Mutagenesis Studies and Structure-function Relationships for GalNAc/Gal-Specific Lectin from the Sea Mussel Crenomytilus grayanus
Abstract
1. Introduction
2. Results
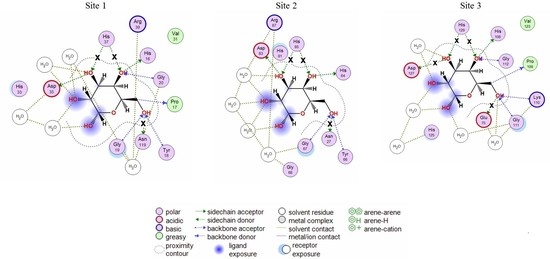
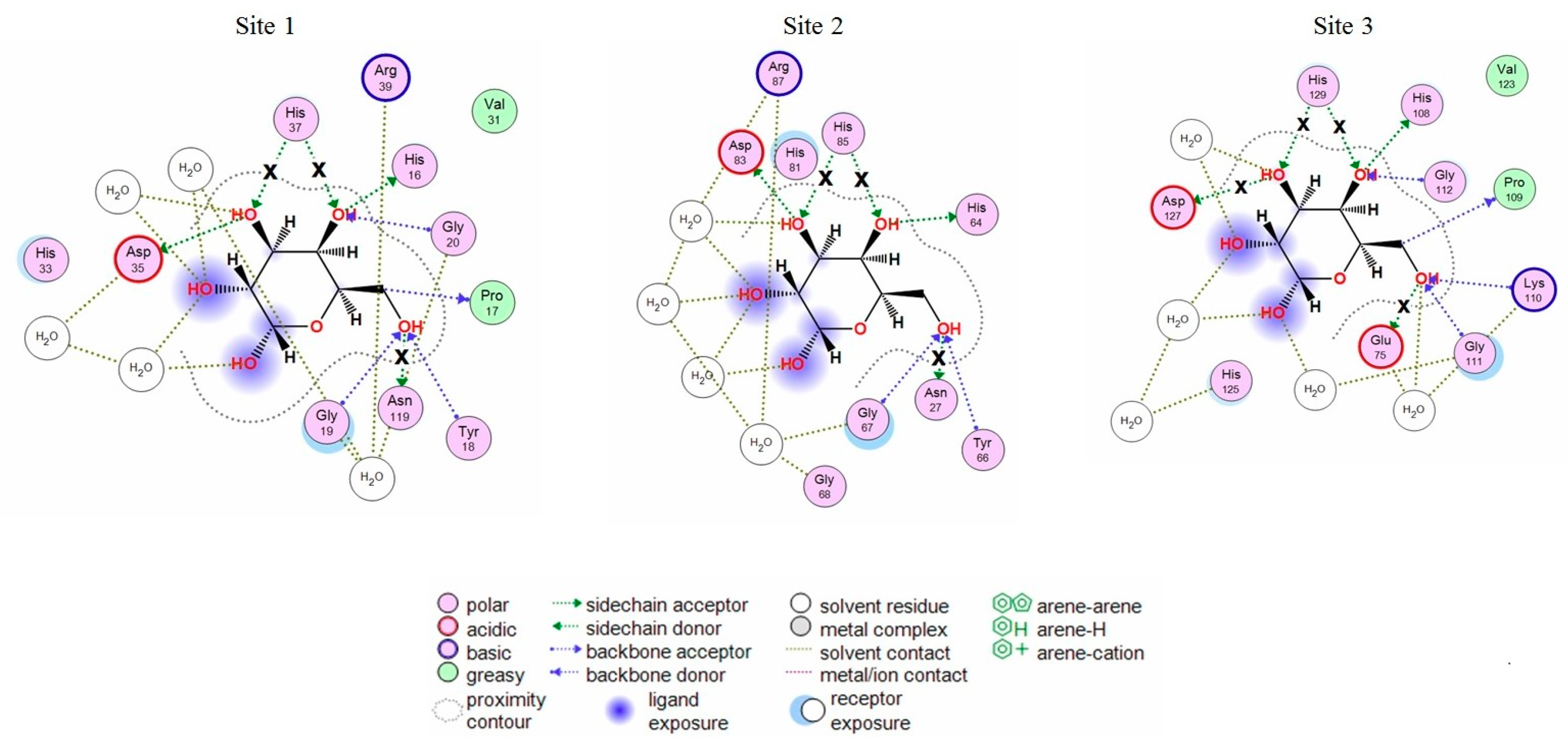
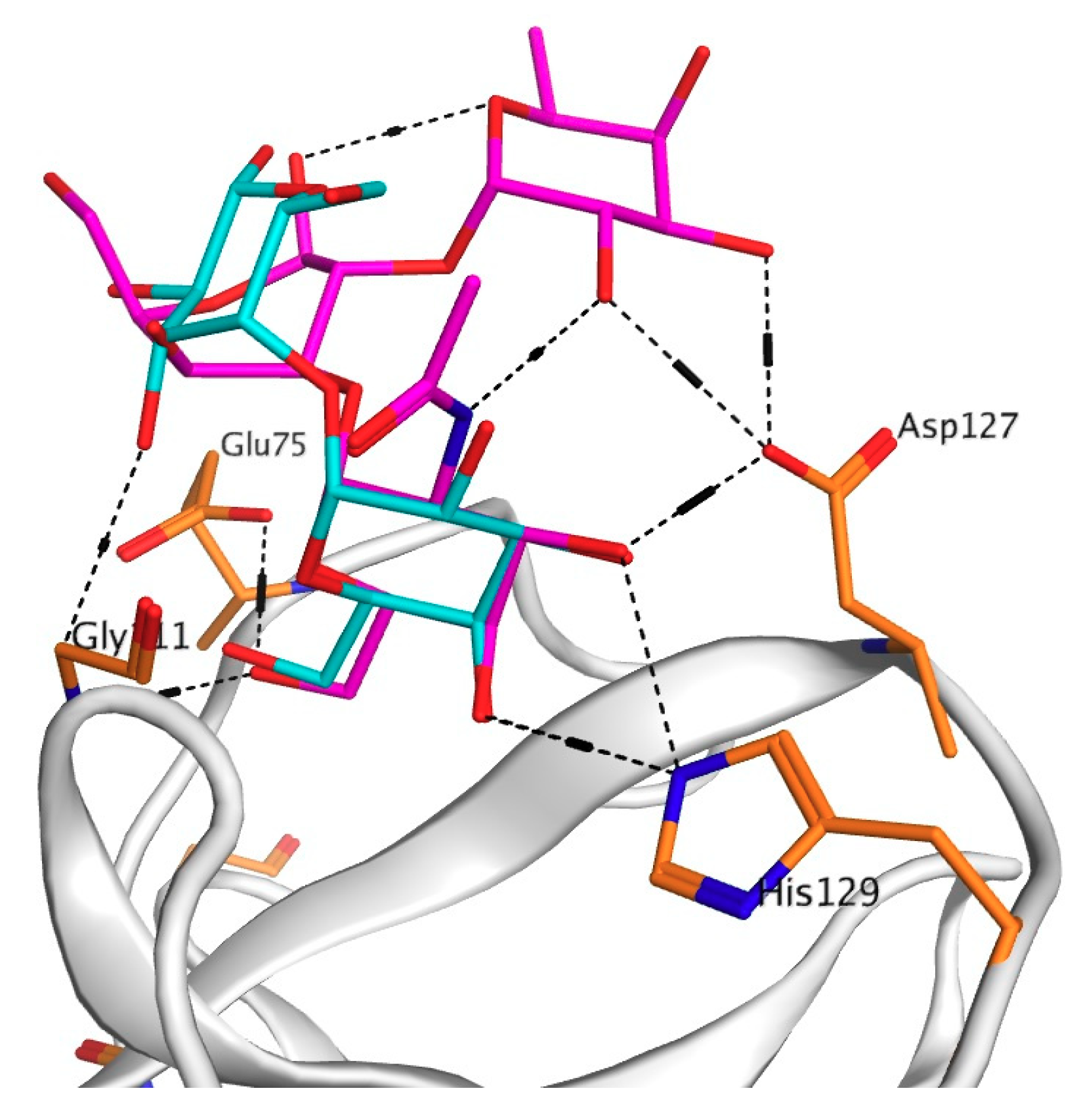
2.1. Analysis of CGL Contacts with Galactose/Galactosamine for Mutagenesis
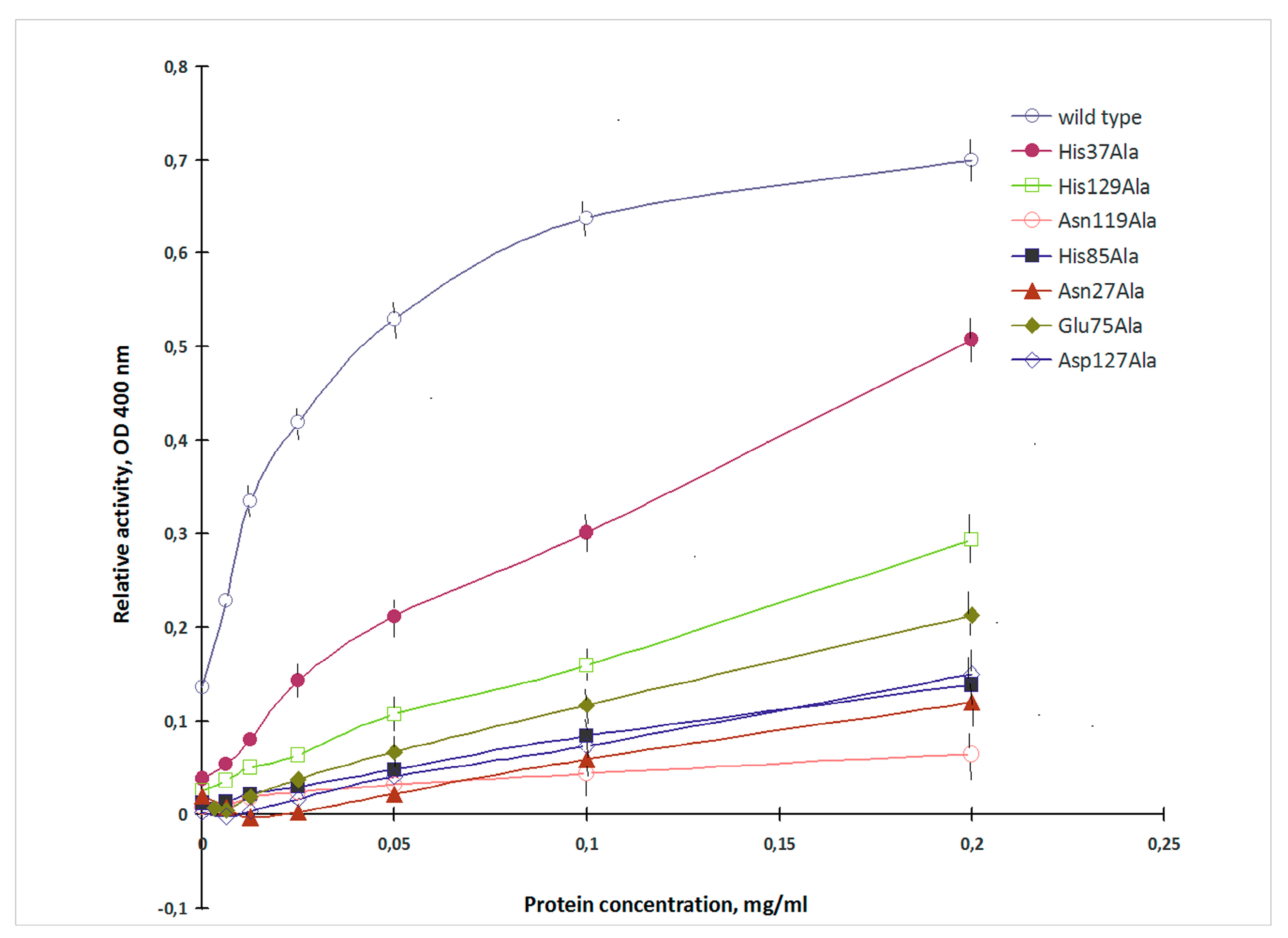
2.2. Mutagenesis Studies
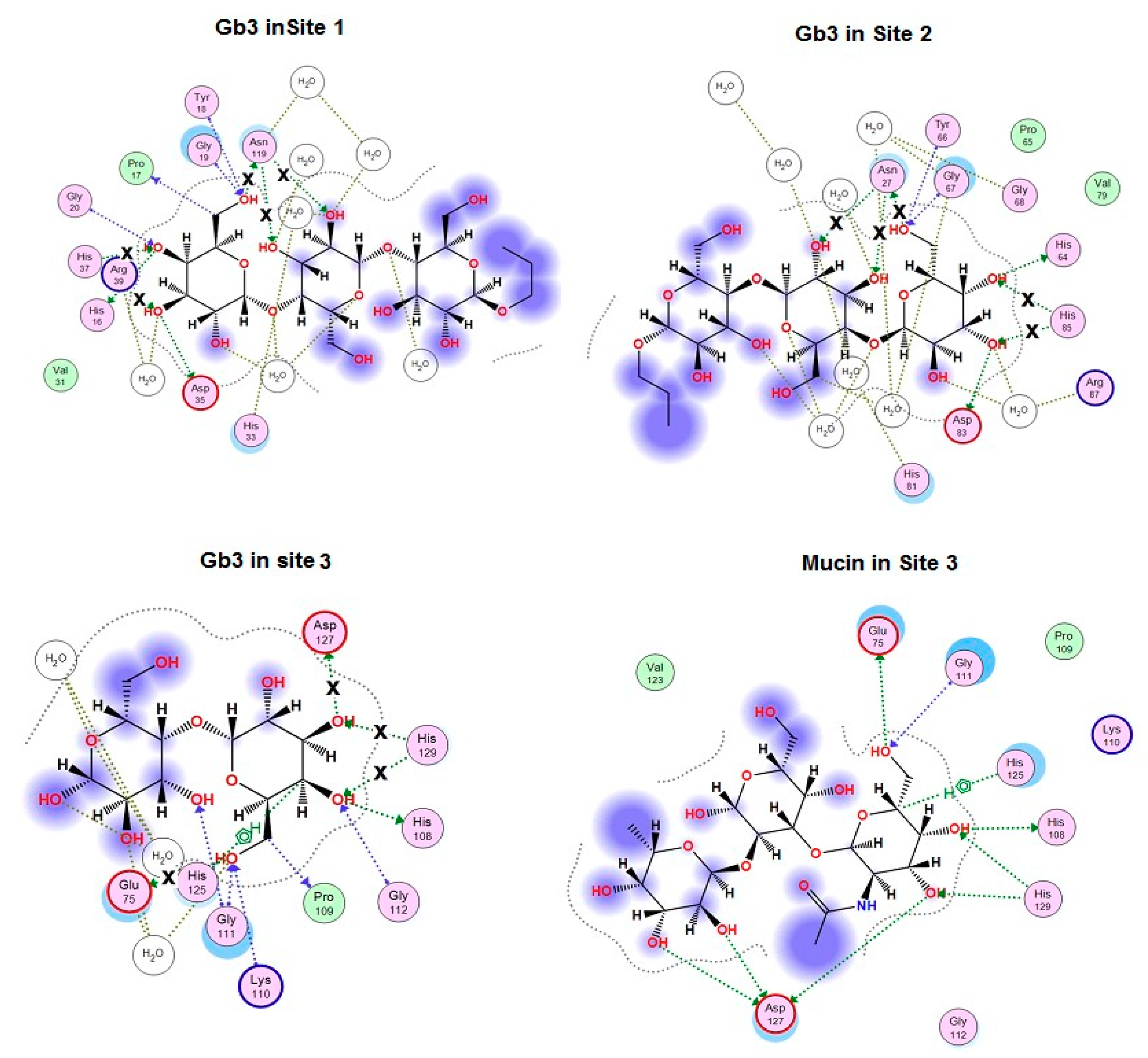
2.3. Analysis of Contacts in Complexes of CGL and Its Mutants with Oligosaccharides
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis of Contacts between CGL and Ligands and Mutagenesis
4.2. Construction of Recombinant Plasmids, Protein Expression and Purification
4.3. Lectin Activity Assay
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharon, N.; Lis, H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology 2004, 14, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, D.C. Animal lectins: A historical introduction and overview. Biochim. Biophys. Acta 2002, 1572, 187–197. [Google Scholar] [CrossRef]
- Iwanaga, S.; Lee, B.L. Recent advances in the innate immunity of invertebrate animals. J. Biochem. Mol. Biol. 2005, 38, 128–150. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R.; Ahmed, H. Animal Lectins: A Functional View; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9780849372698. [Google Scholar]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H. (Eds.) Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015–2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK310274 (accessed on 5 September 2017).
- Classification of Animal Lectins. Part I: Structures and Functions of Animal Lectins. Available online: http://www.imperial.ac.uk/research/animallectins/ctld/lectins.html (accessed on 1 January 2014).
- Fujimoto, Z.; Tateno, H.; Hirabayashi, J. Lectin Structures: classification based on the 3-D structures. In Lectins; Hirabayashi, J., Ed.; Methods in Molecular Biology (Methods and Protocols); Humana Press: New York, NY, USA, 2014; Volume 1200. [Google Scholar]
- Belogortseva, N.I.; Molchanova, V.I.; Kurika, A.V.; Skobun, A.S.; Glazkova, V.E. Isolation and characterization of new GalNAc/Gal-specific lectin from the sea mussel Crenomytilus grayanus. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998, 119, 45–50. [Google Scholar] [CrossRef]
- Kovalchuk, S.N.; Chikalovets, I.V.; Chernikov, O.V.; Molchanova, V.I.; Li, W.; Rasskazov, V.A.; Lukyanov, P.A. cDNA cloning and structural characterization of a lectin from the mussel Crenomytilus grayanus with a unique amino acid sequence and antibacterial activity. Fish. Shellfish Immunol. 2013, 35, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, S.N.; Golotin, V.A.; Balabanova, L.A.; Buinovskaya, N.S.; Likhatskaya, G.N.; Rasskazov, V.A. Carbohydrate-binding motifs in a novel type lectin from the sea mussel Crenomytilus grayanus: Homology modeling study and site-specific mutagenesis. Fish. Shellfish Immunol. 2015, 47, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Jakób, M.; Lubkowski, J.; O’Keefe, B.R.; Wlodawer, A. Structure of a lectin from the sea mussel Crenomytilus grayanus (CGL). Acta Crystallogr. F Struct. Biol. Commun. 2015, 71, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.H.; Chien, C.T.; Wu, H.Y.; Huang, K.F.; Wang, I.; Ho, M.R.; Tu, I.F.; Lee, I.M.; Li, W.; Shih, Y.L.; et al. Multivalent marine lectin from Crenomytilus grayanus possesses anti-cancer activity through recognizing globotriose Gb3. J. Am. Chem. Soc. 2016, 138, 4787–4795. [Google Scholar] [CrossRef] [PubMed]
- Terada, D.; Kawai, F.; Noguchi, H.; Unzai, S.; Hasan, I.; Fujii, Y.; Tame, J.R.H. Crystal structure of MytiLec, a galactose-binding lectin from the mussel Mytilus galloprovincialis with cytotoxicity against certain cancer cell types. Sci. Rep. 2016, 6, 28344. [Google Scholar] [CrossRef] [PubMed]
- Balabanova, L.; Golotin, V.; Kovalchuk, S.; Bulgakov, A.; Likhatskaya, G.; Son, O.; Rasskazov, V.A. A novel bifunctional hybrid with marine bacterium alkaline phosphatase and Far Eastern holothurian mannan-binding lectin activities. PLoS ONE 2014, 9, e112729. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Dohmae, N.; Takio, K.; Kawsar, S.M.; Matsumoto, R.; Hasan, I.; Koide, Y.; Kanaly, R.A.; Yasumitsu, H.; Ogawa, Y.; et al. A lectin from the mussel Mytilus galloprovincialis has a highly novel primary structure and induces glycan-mediated cytotoxicity of globotriaosyl ceramide expressing lymphoma cells. J. Biol. Chem. 2012, 287, 44772–44783. [Google Scholar] [CrossRef] [PubMed]
- Chikalovets, I.V.; Kovalchuk, S.N.; Litovchenko, A.P.; Molchanova, V.I.; Pivkin, M.V.; Chernikov, O.V. A new Gal/GalNAc-specific lectin from the mussel Mytilus trossulus: Structure, tissue specificity, antimicrobial and antifungal activity. Fish. Shellfish Immunol. 2016, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; Venier, P. An updated molecular basis for mussel immunity. Fish. Shellfish Immunol. 2015, 1, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Hasan, I.; Gerdol, M.; Fujii, Y.; Rajia, S.; Koide, Y.; Yamamoto, D.; Kawsar, S.M.; Ozeki, Y. cDNA and gene structure of MytiLec-1, a bacteriostatic R-type lectin from the Mediterranean mussel (Mytilus galloprovincialis). Mar. Drugs 2016, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- García-Maldonado, E.; Cano-Sánchez, P.; Hernández-Santoyo, A. Molecular and functional characterization of a glycosylated galactose-binding lectin from Mytilus californianus. Fish. Shellfish Immunol. 2017, 66, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Murzin, A.G.; Lesk, A.M.; Chothia, C. Beta-trefoil fold. Patterns of structure and sequence in the Kunitz inhibitors interleukins—1 beta and 1 alpha and fibroblast growth factors. J. Mol. Biol. 1992, 223, 531–543. [Google Scholar] [CrossRef]
- SCOPe 2.06. Available online: http://scop.berkeley.edu/sunid=50352 (accessed on 2 March 2018).
- Molecular Operating Environment (MOE), 2018.01; Chemical Computing Group ULC: Montreal, QC, Canada, 2018.
- Tian, P.; Engelbrektson, A.; Mandrell, R. Two-log increase in sensitivity for detection of norovirus in complex samples by concentration with porcine gastric mucin conjugated to magnetic beads. Appl. Environ. Microbiol. 2008, 74, 4271–4276. [Google Scholar] [CrossRef] [PubMed]
- Labute, P. The generalized born/volume integral (GB/VI) implicit solvent model: Estimation of the free energy of hydration using london dispersion instead of atomic surface area. J. Comput. Chem. 2008, 29, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
| Lectin | Gal Binding ΔE a, kcal/mol | Globotriose Binding ΔE b, kcal/mol | Mucin-Binding Activity c, % |
|---|---|---|---|
| Asn119Ala * | 1.9 | 4.9 | 9 |
| Asn27Ala ** | 2.0 | 5.2 | 17 |
| Asp127Ala *** | 4.0 | 4.3 | 22 |
| His85Ala ** | 3.6 | 3.4 | 20 |
| Glu75Ala *** | 3.4 | 4.7 | 31 |
| His129Ala *** | 3.5 | 3.6 | 43 |
| His37Ala * | 3.5 | 3.1 | 73 |
| Mutation | Sense Primer | Antisense Primer |
|---|---|---|
| Asn27Ala | 5′–AGTAGCAACCCTGCTAACGCCACTAAGTTG−3′ | 5′–GCAGGACCAACTTAGTGGCGTTAGCAGGGT−3′ |
| His37Ala | 5′–GTCCTGCATAGCGATATCGCTGAAAGAATG−3′ | 5′–GGAAGTACATTCTTTCAGCGATATCGCTAT−3′ |
| Glu 75Ala | 5′–AGCTAATCCACCAAATGCCACCAATATGGTTC−3′ | 5′–TGATGCAGAACCATATTGGTGGCATTTGGTG−3′ |
| His85Ala | 5′–GTTCTGCATCAAGATCGTGCTGATCGGGCA−3′ | 5′–GAATAGTGCCCGATCAGCACGATCTTGAT–3′ |
| Asn119Ala | 5′–ATCCCCGAATCCACCGAATGCTACCGAAACAG−3′ | 5′–GTATAACTGTTTCGGTAGCATTCGGTGGAT−3′ |
| Asp127Ala | 5′–CAGTTATACATGGAGCTAAACATGCAGCCA−3′ | 5′−GAATTCCATGGCTGCATGTTTAGCTCCATGTA–3′ |
| His129Ala | 5′–ATACATGGAGATAAAGCTGCAGCCATGGAA−3′ | 5′–CAAAAATGAATTCCATGGCTGCAGCTTTATCT−3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalchuk, S.N.; Buinovskaya, N.S.; Likhatskaya, G.N.; Rasskazov, V.A.; Son, O.M.; Tekutyeva, L.A.; Balabanova, L.A. Mutagenesis Studies and Structure-function Relationships for GalNAc/Gal-Specific Lectin from the Sea Mussel Crenomytilus grayanus. Mar. Drugs 2018, 16, 471. https://doi.org/10.3390/md16120471
Kovalchuk SN, Buinovskaya NS, Likhatskaya GN, Rasskazov VA, Son OM, Tekutyeva LA, Balabanova LA. Mutagenesis Studies and Structure-function Relationships for GalNAc/Gal-Specific Lectin from the Sea Mussel Crenomytilus grayanus. Marine Drugs. 2018; 16(12):471. https://doi.org/10.3390/md16120471
Chicago/Turabian StyleKovalchuk, Svetlana N., Nina S. Buinovskaya, Galina N. Likhatskaya, Valery A. Rasskazov, Oksana M. Son, Liudmila A. Tekutyeva, and Larissa A. Balabanova. 2018. "Mutagenesis Studies and Structure-function Relationships for GalNAc/Gal-Specific Lectin from the Sea Mussel Crenomytilus grayanus" Marine Drugs 16, no. 12: 471. https://doi.org/10.3390/md16120471
APA StyleKovalchuk, S. N., Buinovskaya, N. S., Likhatskaya, G. N., Rasskazov, V. A., Son, O. M., Tekutyeva, L. A., & Balabanova, L. A. (2018). Mutagenesis Studies and Structure-function Relationships for GalNAc/Gal-Specific Lectin from the Sea Mussel Crenomytilus grayanus. Marine Drugs, 16(12), 471. https://doi.org/10.3390/md16120471