, 15, 2593–2608. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.J.; Pattabhiraman, T. Chemistry of coelenterates. XXII. New marine sterol possessing a side chain cyclopropyl group: 23-demethylgorgosterol. J. Am. Chem. Soc. 1970, 92, 6073–6074. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, A.; Teshima, S.I.; Ando, T. Sterols of coelenterates. Comp. Biochem./Physiol. B Biochem. Mol. Biol. 1977, 57, 317–323. [Google Scholar] [CrossRef]
- Anderson, G.D.; Powers, T.J.; Djerassi, C.; Fayos, J.; Clardy, J. A stereoselective synthesis of two stereoisomers of demethylgorgosterol. J. Am. Chem. Soc. 1975, 97, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.C.; Hale, R.L.; Djerassi, C. The structure and absolute configuration of the marine sterol gorgosterol. J. Am. Chem. Soc. 1970, 92, 5281–5282. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shao, C.L.; Qi, X.; Li, X.B.; Li, J.; Sun, L.L.; Wang, C.Y. Polyoxygenated sterols from the South China Sea soft coral Sinularia sp. Mar. Drugs 2012, 10, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wang, Y.; Han, L. 4,5-Epoxycholestane-3,6-diols: Templates for generating the full set of eight cholestane-3,5,6-triol stereoisomers in multigram scales, but not for a cholestane-3,4,6-triol. Steroids 2007, 72, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.R.; Huang, C.Y.; Chen, B.W.; Tsai, Y.Y.; Shih, S.P.; Hwang, T.L.; Dai, C.F.; Wang, S.Y.; Sheu, J.H. New bioactive steroids from the soft coral Klyxum flaccidum. RSC Adv. 2015, 5, 12546–12554. [Google Scholar] [CrossRef]
- Chao, C.H.; Huang, L.F.; Wu, S.L.; Su, J.H.; Huang, H.C.; Sheu, J.H. Steroids from the gorgonian. Isis hippuris. J. Nat. Prod. 2005, 68, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Hsieh, P.W.; Chang, Y.J.; Chung, P.J.; Kuo, L.M.; Hwang, T.L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]

| 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| Position | δC a, Mult. b | δH c, Mult. (J) d | δC e, Mult. | δH f, Mult. (J) | δC a, Mult. | δH c, Mult. (J) |
| 1 | 25.2, CH2 | 1.35 m; 1.81 m | 25.1, CH2 | 1.33 m; 1.81 m | 25.1, CH2 | 1.34 m; 1.82 m |
| 2 | 27.7, CH2 | 1.59 m; 1.65 m | 27.8, CH2 | 1.59 m | 27.6, CH2 | 1.59 m |
| 3 | 67.7, CH | 4.27 br s | 67.7, CH | 4.25 br s | 66.9, CH | 4.11 br s |
| 4 | 30.0, CH2 | α: 1.93 m; β: 1.87 m | 29.9, CH2 | 1.81 m; 1.92 m | 31.1, CH2 | 1.92 br s |
| 5 | 78.1, C | 77.8, C | 77.4, C | |||
| 6 | 71.8, CH | 3.82 dd (12.0, 4.8) | 71.7, CH | 3.80 dd (12.0, 5.0) | 75.8, CH | 4.97 dd (12.0, 4.8) |
| 7 | 34.5, CH2 | α: 1.09 q (12.0) β: 1.85 m | 34.6, CH2 | 1.03 m; 1.88 m | 33.6, CH2 | 1.07 m 1.81 m |
| 8 | 32.4, CH | 1.76 m | 33.0, CH | 1.63 m | 33.6, CH | 1.56 m |
| 9 | 43.3, CH | 1.34 m | 42.9, CH | 1.27 m | 42.7, CH | 1.25 m |
| 10 | 41.1, C | 40.9, C | 41.6, C | |||
| 11 | 21.5, CH2 | 1.36 m; 1.51 m | 22.0, CH2 | 1.38 m; 1.52 m | 21.4, CH2 | 1.29 m; 1.42 m |
| 12 | 35.8, CH2 | α: 2.06 m; β: 1.66 m | 38.7, CH2 | 1.44 m; 2.27 m | 39.7, CH2 | 1.13 m; 1.99 br d (12.8) |
| 13 | 47.4, C | 44.1, C | 42.6, C | |||
| 14 | 57.8, CH | 1.52 m | 51.7, CH | 1.09 m | 56.2, CH | 1.07 m |
| 15 | 31.0, CH2 | 1.90 m; 2.12 m | 29.4, CH2 | 1.69 m; 2.08 m | 24.0, CH2 | 1.05 m; 1.53 m |
| 16 | 127.0, CH | 5.70 d (2.0) | 86.1, CH | 4.96 dd (7.5, 7.5) | 28.0, CH2 | 1.28 m; 1.84 m |
| 17 | 157.6, C | 143.6, C | 55.8, CH | 1.13 m | ||
| 18 | 17.9, CH3 | 0.96 s | 17.7, CH3 | 0.99 s | 11.9, CH3 | 0.64 s |
| 19 | 17.2, CH3 | 0.96 s | 17.0, CH3 | 0.94 s | 17.0, CH3 | 0.97 s |
| 20 | 85.6, C | 132.8, C | 36.2, CH | 1.36 m | ||
| 21 | 22.4, CH3 | 1.31 s | 19.4, CH3 | 1.71 s | 18.9, CH3 | 0.92 d (6.4) |
| 22 | 44.5, CH2 | α: 1.99 m β: 1.42 m | 41.2, CH2 | 1.76 m; 2.43 dd (10.0, 13.5) | 34.8, CH2 | 0.92 m; 1.50 m |
| 23 | 26.3, CH | 2.05 m | 29.6, CH | 2.10 m | 27.8, CH2 | 0.77 m; 1.68 m |
| 24 | 46.1, CH | 2.28 m | 40.7, CH | 2.24 m | 45.1, CH | 1.27 m |
| 25 | 87.2, C | 87.0, C | 73.6, C | |||
| 26 | 24.1, CH3 | 1.41 s | 24.0, CH3 | 1.41 s | 26.0, CH3 | 1.14 s |
| 27 | 25.8, CH3 | 1.50 s | 25.3, CH3 | 1.43 s | 27.2, CH3 | 1.15 s |
| 28 | 9.3, CH3 | 0.88 d (7.2) | 9.3, CH3 | 0.81 d (7.0) | 14.8, CH3 | 0.88 d (6.8) |
| 29 | 18.0, CH3 | 0.93 d (6.8) | 16.7, CH3 | 0.86 d (7.0) | ||
| OAc | 22.7, CH3 | 2.03 s | 22.7, CH3 | 2.00 s | 21.3, CH3 | 2.03 s |
| 171.9, C | 171.2, C | 171.9, C | ||||
| 6-OH | 4.11 br s | 4.01 br s | ||||
| 20-OOH | 8.06 br s | 8.91 br s | ||||
| Michosterol A (1) | 5α-Cholestane-3β,5,6α-Triol | 5α-Cholestane-3β,5,6β-Triol | 5β-Cholestane-3β,5,6α-Triol | 5β-Cholestane-3β,5,6β-Triol | |
|---|---|---|---|---|---|
| H-3 | 4.27, br s | 4.06, m | 4.09, m | 4.24, s | 4.14, s |
| H-6 | 3.82, dd, J = 12.0, 4.8 Hz | 3.64, dd | 3.53, s | 3.74–3.81, dd, J = 12.0, 4.8 Hz | 3.56, m |
| Compound | Superoxide Anion Generation | Elastase Release | ||||
|---|---|---|---|---|---|---|
| IC50 (μM) a | Inh% b | IC50 (μM) a | Inh% b | |||
| 1 | 7.1 ± 0.3 | 63.1 ± 1.1 | *** | 4.5 ± 0.9 | 91.7 ± 3.1 | *** |
| 2 | >10 | 14.7 ± 5.7 | >10 | 31.8 ± 5.0 | ** | |
| 3 | >10 | 17.8 ± 2.8 | ** | 0.9 ± 0.1 | 95.4 ± 3.6 | *** |
| Idelalisib | 0.07 ± 0.01 | 102.8 ± 2.2 | *** | 0.3 ± 0.1 | 99.6 ± 4.2 | *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Tseng, W.-R.; Ahmed, A.F.; Chiang, P.-L.; Tai, C.-J.; Hwang, T.-L.; Dai, C.-F.; Sheu, J.-H. Anti-Inflammatory Polyoxygenated Steroids from the Soft Coral Lobophytum michaelae. Mar. Drugs 2018, 16, 93. https://doi.org/10.3390/md16030093
Huang C-Y, Tseng W-R, Ahmed AF, Chiang P-L, Tai C-J, Hwang T-L, Dai C-F, Sheu J-H. Anti-Inflammatory Polyoxygenated Steroids from the Soft Coral Lobophytum michaelae. Marine Drugs. 2018; 16(3):93. https://doi.org/10.3390/md16030093
Chicago/Turabian StyleHuang, Chiung-Yao, Wan-Ru Tseng, Atallah F. Ahmed, Pei-Lun Chiang, Chi-Jen Tai, Tsong-Long Hwang, Chang-Feng Dai, and Jyh-Horng Sheu. 2018. "Anti-Inflammatory Polyoxygenated Steroids from the Soft Coral Lobophytum michaelae" Marine Drugs 16, no. 3: 93. https://doi.org/10.3390/md16030093
APA StyleHuang, C.-Y., Tseng, W.-R., Ahmed, A. F., Chiang, P.-L., Tai, C.-J., Hwang, T.-L., Dai, C.-F., & Sheu, J.-H. (2018). Anti-Inflammatory Polyoxygenated Steroids from the Soft Coral Lobophytum michaelae. Marine Drugs, 16(3), 93. https://doi.org/10.3390/md16030093