Abstract
Five new oxygenated sesquiterpenes, molestins A–D (1, 3–5) and epi-gibberodione (2), three new cyclopentenone derivatives, ent-sinulolides C, D, and F ((+)-9–(+)-11), one new butenolide derivative, ent-sinulolide H ((+)-13), and one new cembranolide, molestin E (14), together with 14 known related metabolites (6–8, (–)-9–(–)-11, (±)-12, (–)-13, 15–19) were isolated from the Paracel Islands soft coral Sinularia cf. molesta. The structures and absolute configurations were elucidated based on comprehensive spectroscopic analyses, quantum chemical calculations, and comparison with the literature data. Compound 5 is the first example of a norsesquiterpene with a de-isopropyl guaiane skeleton isolated from the genus Sinularia. Molestin E (14) exhibited cytotoxicities against HeLa and HCT-116 cell lines with IC50 values of 5.26 and 8.37 μM, respectively. Compounds 4, 5, and 8 showed significant inhibitory activities against protein tyrosine phosphatase 1B (PTP1B) with IC50 values of 218, 344, and 1.24 μM, respectively.
1. Introduction
Soft corals of the genus Sinularia (phylum Cnidaria, class Anthozoa, subclass Octocorallia, order Alcyonacea, suborder Alcyoniina, family Alcyoniidae), comprising more than 100 species, constitute an important invertebrate group occurring widely in different coral reefs of the world. The genus Sinularia is well known for being a rich source of valuable secondary metabolites as they have produced many structurally unique and biologically active compounds. Since 1975 [1,2], over 50 species of the genus Sinularia have been chemically investigated, showing a wide range of structure diversities in diterpenes (especially cembrane diterpenes) [3,4], sesquiterpenes [5,6], polyhydroxylated steroids [7,8], polyamines [9,10], cyclopentenones [6,8,11,12], and butenolides [11,12]. Many of these metabolites exhibited a wide range of biological activities, including cytotoxic [13,14], anti-inflammatory [15], antimicrobial [16], antifouling [17], and antifeedant activities [18].
Up to now, there have been few reports on the soft coral S. cf. molesta and S. molesta, except for one report on the Moyli Island S. intacta (a synonym of S. molesta) in 1999, resulting in a series of africane-type sesquiterpenes [19]. In addition, our first and preliminary study on S. cf. molesta yielded some terpenoids and sterols [20]. In our continuing interest in bioactive natural products from Paracel Islands invertebrates [8,21,22], we investigated extracts of the Paracel Islands soft coral S. cf. molesta that exhibited cytotoxic activities against human leukemia cell lines (K562) and human myeloid leukemia cell lines (HL-60), with the inhibition ratios of 60.0% and 72.8%, respectively, at the concentration of 50 μM. That led to the isolation of two new secoguaiane-type sesquiterpenes (1 and 2), three new guaiane-type sesquiterpenes (3–5), three new cyclopentenone derivatives ((+)-9–(+)-11), one new butenolide derivative ((+)-13), and one new furanocembranolide (14), together with 14 known analogs (6–8, (–)-9–(–)-11, (±)-12, (–)-13, and 15–19) (Figure 1). Their cytotoxic and inhibitory activities against protein tyrosine phosphatase 1B (PTP1B) were evaluated. Herein, we report the isolation, structure elucidation, and bioactivities of these compounds.
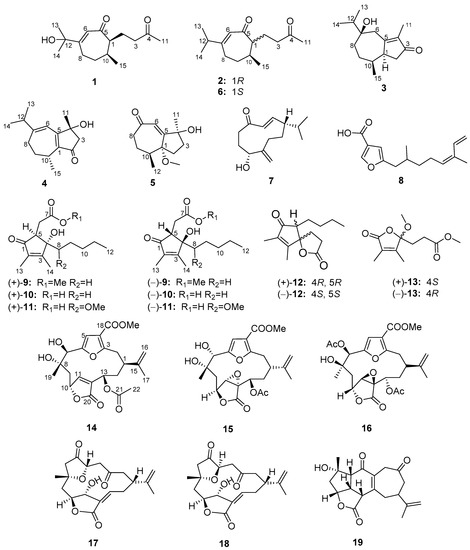
Figure 1.
Structures of compounds 1–19.
2. Results and Discussion
Molestin A (1) was obtained as a colorless oil. Its molecular formula of C15H24O3 was established through a protonated molecular [M + H]+ at m/z 253.1803 (calcd for C15H25O3, 253.1798) in the (+)-high-resolution electrospray ionization mass spectrometry (HRESIMS) spectrum, indicating four degrees of unsaturation. The infrared radiation (IR) spectrum displayed absorption bands for hydroxy (3151 cm−1), carbonyl (1712 cm−1), and olefinic (1649 cm−1) functionalities. The 13C NMR and DEPT data (Table 1) of 1 exhibited 15 carbon resonances corresponding to four methyls, four methylenes, three methines, and four quaternary carbons (including one oxygenated, one olefinic, and two carbonyl). The olefinic carbons at δC 168.7 (C-7) and 127.1 (C-6) and the carbonyl carbon at δC 203.9 (C-5) showed characteristic chemical shifts for an α,β-unsaturated ketone (Table 1). The presence of a 3-oxobutyl group was readily verified by 1H–1H COSY correlations of H-1/H2-2/H2-3 and HMBC correlations from H3-11 to C-3 and C-4 (Figure 2). Furthermore, 1H–1H correlation spectroscopy (1H–1H COSY) correlations of H2-8/H2-9/H-10/H-1 and heteronuclear multiple-bond correlation (HMBC) correlations from H-6 to C-1 and C-8, and from H2-9 to C-1 and C-7 suggested that compound 1 should possess a conjugated cycloheptenone core with a 3-oxobutyl side chain linked to C-1. Additionally, a doublet methyl at δH 0.80 (3H, H3-15) was connected to C-10 by 1H–1H COSY correlation of H-10/H3-15 and HMBC correlations from H3-15 to C-1, C-9, and C-10. Besides, signals of two downfield singlet methyls at δH 1.38 (6H, H3-13 and H3-14) and an additional oxygenated quaternary carbon at δC 74.0 (C-12), in combination with the molecular formula of 1, indicated the presence of a 2-hydroxyprop-2-yl group, which was attached to C-7 by HMBC correlations from H3-13 and H3-14 to C-7, and from H-6 and H2-8 to C-12. Accordingly, compound 1 was thus elucidated as the 12-hydroxylated derivative of the co-isolated secoguaiane-type sesquiterpene 6 (gibberodione), which was previously isolated from a Formosan soft coral S. gibberosa [5].

Table 1.
13C NMR data for compounds 1−5 (δ in ppm).
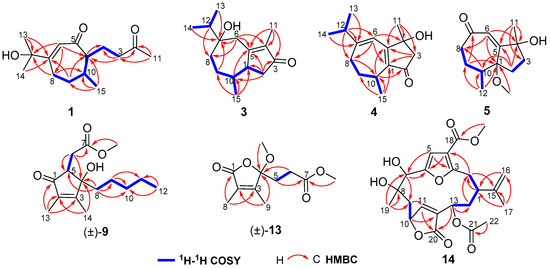
Figure 2.
1H−1H COSY and key HMBC correlations of compounds 1, 3, 4, 5, (±)-9, (±)-13, and 14.
The absolute configuration of 1 was determined to be the same as that of gibberodione, according to the nuclear overhauser effect spectroscopy (NOESY) experiment, comparison of corresponding chemical shifts and optical rotations with those reported analogs, and electronic circular dichroism (ECD) calculations. The E-geometry of the Δ6(7)-double bond was assigned by NOESY correlations of H-6/H3-13 and H-6/H3-14 (Figure 3). NOESY correlations of H-1/H-10 and H2-2/H3-15 as well as the absence of H-1/H3-15 indicated the cis-orientation of H-1 and H-10, which was further supported by the chemical shifts of H3-15 at δH 0.80 and δC 16.3 recorded in CDCl3 (Ahmed et al. concluded that the chemical shifts of the secondary methyl at δH 0.83 and δC 15.7 in (1S, 10S) isomer were upfield, compared to δH 1.09 and δC 19.9 in (1R, 10S) isomer) [5]). The absolute configuration of 1 was determined by ECD calculations performed by the time dependent density functional theory (TDDFT)/ECD method [23]. The experimental ECD spectrum of 1, which matched well with the calculated ECD spectrum for (1S, 10S) isomer, and showed mirror-image-like relationship with calculated ECD spectra for (1R, 10R) isomer (Figure 4), proved the (1S, 10S) absolute configuration for 1, corresponding to the same sign of optical rotation of 1 ([α +10. 0, c 0.1, MeOH) as that of gibberodione ([α +20.8, c 0.72, CHCl3).
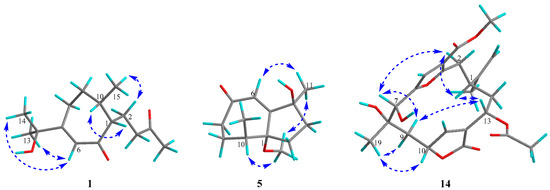
Figure 3.
Key NOESY correlations of compounds 1, 5, and 14.
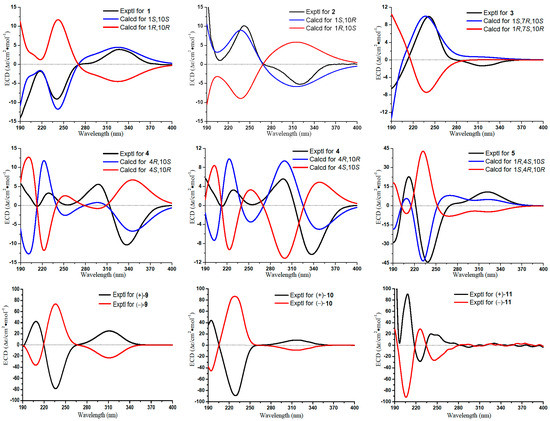
Figure 4.
Experimental and calculated ECD spectra of compounds 1–5 and experimental ECD spectra of compounds (±)-9–(±)-11.
Epi-gibberodione (2), afforded as a colorless oil, was assigned a molecular formula of C15H24O2 by (+)-HRESIMS ion at m/z 237.1852 [M + H]+ (calcd for C15H25O2, 237.1849) and 13C NMR data (Table 1). 1D NMR (Table 1 and Table 2) together with 1H–1H COSY data of 2 revealed the presence of an α,β-unsaturated ketone, a 3-oxobutyl moiety, and an isopropyl group, implying compound 2 could be an analog of gibberodione [5]. That speculation was subsequently confirmed by detailed analyses of the 1H–1H COSY, heteronuclear singular quantum correlation (HSQC), and HMBC spectra, which established that compound 2 possessed an identical planar structure as gibberodione. NOESY correlations of H-6/H3-13 and H-6/H3-14 defined E-geometry of the Δ6(7)-double bond. Besides, NOESY correlations of H-1/H3-15 and the absence of H-1/H-10 indicated the trans-orientation of H-1 and H-10, which were confirmed by the chemical shift of H3-15 at δH 1.10 recorded in CDCl3 (see Section 3.3), because of the previous report that the cis-orientation isomer showed marked upfield shift for H3-15 relative to that of trans-orientation isomer and the upfield shift of H3-15 in gibberodione (δH 0.79) was detected [5]. The overall pattern of experimental ECD spectrum of compound 2 exhibiting positive Cotton effect at 241 nm and negative Cotton effect at 320 nm matched well with the (1R, 10S) isomer and mirrored with (1S, 10R) isomer in the calculated ECD spectra (Figure 4), permitting assignment of the (1R, 10S) absolute configuration for compound 2.

Table 2.
1H NMR data for compounds 1−5 (δ in ppm, J in Hz).
Molestin B (3) was obtained as a colorless oil having a molecular formula of C15H24O2 as deduced from the protonated molecular [M + H]+ at m/z 237.1853 (calcd for C15H25O2, 237.1849) in the (+)-HRESIMS spectrum and 13C NMR data (Table 1). The 13C NMR and DEPT spectra showed 15 carbon signals, which were assigned as four methyls, four methylenes, three methines, and four quaternary carbons (including two olefinic, one oxygenated, and one conjugated carbonyl). Additionally, the characteristic carbon resonances at δC 174.0 (C-5), 138.6 (C-4), and 208.7 (C-3) attributed to an α,β-unsaturated ketone group were also observed. Considering all these data as well as four degrees of unsaturation, compound 3 was predicted to be a bicyclic sesquiterpene. The presence of an isopropyl group was verified by the 1H–1H COSY correlations of H3-13/H-12/H3-14 (Figure 2). The aforementioned information together with the sequential 1H–1H COSY correlations of H2-8/H2-9/H-10/H-1/H2-2 implied that the structure of 3 was analogous to the guaiane-type sesquiterpene torilolone [24]. The HMBC correlations from H3-11 to C-3, C-4, and C-5; from H3-13 and H3-14 to C-7; from H3-15 to C-1, C-9, and C-10; from H2-2 to C-1, C-3, C-5, and C-10; and from H2-6 to C-4, C-5, C-7, and C-8 further constructed the planar structure of compound 3 (Figure 2). The NOESY correlations of H-10/H3-13 suggested the cofacial arrangement of H3-15 and 7-OH. In addition, the cis-disposition of H-1 and H-10 was deduced from chemical shift of H-10 (δH 2.08) and H3-15 (δH 0.65) in 1H NMR spectrum recorded in CDCl3 [5,25]. The overall pattern of experimental ECD spectrum of compound 3 matched well with the calculated ECD curve for (1S, 7R, 10S) isomer (Figure 4), demonstrating the (1S, 7R, 10S) absolute configuration for compound 3.
Molestin C (4), a yellow oil, had a molecular formula of C15H22O2 based on its (+)-HRESIMS ion at m/z 235.1693 [M + H]+ (calcd for C15H23O2, 235.1693), requiring five degrees of unsaturation. Compound 4 shared a similar guaiane-type sesquiterpene skeleton with compound 3 by comparison of their 1D NMR data (Table 1 and Table 2). The spin-spin coupling system of H2-8/H2-9/H-10/H3-15 and H3-13/H-12/H3-14 deduced from 1H–1H COSY spectrum and HMBC correlations from H3-11 to C-3, C-4 and C-5; from H-12 to C-6 and C-8; from H3-15 to C-1, C-9 and C-10; from H2-3 to C-1 and C-5; from H-6 to C-1, C-4, and C-8; and from H-10 to C-2 and C-5 (Figure 2) completed the planner structure assignment as depicted for compound 4. The E-geometry of the Δ6(7)-double bond was verified by NOESY correlations of H-6/H3-13 and H-6/H3-14. To determine the absolute configuration of compound 4, ECD spectra of the four configurational isomers were calculated. Results revealed that the experimental ECD spectrum of compound 4 was consistent with the calculated ECD spectrum for (4R, 10R) configuration only (Figure 4). Accordingly, the (4R, 10R) absolute configuration was assigned for compound 4.
Molestin D (5) was isolated as a yellow oil, with a molecular formula of C13H20O3 based on (+)-HRESIMS ion at m/z 242.1753 [M + NH4]+ (calcd for C13H24O3N, 242.1751) and 13C NMR data (Table 1), implying four degrees of unsaturation. Typical resonances at δC162.7 (C-5), 127.1 (C-6), and 205.3 (C-7) in 13C NMR spectrum indicated the presence of an α,β-unsaturated ketone group. The comparisons of 1D NMR spectroscopic data of compound 5 with those of 1–4 (Table 1 and Table 2) indicated that compound 5 was likely to be a de-isopropyl guaiane-type sesquiterpene. That speculation was confirmed by 1H–1H COSY correlations of H2-8/H2-9/H-10/H3-12 and HMBC correlations from H2-2 to C-4, C-5, and C-10; from H-6 to C-1, C-4, and C-8; from H3-11 to C-3, C-4, and C-5; and from H3-12 to C-1, C-9, and C-10 (Figure 2). Additionally, HMBC correlation from 1-OCH3 to C-1 demonstrated the location of the methoxyl at C-1 in compound 5. NOESY correlation of H-6/H3-11 suggested E-geometry of the Δ5-double bond (Figure 3). Besides, NOESY correlations of 1-OCH3/H-10 and 1-OMe/H3-11, along with the absence of 1-OCH3/H3-12 suggested that 1-OCH3, H-10, and H3-11 were on the same side of the ring system. On the other hand, the cis-oritation of 1-OCH3 and H-10 was indicated by the chemical shift of H-10 (δH 2.30) in compound 5, because it was reported that the chemical shifts for trans-oritation of 1-OH and H-10 in its analogs are more upfield [26,27]. The (1R, 4S, 10S) absolute configuration for molestin D (5) was identified through ECD calculations, in which the experimental ECD spectrum of compound 5 showed good agreement with the calculated ECD spectrum for (1R, 4S, 10S) configuration (Figure 4).
ent-sinulolide C ((+)-9), a colorless oil, was deduced to have a molecular formula of C15H24O4 according to the (+)-HRESIMS ions at m/z 269.1747 [M + H]+ (calcd for C15H25O4, 269.1747) and m/z 291.1565 [M + Na]+ (calcd for C15H24O4Na, 291.1567). The 1H NMR spectrum (see Section 3.3) exhibited one methoxyl at δH 3.77 (3H, s, 7-OCH3), two olefinic singlet methyls at δH 2.02 (3H, s, H3-14) and 1.73 (3H, s, H3-13), and one terminal triplet methyl at δH 0.83 (3H, t, J = 6.7 Hz, H3-12). The characteristic carbon signals at δC 168.8 (C-3), 135.7 (C-2), and 202.9 (C-1) in 13C NMR spectrum (see Section 3.3) indicated the presence of an α,β-unsaturated ketone group in compound 9. Besides, the consecutive correlations of H2-8/H2-9/H2-10/H2-11/H3-12 observed in 1H–1H COSY spectrum formed a pentane linear side chain in 9 (Figure 2). The aforementioned data bear remarkable similarities to those of sinulolide C [11]. In addition, further comparative analyses of 1D and 2D NMR data of compound 9 and sinulolide C suggested that the two compounds shared an identical planner structure. NOESY correlation of H-6b (δH 2.38)/H-8b (δH 1.53) in compound 9 clarified the same relative configuration as sinulolide C. However, just as the natural occurring analogs (±)-foedanolide ((±)-12) [28], compound 9 was optically inactive, indicating 9 to be a racemate. The chiral HPLC separation of 9 yielded optical pure compounds (+)-9 and (−)-9 (sinulolide C), with a ratio of approximately 1:1 (Figure S79, Supplementary Materials). The opposite optical rotations of respective +17.8 (c 0.05, MeOH) and −23.5 (c 0.05, MeOH), as well as mirror-image-like experimental ECD curves (Figure 4) of compounds (+)-9 and (−)-9, suggested the (4S, 5S) absolute configuration for ent-sinulolide C ((+)-9), by comparison with those of sinulolide C, sinularone B [12], and (±)-sinularone J [8].
ent-sinulolide D ((+)-10) was obtained as a colorless oil. Its molecular formula of C14H22O4 was determined by the (−)-HRESIMS ion at m/z 253.1440 [M − H]− (calcd for C14H21O4, 253.1445). The 1D NMR data of 10 (see Section 3.3) revealed that its structural features were very close to those of 9, differing only in the absence of a methoxyl at C-7 in 10. The planar structure of compound 10 was unambiguously proved to be the same with sinulolide D by 1H–1H COSY and HMBC correlations [11]. Moreover, NOESY correlation of H-6b (δH 2.64)/H-8b (δH 1.79) in compound 10 disclosed the same relative configuration as sinulolide D. Similarly, Compound 10 was initially isolated as a racemic mixture, and was separated by chiral HPLC to afford (+)-10 ([α +44.6, c 0.2, MeOH) and (–)-10 (sinulolide D, [α −42.1, c 0.2, MeOH) with a ratio of 1:1 (Figure S80, Supplementary Materials). Comparing the optical rotations as well as experimental ECD spectra of compounds (+)-10 and (−)-10 (Figure 4) with those of analogs [8,11,12], the absolute configuration for ent-sinulolide D ((+)-10) was clearly assigned as (4S, 5S). The co-occurrence of compounds 9 and 10 in the extract and the fact that MeOH was used during the extraction process suggested that compound 9 is likely a 7-O-methyl artifact of 10.
ent-sinulolide F ((+)-11), obtained as a colorless oil, was defined a molecular formula of C15H24O5 by the (+)-HRESIMS ion at m/z 307.1511 [M + Na]+ (calcd for C15H24O5Na, 307.1516). The 1D NMR spectroscopic data of compound 11 (see Section 3.3) were very close to those of compound 9, except for the presence of a methoxyl (δH 3.39, δC 60.4) and an oxygenated methane (δH 3.60, δC 80.9), instead of one methylene (δH 1.77 and 1.53, δC 37.1) in 9. The 1H–1H COSY and HMBC data finally defined the planner structure of compound 11, which was identical to sinulolide F [11]. The relative configuration of 11 was shown to be the same as that of sinulolide F based on equivalent NMR data and NOESY correlation of H-6a (δH 3.06)/H-8 (δH 3.60). Compound 11 was also separated by chiral HPLC to obtain (+)-11 and (–)-11 (sinulolide F), with an enantiomeric ratio of 1:1 (Figure S81, Supplementary Materials). Comparison of the experimental ECD spectra for compounds (±)-11 (Figure 4) and sinulolide F suggested the (4S, 5S) configuration for ent-sinulolide F ((+)-11). However, the configurations at C-8 in compounds (±)-11 were still undetermined.
ent-sinulolide H ((+)-13), a colorless oil, had a molecular formula of C11H16O5 based on the (+)-HRESIMS ion at m/z 251.0895 [M + Na]+ (calcd for C11H16O5Na, 251.0890), implying four degrees of unsaturation. In the 13C NMR spectrum, three quaternary signals at δC 155.9 (C-3), 127.8 (C-2), and 171.4 (C-1) attributed to an α,β-unsaturated ester group were exhibited (see Section 3.3). The 1H NMR data showed two downfield singlet methyls at δH 1.88 (3H, s, H3-9) and 1.85 (3H, s, H3-8), which were attached to C-2 and C-3 by HMBC correlations from H3-8 to C-1 and C-3, and from H3-9 to C-2 and C-4, respectively. The presence of a methyl propionate fragment was confirmed by the 1H–1H COSY correlation of H2-5/H2-6 and HMBC correlations from H2-5 and 7-OCH3 to C-7 (Figure 2). In addition, resonances of one notable downfield quaternary carbon at δC 109.0 (C-4) as well as a methoxyl at δC 50.4 (4-OCH3) were observed in the 13C NMR spectrum. The above information strongly indicated that compound 13 was likely to have the same planner structure as the 2,3-dimethyl butenolide derivative sinulolide H [11], which was then confirmed by 1H–1H COSY and HMBC correlations (Figure 2). Compound 13 was further purified by chiral HPLC chromatography to obtain (+)-13 ([α +8.6, c 0.01, MeOH) and (–)-13 (sinulolide H, [α –10.3, c 0.01, MeOH) (Figure S83, Supplementary Materials). The absolute configuration of ent-sinulolide H ((+)-13) was determined as 4S by comparing the optical rotations with those of the analogs sinulolide H ([α –3.2, c 0.03, MeOH) and sinularone H ([α +3.7, c 0.12, MeOH) [12].
Molestin E (14) was isolated as a yellow oil and had a molecular formula of C23H28O9 as determined by (+)-HRESIMS ion at m/z 466.2063 [M + NH4]+ (calcd for C23H32O9N, 466.2072). Absorption for hydroxy and carbonyl groups at 3370, 1751, and 1698 cm–1 were observed in the IR spectrum. The 1D NMR data of compound 14 (Table 3) were similar to those of the co-occurring compounds 15 and 16. 1H NMR spectrum of 14 displayed signals at δH 6.75 (1H, s, H-5), 6.10 (1H, s, H-11), and 4.81 (2H, d, J = 6.1 Hz, H2-16) ascribed to a trisubstituted furan, a disubstituted butyrolactone ring, and a terminal olefin, respectively, as found in sinulacembranolide A isolated from the soft coral S. gaweli [29]. The 13C NMR and DEPT spectra of 14 showed the presence of 23 carbon signals which were assigned to four methyls, four methylenes (including one terminal olefinic), six methines (including two oxygenated, two olefinic), and nine quaternary carbons (including one oxygenated, four olefinic, and three carbonyl), indicating compound 14 could be the deacetyl analog of sinulacembranolide A. Connectivity information obtained from 2D NMR, especially 1H–1H COSY and HMBC experiments, unambiguously confirmed the above speculation, and determined the planar structure of 14 (Figure 2). The relative configuration of 14 was elucidated by NOESY spectrum. NOESY correlation of H-1/H-13 suggested that H-1 and H-13 were on the same side of the ring system. NOESY correlations of H-1/H-2α (δH 2.75) and H-7/H-2α assigned the α-orientation of H-1, H-7, and H-13 (Figure 3). The same side of H3-19 and H-10 were determined based on NOESY correlations of H3-19/H-10, and their β-orientation were finally defined by NOESY correlations of H3-19/H-9β (δH 2.60), H-10/H-9β, H-7/H-9α (δH 1.80), and H-13/H-9α. That allowed the assignment of the relative configuration of 14 as (1S*, 7R*, 8S*, 10R*, 13R*).

Table 3.
1H and 13C NMR data for compound 14 (δ in ppm, J in Hz).
The known compounds, including three sesquiterpenes (6–8), five cyclopentenone derivatives ((–)-9–(–)-11, and (±)-12), one butenolide derivative ((–)-13), and five diterpenes (15–19) were identified as gibberodione [5], polydactin A [30], (5′Z)-5-(2′,6′-Dimethylocta-5′,7′-dienyl)-furan-3- carboxylic acid [31], sinulolide C [11], sinulolide D [11], sinulolide F [11], (±)-foedanolide ((±)-sinularone D) [12,28], sinulolide H [11], leptodiol [32], (–)-leptodiol acetate [29], 5-epi-sinuleptolide [33], sinuleptolide [34,35], and scabrolide A [36], by comparing their spectroscopic data with those reported earlier.
Considering the reported cytotoxic activities of some sesquiterpenes [30], cyclopentenone derivatives [28], and cembranoid diterpenes [36], all the compounds were evaluated for their cytotoxic activities against HeLa (human cervical carcinoma), HCT-116 (human colon carcinoma), BEL-7402 (human hepatocellular carcinoma), K562 (human leukemia), and Jurkat (human acute leukemia T) tumor cell lines. Only three cembranoid diterpenes showed valuable cytotoxicities against the selected cell lines (IC50 < 10 μM). Molestin E (14) exhibited cytotoxicities against HeLa and HCT-116 cell lines with IC50 values of 5.26 and 8.37 μM, respectively. Compounds 17 and 18 showed cytotoxicities against HeLa cell lines with IC50 values of 6.66 and 6.10 μM, respectively.
Considering some guaiane-type sesquiterpenes and furanoterpenoids previously reported showed inhibitory activities against protein tyrosine phosphatase 1B (PTP1B) [37,38,39], a major negative regulator in insulin signaling pathways [40]. Inhibiting PTP1B activity could increase insulin sensitivity and is expected to be a potential promising therapeutic for type 2 diabetes and obesity [41]. These isolated sesquiterpenes were also assessed for their inhibitory activities against PTP1B. In addition, the results revealed that two guaiane-type sesquiterpenes (4 and 5) and a furanosesquiterpene (8) displayed strong inhibitory activities against PTP1B with IC50 values of 218, 344, and 1.24 μM, respectively, lower than the positive control (the IC50 value of sodium orthovanadate was 881 μM).
3. Materials and Methods
3.1. General Methods
Optical rotations were measured on a JASCO P-1020 digital polarimeter. Ultra-violet (UV) spectra were measured on a Beckman DU640 spectrophotometer. ECD spectra were obtained on a JASCO J-810 spectropolarimeter. IR spectra were recorded on a Nicolet Nexus 470 FT-IR spectrophotometer in KBr discs. 1D and 2D NMR spectra were recorded on a JEOL JNMECP 500 spectrometer (500 MHz for 1H and 125 MHz for 13C) using tetramethylsilane as an internal standard. Chemical shifts are expressed in δ (ppm) referring to the solvent peaks at δH 7.26 and δC 77.16 for CDCl3, and δH 3.31 and δC 49.50 for methanol-d4 and coupling constant J in Hz. HRESIMS data were acquired on a Thermo Scientific LTQ Orbitrap XL mass spectrometer or a Micromass Q-Tof Ultima GLOBAL GAA076 mass spectrometer, equipped with an electrospray ionization (ESI) source, and the ionization mode was positive or negative. Semipreparative HPLC separations were carried out using an Agilent 1100 series instrument with a diode array detector (DAD) detector, equipped with a reversed-phase column (YMC-Pack ODS-A, 5 μm, 250 × 10 mm). Chiral HPLC analysis and resolution were conducted on a chiral analytical column (Daicel Chiralpack IC, 5 μm, 250 × 4.6 mm). Silica gel (200–300 mesh, 300–400 mesh; Qingdao Marine Chemical Co. Ltd., Qingdao, China), ODS silica gel (50 μm, Merck, Darmstadt, Germany) and Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) were used to perform column chromatography (CC). Precoated silica gel plates (GF254, Qingdao Marine Chemical Co. Ltd., Qingdao, China) were used for thin layer chromatography (TLC) analyses, and spots were visualized under UV light and by spraying with 10% H2SO4 in EtOH.
3.2. Animal Material
The soft coral S. cf. molesta was collected from the Paracel Islands of the South China Sea in October 2012, at a depth of about 10 m, and was frozen immediately until it was examined. The specimen was identified by Dr. Leen van Ofwegen, a co-author of this paper.. A voucher specimen (NO. XS-2012-22) was deposited in the school of Medicine and Pharmacy, Ocean University of China, China.
3.3. Extraction and Isolation
The frozen organism (5.0 kg, wet weight; 2.2 kg, dry weight) was homogenized and extracted with MeOH four times (each time, 5 L, 3 d) at room temperature. The combined solutions were concentrated in vacuum and desalted by anhydrous MeOH three times (0.6 L, 0.5 L, 0.4 L) to yield a residue (95.0 g). The crude extract was subjected to silica gel vacuum liquid chromatography (VLC), eluting with a gradient of petroleum ether (PE)/acetone (150:1–1:1) and subsequently CH2Cl2/MeOH (20:1–1:1) to obtain seven fractions (Frs. 1–7). Fr. 2 was subjected to silica gel CC using PE/acetone (100:1–5:1 gradient) as eluent to give four subfractions (Frs. 2.1–2.4). Fr. 2.2 was separated by ODS CC (MeOH/H2O, 60:40−100:0 gradient) to yield four subfractions (Frs. 2.2.1–2.2.4). Fr. 2.2.2 was further purified by semipreparative HPLC (ODS; MeOH/H2O, 60:40) to afford compounds 1 (3.5 mg) and 2 (1.7 mg). Fr. 2.2.3 was purified by semipreparative HPLC (ODS; MeOH/H2O, 40:60) to afford compounds 6 (10.0 mg) and 7 (2.1 mg). Fr. 3 was applied to silica gel CC (PE/acetone, 50:1–1:1 gradient) to yield six subfractions (Frs. 3.1–3.6). Fr. 3.3 was further separated by CC (Sephadex LH-20; CHCl3/MeOH, 1:1), and then semipreparative HPLC (ODS; MeOH/H2O, 40:60) to afford compound 13 (2.5 mg). Fr. 3.5 was purified by ODS CC (MeOH/H2O, 40:60−80:20 gradient) and then semipreparative HPLC (ODS; MeOH/H2O, 40:60) to yield compounds 3 (32.0 mg), 4 (2.1 mg), and 5 (2.5 mg). Fr. 4 was fractionated into six subfractions (Fr. 4.1−4.6) by silica gel CC (PE/acetone, 40:1–1:1 gradient). Fr. 4.2 was fractionated by ODS CC, eluted with MeOH/H2O (30:70−80:20 gradient), followed by semipreparative HPLC (MeOH/H2O, 55:45), to afford compounds 10 (5.0 mg) and 12 (18.0 mg). Repeated Sephadex LH-20 CC (CHCl3/MeOH, 1:1), followed by ODS CC (MeOH/H2O, 30:70−80:20 gradient) and then semipreparative HPLC (MeOH/H2O, 45:55), gave compounds 14 (14.0 mg), 15 (33.0 mg), 16 (1.8 mg), 17 (55.0 mg), 18 (43.0 mg), and 19 (1.5 mg) from Fr. 4.5. Fr. 5 was separated by silica gel CC (PE/acetone, 20:1−1:1 gradient) to provide five subfractions (Fr. 5.1−5.5). Fr. 5.5 was separated by Sephadex LH-20 CC (CH2Cl2/MeOH, 1:1), followed by semipreparative HPLC (MeOH/H2O, 55:45), to yield compounds 8 (4.5 mg), 9 (1.6 mg), and 11 (1.5 mg). Chiral separations of (±)-9−(±)-13 (chiral HPLC column; n-hexane/i-PrOH 80:20, 90:10, 70:30, 97:3, and 85:15, respectively) to resolved into optically pure compounds (+)-9 (0.8 mg), (–)-9 (0.8 mg), (+)-10 (2.1 mg), (−)-10 (2.2 mg), (+)-11 (0.7 mg), (−)-11 (0.7 mg), (+)-12 (8.7 mg), (−)-12 (8.8 mg), (+)-13 (1.2 mg), and (−)-13 (1.2 mg). During the HPLC separations, the injection volume was less than 40 μL (semipreparative HPLC) or 5 μL (chiral HPLC) and the column temperature was maintained at about 30 °C.
Molestin A (1): colorless oil; [α +10.0 (c 0.1, MeOH); ECD (MeOH) λmax (Δε) 241 (−9.1), 328 (3.9) nm; IR (KBr) νmax 3151, 2975, 2932, 2872, 1712, 1649,1453, 1379, 1217, 1096 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS m/z 253.1803 [M + H]+ (calcd for C15H25O3, 253.1798).
Epi-gibberodione (2): colorless oil; [α +9.8 (c 0.1, MeOH); ECD (MeOH) λmax (Δε) 242 (10.1), 320 (−5.2) nm; IR (KBr) νmax 2954, 2803, 1703, 1652, 1465, 1382, 1190 cm−1; 1H NMR (500 MHz, CDCl3) δ 5.84 (1H, br d, J = 2.1 Hz, H-6), 2.11 (3H, s, H3-11), 1.10 (3H, d, J = 6.2 Hz, H3-15), 1.07 (3H, d, J = 6.6 Hz, H3-13), 1.06 (3H, d, J = 6.8 Hz, H3-14). 1H and 13C NMR data (methanol-d4), see Table 1 and Table 2; HRESIMS m/z 237.1852 [M + H]+ (calcd for C15H25O2, 237.1849).
Molestin B (3): colorless oil; [α +37.9 (c 0.1, MeOH); ECD (MeOH) λmax (Δε) 242 (9.9), 316 (−1.4) nm; IR (KBr) νmax 3451, 2972, 2850, 1685, 1496, 1383, 1167, 996 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS m/z 237.1853 [M + H]+ (calcd for C15H25O2, 237.1849).
Molestin C (4): yellow oil; [α +110.9 (c 0.1, MeOH); ECD (MeOH) λmax (Δε) 213 (−0.2), 229 (3.3), 298 (5.6), 337 (−10.3) nm; IR (KBr) νmax 3450, 2961, 2780, 1683,1506, 1381, 1257,1035 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS m/z 235.1693 [M + H]+ (calcd for C15H23O2, 235.1693).
Molestin D (5): yellow oil; [α +39.4 (c 0.2, MeOH); ECD (MeOH) λmax (Δε) 212 (22.8), 238 (−44.2), 322 (10.9) nm; IR (KBr) νmax 3417, 2966, 1666, 1458, 1052, 916, 870 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS m/z 242.1753 [M + NH4]+ (calcd for C13H24O3N, 242.1751).
ent-sinulolide C ((+)-9): colorless oil; [α +17.8 (c 0.05, MeOH); ECD (MeOH) λmax (Δε) 210 (40.9), 236 (−79.5), 314 (22.1) nm; UV (MeOH) λmax (log ε) 224 (2.16) nm; IR (KBr) νmax 3445, 2985, 2921, 1730, 1643, 1457, 1370, 1163 cm−1; 1H NMR (500 MHz, CDCl3) δ 2.85 (1H, dd, J = 11.7, 2.9 Hz, H-5), 3.15 (1H, dd, J = 18.5, 3.2 Hz, H-6a), 2.38 (1H, dd, J = 18.6, 11.8 Hz, H-6b), 1.77 (1H, td, J = 13.5, 5.3 Hz, H-8a), 1.53 (1H, td, J = 12.1, 3.8 Hz, H-8b), 0.76 (1H, m, H-9a), 0.64 (1H, m, H-9b), 1.20 (4H, m, H2-10, H2-11), 0.83 (3H, t, J = 6.7 Hz, H3-12), 1.73 (3H, s, H3-13), 2.02 (3H, s, H3-14), 3.77 (3H, s, 7-OCH3); 13C NMR (125 MHz, CDCl3) δ 202.9 (C, C-1), 135.7 (C, C-2), 168.8 (C, C-3), 80.6 (C, C-4), 55.2 (CH, C-5), 30.0 (CH2, C-6), 175.4 (C, C-7), 37.1 (CH2, C-8), 25.2 (CH2, C-9), 32.1 (CH2, C-10), 22.5 (CH2, C-11), 14.1 (CH3, C-12), 8.0 (CH3, C-13), 11.7 (CH3, C-14), 52.6 (CH3, 7-OCH3); HRESIMS m/z 269.1747 [M + H]+ (calcd for C15H25O4, 269.1747), m/z 291.1565 [M + Na]+ (calcd for C15H24O4Na, 291.1567).
ent-sinulolide D ((+)-10): colorless oil; [α +44.6 (c 0.2, MeOH); UV (MeOH) λmax (log ε) 228 (2.60) nm; ECD (MeOH) λmax (Δε) 229 (−89.1), 320 (9.0) nm; IR (KBr) νmax 3422, 2959, 2860, 1735, 1610, 1469, 1365, 1103 cm−1; 1H NMR (500 MHz, CDCl3) δ 2.94 (1H, overlap, H-5), 2.96 (1H, overlap, H-6a), 2.64 (1H, d, J = 15.5 Hz, H-6b), 2.01 (1H, td, J = 14.4, 4.2 Hz, H-8a), 1.79 (1H, m, H-8b), 135−1.25 (5H, m, H-9a, H2-10, and H2-11), 1.11 (1H, m, H-9b), 0.89 (3H, t, J = 6.8 Hz, H3-12), 1.76 (3H, s, H3-13), 2.07 (3H, s, H3-14); 13C NMR (125 MHz, CDCl3) δ 204.7 (C, C-1), 139.2 (C, C-2), 167.2 (C, C-3), 92.4 (C, C-4), 46.7 (CH, C-5), 32.6 (CH2, C-6), 174.5 (C, C-7), 34.7 (CH2, C-8), 23.4 (CH2, C-9), 31.9 (CH2, C-10), 22.6 (CH2, C-11), 14.1 (CH3, C-12), 8.3 (CH3, C-13), 12.4 (CH3, C-14); HRESIMS m/z 253.1440 [M − H]+ (calcd for C14H21O4, 253.1445).
ent-sinulolide F ((+)-11): colorless oil; [α +9.8 (c 0.04, MeOH); UV (MeOH) λmax (log ε) 229 (2.93) nm; ECD (MeOH) λmax (Δε) 208 (90.9), 226 (−28.5), 246 (26.9) nm; IR (KBr) νmax 3385, 2927, 1722, 1635, 1480, 1377, 1145 cm−1; 1H NMR (500 MHz, CDCl3) δ 2.91 (1H, dd, J = 19.1, 12.5 Hz, H-5), 3.06 (1H, dd, J = 12.5, 4.6 Hz, H-6a), 2.59 (1H, dd, J = 19.1, 4.6 Hz, H-6b), 3.60 (1H, dd, J = 8.8, 2.9 Hz, H-8), 1.52 (1H, m, H-9a), 1.34 (5H, m, H-9b, H2-10, H2-11), 0.92 (3H, t, J = 7.1 Hz, H3-12), 1.78 (3H, s, H3-13), 2.13 (3H, s, H3-14), 3.39 (3H, s, 8-OCH3); 13C NMR (500 MHz, CDCl3) δ 204.6 (C, C-1), 140.1 (C, C-2), 165.8 (C, C-3), 93.0 (C, C-4), 44.2 (CH, C-5), 32.3 (CH2, C-6), 174.4 (C, C-7), 80.9 (CH, C-8), 30.7 (CH2, C-9), 28.8 (CH2, C-10), 23.0 (CH2, C-11), 14.2 (CH3, C-12), 8.5 (CH3, C-13), 13.2 (CH3, C-14), 60.4 (CH3, 8-OCH3); HRESIMS m/z 307.1511 [M + Na]+ (calcd for C15H24O5Na, 307.1516).
ent-sinulolide H ((+)-13): colorless oil, [α +8.6 (c 0.01, MeOH); UV (MeOH) λmax (log ε) 233 (2.10) nm; IR (KBr) νmax 2815, 2700, 1750, 1485, 1311, 1084 cm−1; 1H NMR (500 MHz, CDCl3) δ 2.52 (1H, m, H-5a), 2.38 (1H, m, H-5b), 2.30 (1H, m, H-6a), 1.96 (1H, m, H-6b), 1.85 (3H, s, H3-8), 1.88 (3H, s, H3-9), 3.08 (3H, s, 4-OCH3), 3.66 (3H, s, 7-OCH3); 13C NMR (125 MHz, CDCl3) δ 171.4 (C, C-1), 127.8 (C, C-2), 155.9 (C, C-3), 109.0 (C, C-4), 28.0 (CH2, C-5), 31.4 (CH2, C-6), 173.4 (C, C-7), 8.6 (CH3, C-8), 10.9 (CH3, C-9), 50.4 (CH3, 4-OCH3), 51.9 (CH3, 7-OCH3); HRESIMS m/z 251.0895 [M + Na]+ (calcd for C11H16O5Na, 251.0890).
Molestin E (14): yellow oil; [α +13.2 (c 0.2, MeOH); UV (MeOH) λmax (log ε) 264 (2.96) nm; IR (KBr) νmax 3481, 1756, 1721, 1580, 1396, 1185 cm−1; 1H and 13C NMR data, see Table 3; HRESIMS m/z 466.2063 [M + NH4]+ (calcd for C23H32O9N, 466.2072).
3.4. ECD Calculations of Compounds 1–5
The quantum chemical calculations were performed by using the density functional theory (DFT) as implemented in Gaussian 09 [42]. The initial structures of compounds 1–5 were built with Spartan 10 software and all trial structures were first minimized based on molecular mechanics calculations. Conformational searches were performed by Spartan 10 software using Merck Molecular Force Field (MMFF), and conformers occurring within a 10 kcal/mol energy window from the global minimum were chosen for geometry optimization in the gas phase with the DFT method at the B3LYP/6-31G (d,p) level. The stable conformations of 1–5 were calculated for ECD spectra using TD-DFT method with the basis set RB3LYP/ DGDZVP [23]. Solvent effects of MeOH were evaluated at the same DFT level by using the SCRF/PCM method. The ECD spectra were combined after Boltzmann weighting according to their population contribution (Figure S84–S88, Supplementary Materials).
3.5. Cytotoxicity Assay
In vitro cytotoxicities were determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H- tetrazolium bromide) colorimetric method [43] against K562 and Jurkat cell lines, and SRB (Sulforhodamine B) method [44] against HeLa, HCT-116, and BEL-7402 cell lines, with adriamycin as a positive control, and compounds with IC50 values > 50 μM were considered to be inactive in cytotoxicity assays.
3.6. PTP1B Inhibitory Assay
The PTP1B inhibitory activities of the isolates were evaluated by the method of pNPP [45], using sodium orthovanadate as a positive control.
4. Conclusions
During the first chemical and biological investigation on the soft coral S. cf. molesta, ten new compounds (1–5, (+)-9–(+)-11, (+)-13, and 14) and 14 known related metabolites (6–8, (–)-9–(–)-11, (±)-12, (–)-13, 15–19) were obtained. Since 1975, only seven guaiane-type sesquiterpenes and two secoguaiane-type sesquiterpenes have been isolated from the soft corals of the genus Sinularia, including the Red Sea soft corals S. gardineri [46] and S. terspilli [47], the Formosan soft corals S. leptoclados [48] and S. gibberosa [5], the Hainan Islands soft corals S. numerosa [49] and Sinularia sp. [50], and the South China Sea soft corals Sinularia sp. [51,52]. Our present study on the soft coral S. cf. molesta collected from the Paracel Islands of the South China Sea yielded two new secoguaiane-type sesquiterpenes (1 and 2), and three new guaiane-type sesquiterpenes (3–5), which were distinctly different from the africane-type sesquiterpenes from Moyli Island soft coral S. intacta [19]. Compounds (+)-12 and 15 were first encountered in the genus of Sinularia. That enriched the specific chemo-diversities of the genus Sinularia. The cytotoxicities of three cembranoid diterpenes (14, 17, and 18) and PTP1B inhibitory activities of two guaiane-type sesquiterpenes (4, 5) and one furanosesquiterpene (8) were unveiled for pharmaceutical potentials.
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-3397/16/12/517/s1. The Supplementary Materials including 1D and 2D NMR, and HRESIMS spectra of the ten new compounds, chiral HPLC chromatograms of (±)-9−(±)-13, and computational details of 1−5 (PDF) are available online.
Author Contributions
G.-Q.L. and P.-L.L. conceived and designed the experiments; M.-J.C., X.-L.T., and M.-M.J. isolated and identified the compounds. X.H., T.L., L.-Z.L., and G.Z. performed the biological tests; X.-C.L. performed the computational section; L.v.O. identified the soft coral species; M.-J.C. and X.-L.T. wrote the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2018YFC0310903), the National Natural Science Foundation of China (Grant Nos. 41522605, 41476107, 21572210), AoShan Talents Program Supported by Qingdao National Laboratory for Marine Science and Technology (No. 2015ASTP), the Shandong Natural Science Fund for Distinguished Young Scholars (JQ201606), and NSFC-Shandong Joint Fund for Marine Science Research Centers (Grant No. U1606403).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Missakian, M.G.; Burreson, B.J.; Scheuer, P.J. Pukalide, a furanocembranollide from the soft coral Sinularia abrupta. Tetrahedron 1975, 31, 2513–2515. [Google Scholar] [CrossRef]
- Tursch, B.; Braekman, J.C.; Daloze, D.; Herin, M.; Karlsson, R.; Losman, D. Sinulariolide, a new cembranolid diterpene from the soft coral Sinularia flexibilis. Tetrahedron 1975, 31, 129–133. [Google Scholar] [CrossRef]
- Chen, W.T.; Li, J.; Wang, J.R.; Li, X.Y.; Guo, Y.W. Structural diversity of terpenoids in the soft coral Sinularia flexibilis, evidenced by a collection from the South China Sea. RSC Adv. 2015, 5, 23973–23980. [Google Scholar] [CrossRef]
- Huang, C.Y.; Tseng, Y.J.; Chokkalingam, U.; Hwang, T.L.; Hsu, C.H.; Dai, C.F.; Sung, P.J.; Sheu, J.H. Bioactive isoprenoid-derived natural products from a Dongsha atoll soft coral Sinularia erecta. J. Nat. Prod. 2016, 79, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.F.; Kuo, Y.H.; Dai, C.F.; Sheu, J.H. Oxygenated terpenoids from a Formosan soft coral Sinularia gibberosa. J. Nat. Prod. 2005, 68, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.P.; Cheng, S.M.; Fu, W.T.; Zhao, M.; Li, X.B.; Cai, Y.P.; Dong, J.Y.; Huang, K.X.; Gustafson, K.R.; Yan, P.C. Structurally diverse metabolites from the soft coral Sinularia verruca collected in the South China Sea. J. Nat. Prod. 2016, 79, 1124–1131. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Tai, S.H.; Wu, Y.H.; Sheu, J.H. Sinugrandisterols A–D, trihydroxysteroids from the soft coral Sinularia grandilobata. Steroids 2007, 72, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.X.; Tang, X.L.; van Ofwegen, L.; Xue, L.; Song, W.J.; Li, P.L.; Li, G.Q. Cyclopentenone Derivatives and Polyhydroxylated Steroids from the Soft Coral Sinularia acuta. Chem. Biodivers. 2015, 12, 273–283. [Google Scholar] [CrossRef]
- Ojika, M.; Islam, M.K.; Shintani, T.; Zhang, Y.; Okamoto, T.; Sakagami, Y. Three new cytotoxic acylspermidines from the soft coral, Sinularia sp. Biosci. Biotechnol. Biochem. 2003, 67, 1410–1412. [Google Scholar] [CrossRef]
- Su, J.Y.; Kuang, Y.Y.; Zeng, L.M.; Li, H. New tetracyclic diterpenoid and new ceramides from the soft coral Sinularia conferta. J. Asian Nat. Prod. Res. 2006, 7, 107–113. [Google Scholar] [CrossRef]
- Yang, B.; Wei, X.Y.; Huang, J.X.; Lin, X.P.; Liu, J.; Liao, S.R.; Wang, J.F.; Zhou, X.F.; Wang, L.S.; Liu, Y.H. Sinulolides A–H, new cyclopentenone and butenolide derivatives from soft coral Sinularia sp. Mar. Drugs 2014, 12, 5316–5327. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Y.; Yu, S.J.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W.H. Sinularones A–I, new cyclopentenone and butenolide derivatives from a marine soft coral Sinularia sp. and their antifouling activity. Mar. Drugs 2012, 10, 1331–1344. [Google Scholar] [CrossRef]
- Huang, C.Y.; Su, J.H.; Liaw, C.C.; Sung, P.J.; Chiang, P.L.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. Bioactive steroids with methyl ester group in the side chain from a reef soft coral Sinularia brassica cultured in a tank. Mar. Drugs 2017, 15, 280. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.J.; Tseng, Y.J.; Huang, C.Y.; Wen, Z.H.; Dai, C.F.; Sheu, J.H. Cytotoxic and anti-inflammatory diterpenoids from the Dongsha Atoll soft coral Sinularia flexibilis. Tetrahedron 2012, 68, 244–249. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, C.Y.; Lin, Y.F.; Wen, Z.H.; Su, J.H.; Kuo, Y.H.; Chiang, M.Y.; Sheu, J.H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 2008, 71, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Ashimine, R.; Miyazato, H.; Taira, J.; Ueda, K. Endoperoxy and hydroperoxy cadinane-type sesquiterpenoids from an Okinawan soft coral, Sinularia sp. Arch. Pharm. Res. 2016, 39, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.W.; Geng, Z.F.; Deng, Z.W.; van Ofwegen, L.; Proksch, P.; Lin, W.H. Cembranoids from the soft coral Sinularia rigida with antifouling activities. J. Agric. Food Chem. 2013, 61, 4585–4592. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shao, C.L.; Qi, X.; Li, X.B.; Li, J.; Sun, L.L.; Wang, C.Y. Polyoxygenated sterols from the South China Sea soft coral Sinularia sp. Mar. Drugs 2012, 10, 1422–1432. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Gowri, P.M.; Krishna Murthy, M.V.R. New sesquiterpenoids from the soft coral Sinularia intacta of the Indian Ocean. J. Nat. Prod. 1999, 62, 1600–1604. [Google Scholar] [CrossRef]
- Jiang, M.M.; Tang, X.L.; Li, P.L.; Li, G.Q. Study on chemical constituents of the Xisha soft coral Sinalaria cf. molesta. Zhongguo Haiyang Yaowu 2016, 35, 76–80. [Google Scholar]
- Wang, Q.; Tang, X.L.; Luo, X.C.; de Voogd, N.J.; Li, P.L.; Li, G.Q. (+)- and (−)-Spiroreticulatine, a pair of unusual spiro bisheterocyclic quinoline-imidazole alkaloids from the South China Sea sponge Fascaplysinopsis reticulata. Org. Lett. 2015, 17, 3458–3461. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Song, W.J.; Tang, X.L.; de Voogd, N.J.; Wang, Q.; Chu, M.J.; Li, P.L.; Li, G.Q. Alkaloids and polyketides from the South China Sea sponge Agelas aff. nemoechinata. RSC Adv. 2017, 7, 14323–14329. [Google Scholar] [CrossRef]
- McCann, D.M.; Stephens, P.J. Determination of absolute configuration using density functional theory calculations of optical rotation and electronic circular dichroism: Chiral Alkenes. J. Org. Chem. 2006, 71, 6074–6098. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, J.; Suzuki, N.; Tanaka, Y. New guaiane-type sesquiterpenoid glycosides from Torillis japonica fruit. Chem. Pharm. Bull. 1998, 46, 1743–1747. [Google Scholar] [CrossRef]
- Nakashima, K.; Oyama, M.; Ito, T.; Witono, J.R.; Darnaedi, D.; Tanaka, T.; Murata, J.; Iinuma, M. Novel zierane- and guaiane-type sesquiterpenes from the root of Melicope denhamii. Chem. Biodivers. 2012, 9, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Choi, S.; Baek, N.; Kim, S.; Eun, J.S.; Yang, J.H.; Kim, D.K. Guaiane Sesquiterpenoids from Torilis japonica and their cytotoxic effects on human cancer cell Lines. Arch. Pharm. Res. 2006, 29, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Kim, J.A.; Min, B.S.; Jang, T.S.; Na, M.; Lee, S.H. Guaiane sesquiterpenoids isolated from the fruits of Torilis japonica and their cytotoxic activity. Helv. Chim. Acta 2010, 93, 692–697. [Google Scholar] [CrossRef]
- Yang, X.L.; Li, Z.Z. New spiral γ-lactone enantiomers from the plant endophytic fungus Pestalotiopsis foedan. Molecules 2013, 18, 2236–2242. [Google Scholar] [CrossRef]
- Lin, W.J.; Wu, T.Y.; Su, T.R.; Wen, Z.H.; Chen, J.J.; Fang, L.S.; Wu, Y.C.; Sung, P.J. Terpenoids from the octocoral Sinularia gaweli. Int. J. Mol. Sci. 2015, 16, 19508–19517. [Google Scholar] [CrossRef]
- Zhang, C.X.; Zhu, C.C.; Yan, S.J.; Li, J.; Su, J.Y.; Liang, Y.J.; Yang, X.P.; Zheng, K.C.; Zeng, L.M. Two new sesquiterpenoids from the soft coral Sinularia polydactyla (Ehreberg). J. Asian Nat. Prod. Res. 2008, 10, 277–280. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; de Silva, E.D.; de Costa, M.S.L.; Djura, P.J.; Mahendran, M.; Tapiolas, D.M. Studies of Australian soft corals. XXXI Novel furanosesquiterpenes from several Sinularian soft corals (Coelenterata, Octocorallia, Alcyonacea). Aust. J. Chem. 1983, 36, 371–376. [Google Scholar] [CrossRef]
- Díaz-Marrero, A.R.; Porras, G.; Cueto, M.; D’Croz, L.; Lorenzo, M.; San-Martín, A.; Darias, J. Leptogorgolide, a biogenetically interesting 1,4-diketo-cembranoid that reinforces the oxidation profile of C-18 as taxonomical marker for octocorals. Tetrahedron 2009, 65, 6029–6033. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Mitchell, S.J.; Mulder, J.; Stokie, G.J. Studies of Australian Soft Corals. IX A novel nor-diterpene from the soft coral Sinularia leptoclados. Aust. J. Chem. 1978, 31, 2049–2056. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Shiue, R.T.; Wang, G.H.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Five novel norcembranoids from Sinularia leptoclados and S. parva. Tetrahedron 2003, 59, 7337–7344. [Google Scholar] [CrossRef]
- Shoji, N.; Umeyama, A.; Arihara, S. a novel norditerpenoid from the okinawan soft coral Sinularia sp. J. Nat. Prod. 1993, 56, 1651–1653. [Google Scholar] [CrossRef]
- Sheu, J.H.; Ahmed, A.F.; Shiue, R.T.; Dai, C.F.; Kuo, Y.H. Scabrolides, A-D, Four new norditerpenoids isolated from the soft coral Sinularia scabra. J. Nat. Prod. 2002, 65, 1904–1908. [Google Scholar] [CrossRef]
- Saifudin, A.; Tanaka, K.; Kadota, S.; Tezuka, Y. Sesquiterpenes from the rhizomes of Curcuma heyneana. J. Nat. Prod. 2013, 76, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Na, M.; Hwang, I.H.; Lee, S.H.; Bae, E.Y.; Kim, B.Y.; Ahn, J.S. Isolation of betulinic acid, its methyl ester and guaiane sesquiterpenoids with protein tyrosine phosphatase 1B inhibitory activity from the roots of Saussurea lappa C.B.Clarke. Molecules 2009, 14, 266–272. [Google Scholar] [CrossRef]
- Abdjul, D.B.; Yamazaki, H.; Kanno, S.; Wewengkang, D.S.; Rotinsulu, H.; Sumilat, D.A.; Ukai, K.; Kapojos, M.M.; Namikoshi, M. Furanoterpenes, new types of protein tyrosine phosphatase 1B inhibitors, from two Indonesian marine sponges, Ircinia and Spongia spp. Bioorg. Med. Chem. Lett. 2017, 27, 1159–1161. [Google Scholar] [CrossRef]
- Chen, P.J.; Cai, S.P.; Huang, C.; Meng, X.M.; Li, J. Protein tyrosine phosphatase 1B (PTP1B): A key regulator and therapeutic target in liver diseases. Toxicology 2015, 337, 10–20. [Google Scholar] [CrossRef]
- Qian, S.; Zhang, M.; He, Y.Y.; Wang, W.; Liu, S.Y. Recent advances in the development of protein tyrosine phosphatase 1B inhibitors for Type 2 diabetes. Future Med. Chem. 2016, 8, 1239–1258. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hong, D.; Zhou, Y.; Zhang, Y.; Shen, Q.; Li, J.Y.; Hu, L.H.; Li, J. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. BBA-Gen. Subj. 2006, 1760, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, K.A.; Hamann, M.T. A new norcembranoid dimer from the Red Sea soft coral Sinularia gardineri. J. Nat. Prod. 1996, 59, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.; Radwan, M.M.; Ma, G.; Mohamed, T.A.; Seliem, M.A.; Thabet, M.; ElSohly, M.A. Bioactive sterols and sesquiterpenes from the Red Sea soft coral Sinularia terspilli. Med. Chem. Res. 2017, 26, 1647–1652. [Google Scholar] [CrossRef]
- Su, J.H.; Chiang, M.Y.; Wen, Z.H.; Dai, C.F.; Hsu, C.H.; Sheu, J.H. Sesquiterpenoids from the Formosan soft coral Sinularia leptoclados. Chem. Pharm. Bull. 2010, 58, 250–253. [Google Scholar] [CrossRef]
- Qin, M.L.; Li, X.M.; Wang, B.G. Chemical constituents of sesquiterpenes in soft coral Sinularia numerosa. Haiyang Yu Huzhao 2009, 40, 540–544. [Google Scholar]
- Zhang, G.W.; Ma, X.Q.; Su, J.Y.; Zhang, K.; Kurihara, H.; Yao, X.H.; Zeng, L.M. Two new bioactive sesquiterpenes from the soft coral Sinularia sp. Nat. Prod. Res. 2006, 20, 659–664. [Google Scholar] [CrossRef]
- Jia, R.; Guo, Y.W.; Hou, H.X.; Mollo, E.; Cimino, G. Chemical constituents of Sinularia sp. from the South China Sea. Zhongguo Tianran Yaowu 2003, 1, 193–195. [Google Scholar]
- Qin, G.F.; Tang, X.L.; Sun, Y.T.; Luo, X.C.; Zhang, J.; van Ofwegen, L.; Sung, P.T.; Li, P.L.; Li, G.Q. Terpenoids from the soft coral Sinularia sp. collected in Yongxing Island. Mar. Drugs 2018, 16, 127. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).