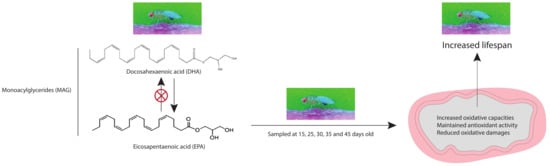
Omega-3 Monoacylglyceride Effects on Longevity, Mitochondrial Metabolism and Oxidative Stress: Insights from Drosophila melanogaster
Abstract
1. Introduction
2. Results
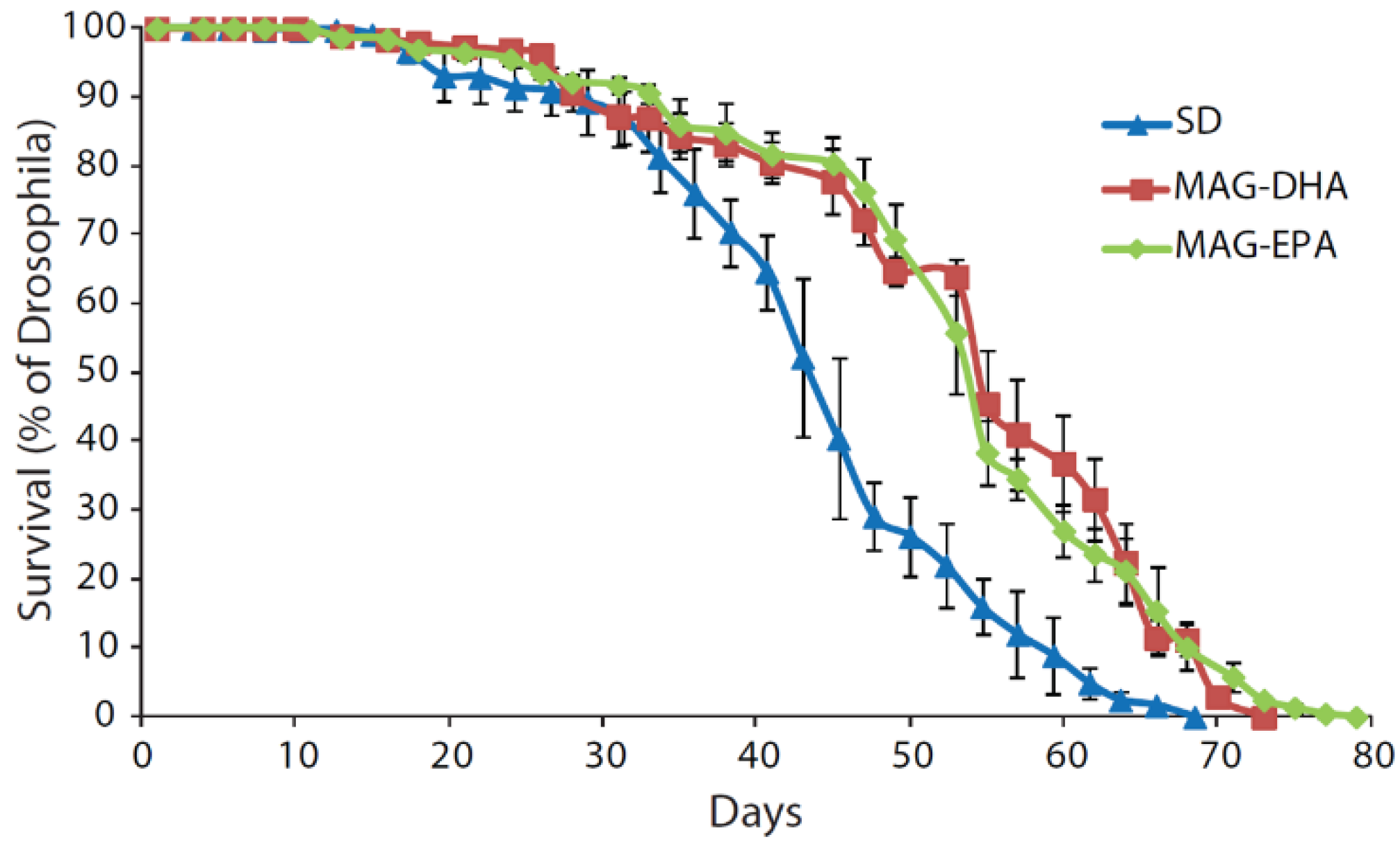
2.1. MAG-DHA and MAG-EPA Extend Longevity in D. melanogaster
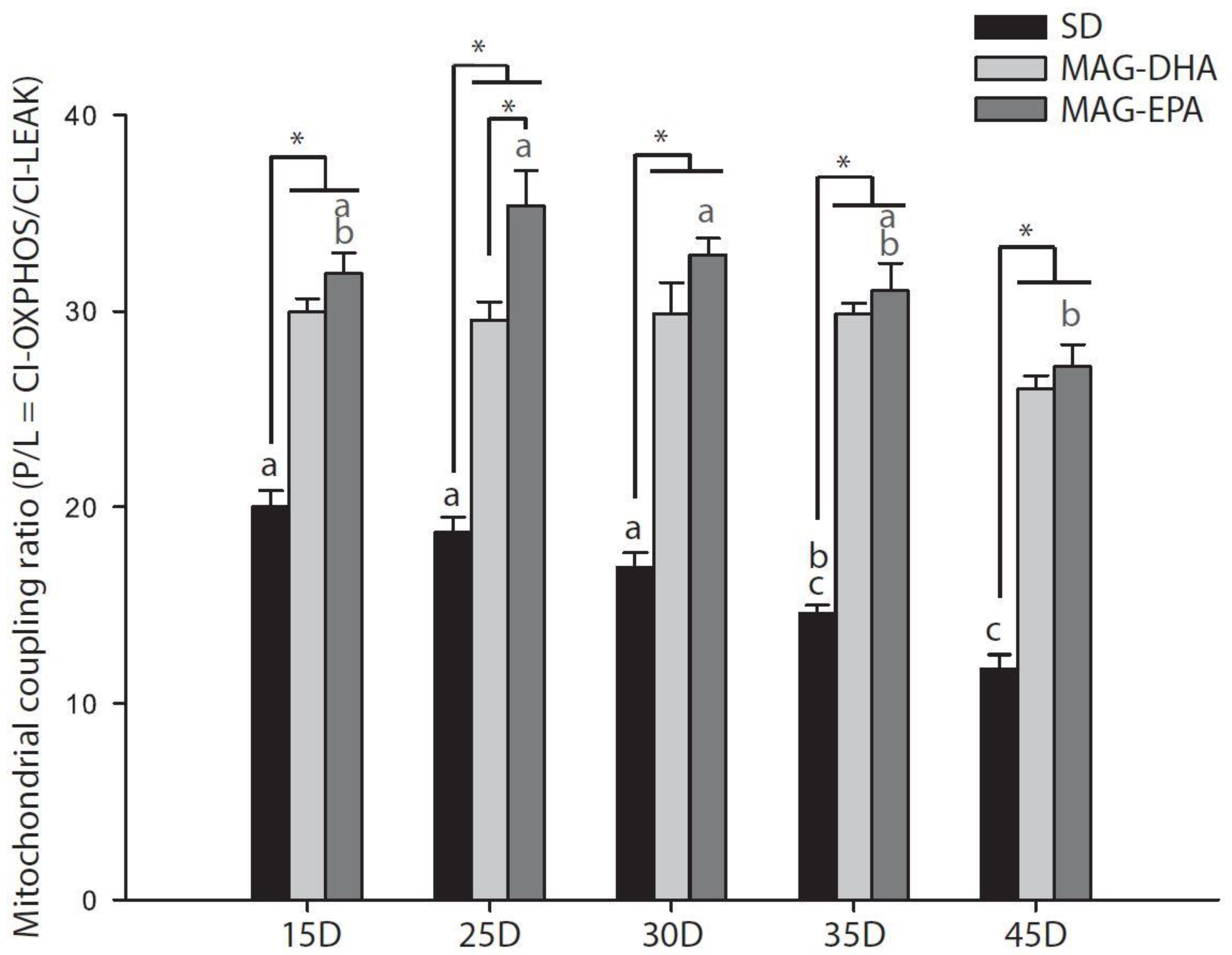
2.2. MAG-DHA and MAG-EPA Decrease Mitochondrial Proton Leak and Ameliorate Mitochondrial Coupling
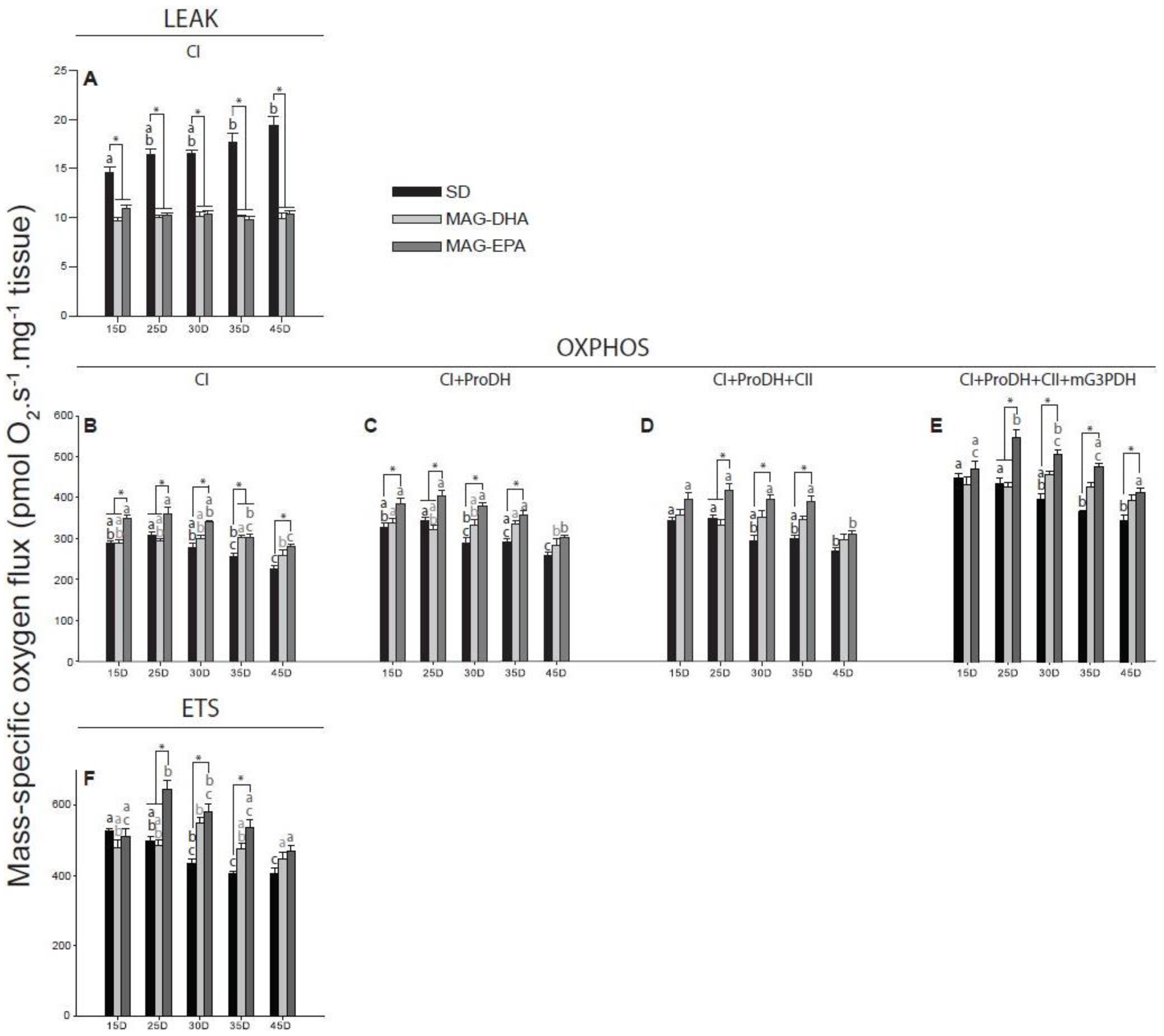
2.3. MAG-EPA Generally Increases Mitochondrial Oxidative Capacities
2.4. Flies Fed MAG-EPA Have Higher Electron Transport System Capacity
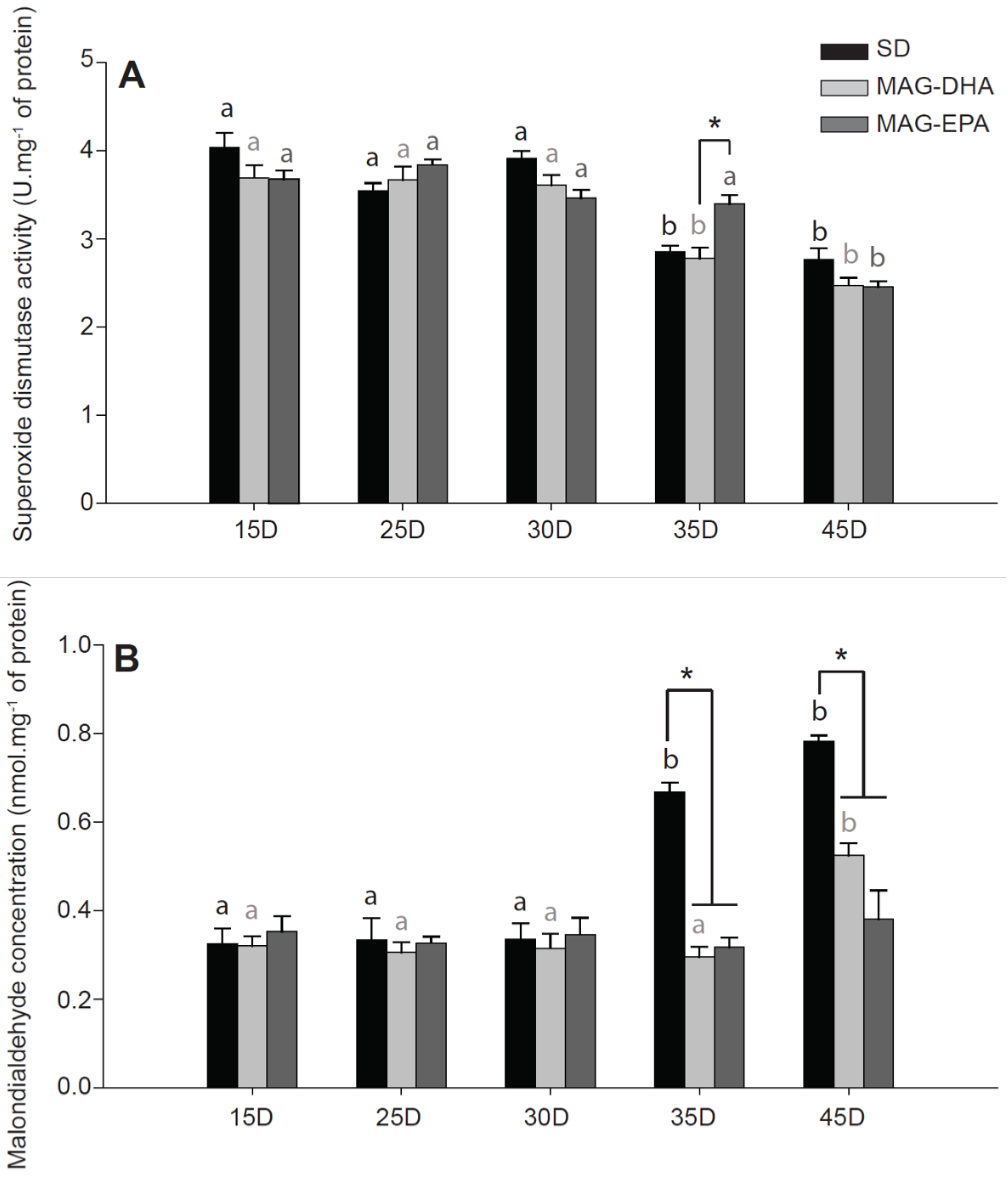
2.5. MAG-EPA Delays the Onset of Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Synthesis of n-3 PUFA Monoacylglycerides
4.2. Drosophila Model and Longevity
4.3. Thorax Permeabilization and Mitochondrial Respiration
4.4. Oxidative Stress Markers
4.4.1. Superoxide Dismutase Activity
4.4.2. MDA Levels
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560S–569S. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Gómez Dumm, I.N.T.; Brenner, R.R. Oxidative desaturation of α-linolenic, linoleic, and stearic acids by human liver microsomes. Lipids 1975, 10, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Monroig, Ó.; Tocher, D.; Navarro, J. Biosynthesis of Polyunsaturated Fatty Acids in Marine Invertebrates: Recent Advances in Molecular Mechanisms. Mar. Drugs 2013, 11, 3998–4018. [Google Scholar] [CrossRef] [PubMed]
- Grønn, M.; Christensen, E.; Hagve, T.A.; Christophersen, B.O. Peroxisomal retroconversion of docosahexaenoic acid (22:6(n−3)) to eicosapentaenoic acid (20:5(n−3)) studied in isolated rat liver cells. Biochim. Biophys. Acta/Lipids Lipid Metab. 1991, 1081, 85–91. [Google Scholar] [CrossRef]
- Park, H.G.; Lawrence, P.; Engel, M.G.; Kothapalli, K.; Brenna, J.T. Metabolic fate of docosahexaenoic acid (DHA; 22:6n−3) in human cells: Direct retroconversion of DHA to eicosapentaenoic acid (20:5n−3) dominates over elongation to tetracosahexaenoic acid (24:6n−3). FEBS Lett. 2016, 590, 3188–3194. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, U.; Taipale, S.J.; Kainz, M.J.; Brett, M.T. Retroconversion of Docosapentaenoic Acid (n-6): An Alternative Pathway for Biosynthesis of Arachidonic Acid in Daphnia magna. Lipids 2014, 49, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Emken, E.A.; Adlof, R.O.; Gulley, R.M. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim. Biophys. Acta/Lipids Lipid Metab. 1994, 1213, 277–288. [Google Scholar] [CrossRef]
- Hussein, N.; Ah-Sing, E.; Wilkinson, P.; Leach, C.; Griffin, B.A.; Millward, D.J. Long-chain conversion of [13 C] linoleic acid and α-linolenic acid in response to marked changes in their dietary intake in men. J. Lipid Res. 2005, 46, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Denis, I.; Potier, B.; Heberden, C.; Vancassel, S. Omega-3 polyunsaturated fatty acids and brain aging. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Beilin, L.J. Omega-3 fatty acids and inflammation. Curr. Atheroscler. Rep. 2004, 6, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A. Omega-3 Fatty Acids in Inflammation and Autoimmune Diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Herbst, E.A.F.; Paglialunga, S.; Gerling, C.; Whitfield, J.; Mukai, K.; Chabowski, A.; Heigenhauser, G.J.F.; Spriet, L.L.; Holloway, G.P. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J. Physiol. 2014, 592, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Jeromson, S.; Gallagher, I.; Galloway, S.; Hamilton, D. Omega-3 Fatty Acids and Skeletal Muscle Health. Mar. Drugs 2015, 13, 6977–7004. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Lalia, A.Z.; Dasari, S.; Pallauf, M.; Fitch, M.; Hellerstein, M.K.; Lanza, I.R. Eicosapentaenoic acid but not docosahexaenoic acid restores skeletal muscle mitochondrial oxidative capacity in old mice. Aging Cell 2015, 14, 734–743. [Google Scholar] [CrossRef] [PubMed]
- de Magalhães, J.P.; Müller, M.; Rainger, G.E.; Steegenga, W. Fish oil supplements, longevity and aging. Aging 2016, 8, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Strong, R.; Miller, R.A.; Antebi, A.; Astle, C.M.; Bogue, M.; Denzel, M.S.; Fernandez, E.; Flurkey, K.; Hamilton, K.L.; Lamming, D.W.; et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 2016, 15, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.T.; de Oliveira Otto, M.C.; Lemaitre, R.N.; McKnight, B.; Song, X.; King, I.B.; Chaves, P.H.; Odden, M.C.; Newman, A.B.; Siscovick, D.S.; et al. Serial circulating omega 3 polyunsaturated fatty acids and healthy ageing among older adults in the Cardiovascular Health Study: Prospective cohort study. BMJ 2018, 363, k4067. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.P.; Nachbar, R.T.; Levada-Pires, A.C.; Hirabara, S.M.; Lambertucci, R.H. Omega-3 fatty acids differentially modulate enzymatic anti-oxidant systems in skeletal muscle cells. Cell Stress Chaperones 2016, 21, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lalia, A.Z.; Dasari, S.; Robinson, M.M.; Abid, H.; Morse, D.M.; Klaus, K.A.; Lanza, I.R. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging 2017, 9, 1096–1129. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.; Fortin, S.; Guibert, C.; Rousseau, E. ω3 and ω6 CYP450 Eicosanoid Derivatives: Key Lipid Mediators in the Regulation of Pulmonary Hypertension. In Pulmonary Hypertension; InTech: Munich, Germany, 2011; pp. 83–108. [Google Scholar]
- Diop, S.B.; Bodmer, R. Drosophila as a model to study the genetic mechanisms of obesity-associated heart dysfunction. J. Cell. Mol. Med. 2012, 16, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.N.S.; Coogan, C.; Chamseddin, K.; Fernandez-Kim, S.O.; Kolli, S.; Keller, J.N.; Bauer, J.H. Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Ansah, E.; Perrimon, N. Modeling metabolic homeostasis and nutrient sensing in Drosophila: Implications for aging and metabolic diseases. Dis. Models Mech. 2014, 7, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Padmanabha, D.; Baker, K.D. Drosophila gains traction as a repurposed tool to investigate metabolism. Trends Endocrinol. Metab. 2014, 25, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Rieder, L.E.; Larschan, E.N. Wisdom from the fly. Trends Genet. 2014, 30, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.R.; Lai, C.Q.; Feng, X.; Parnell, L.D.; Wan, J.B.; Wang, J.D.; Li, D.; Ordovas, J.M.; Kang, J.X. Drosophila lacks C20 and C22 PUFAs. J. Lipid Res. 2010, 51, 2985–2992. [Google Scholar] [CrossRef] [PubMed]
- Vrablik, T.L.; Watts, J.L. Polyunsaturated fatty acid derived signaling in reproduction and development: Insights from Caenorhabditis elegans and Drosophila melanogaster. Mol. Reprod. Dev. 2013, 80, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, J.; Liu, J.; Peng, C.; Suen, Y.L.; Wang, M.; Jiang, Y.; Chen, Z.-Y.; Chen, F. DHA-rich marine microalga Schizochytrium mangrovei possesses anti-ageing effects on Drosophila melanogaster. J. Funct. Foods 2013, 5, 888–896. [Google Scholar] [CrossRef]
- Aguila, J.R.; Suszko, J.; Gibbs, A.G.; Hoshizaki, D.K. The role of larval fat cells in adult Drosophila melanogaster. J. Exp. Biol. 2007, 210, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.E.; Pichaud, N.; Darveau, C.-A. “Alternative” fuels contributing to mitochondrial electron transport: Importance of non-classical pathways in the diversity of animal metabolism. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 224, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Arnold, L.; Orr, W.C. Effect of age on superoxide dismutase, catalase, glutathione reductase, inorganic peroxides, TBA-reactive material, GSH/GSSG, NADPH/NADP+ and NADH/NAD+ in Drosophila melanogaster. Mech. Ageing Dev. 1990, 56, 223–235. [Google Scholar] [CrossRef]
- Faust, J.E.; Verma, A.; Peng, C.; McNew, J.A. An Inventory of Peroxisomal Proteins and Pathways in Drosophila melanogaster. Traffic 2012, 13, 1378–1392. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Else, P.L. Membranes as possible pacemakers of metabolism. J. Theor. Biol. 1999, 199, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Brand, M. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp. Gerontol. 2000, 35, 811–820. [Google Scholar] [CrossRef]
- Serviddio, G.; Bellanti, F.; Romano, A.D.; Tamborra, R.; Rollo, T.; Altomare, E.; Vendemiale, G. Bioenergetics in aging: Mitochondrial proton leak in aging rat liver, kidney and heart. Redox Rep. 2007, 12, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Crescenzo, R.; Bianco, F.; Mazzoli, A.; Giacco, A.; Liverini, G.; Iossa, S. Alterations in proton leak, oxidative status and uncoupling protein 3 content in skeletal muscle subsarcolemmal and intermyofibrillar mitochondria in old rats. BMC Geriatr. 2014, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T.; Mourier, A.; Tain, L.S.; Partridge, L.; Larsson, N.G.; Kühlbrandt, W. Changes of mitochondrial ultrastructure and function during ageing in mice and Drosophila. Elife 2017, 6, e24662. [Google Scholar] [CrossRef] [PubMed]
- Correa, C.C.; Aw, W.C.; Melvin, R.G.; Pichaud, N.; Ballard, J.W.O. Mitochondrial DNA variants influence mitochondrial bioenergetics in Drosophila melanogaster. Mitochondrion 2012, 12, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Mockett, R.J.; Shen, Y.; Orr, W.C.; Sohal, R.S. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J. 2005, 390, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Holmbeck, M.A.; Rand, D.M. Dietary Fatty Acids and Temperature Modulate Mitochondrial Function and Longevity in Drosophila. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Flachs, P.; Horakova, O.; Brauner, P.; Rossmeisl, M.; Pecina, P.; Franssen-van Hal, N.; Ruzickova, J.; Sponarova, J.; Drahota, Z.; Vlcek, C.; et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce β-oxidation in white fat. Diabetologia 2005, 48, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.-Y.; Lee, W.-H.; Tsai, Y.-H.; Chen, C.-Y.; Chao, S.-Y.; Hsieh, R.-H. Functional Modulation of Mitochondria by Eicosapentaenoic Acid Provides Protection against Ceramide Toxicity to C6 Glioma Cells. J. Agric. Food Chem. 2009, 57, 11455–11462. [Google Scholar] [CrossRef] [PubMed]
- Lanza, I.R.; Blachnio-Zabielska, A.; Johnson, M.L.; Schimke, J.M.; Jakaitis, D.R.; Lebrasseur, N.K.; Jensen, M.D.; Sreekumaran Nair, K.; Zabielski, P. Influence of fish oil on skeletal muscle mitochondrial energetics and lipid metabolites during high-fat diet. AJP Endocrinol. Metab. 2013, 304, E1391–E1403. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Taivassalo, T.; Gouspillou, G.; Hepple, R.T. Mitochondria: Isolation, structure and function. J. Physiol. 2011, 589, 4413–4421. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.R.; Nabavi, S.F.; Nabavi, S.M.; Jardim, F.R. Omega-3 polyunsaturated fatty acids and mitochondria, back to the future. Trends Food Sci. Technol. 2017, 67, 76–92. [Google Scholar] [CrossRef]
- Garrel, C.; Alessandri, J.-M.; Guesnet, P.; Al-Gubory, K.H. Omega-3 fatty acids enhance mitochondrial superoxide dismutase activity in rat organs during post-natal development. Int. J. Biochem. Cell Biol. 2012, 44, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Taneda, S.; Honda, K.; Tomidokoro, K.; Uto, K.; Nitta, K.; Oda, H. Eicosapentaenoic acid restores diabetic tubular injury through regulating oxidative stress and mitochondrial apoptosis. Am. J. Physiol. Physiol. 2010, 299, F1451–F1461. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Souza, A.; Couto-Lima, C.A.; Rosa Machado, M.C.; Espreafico, E.M.; Pinheiro Ramos, R.G.; Alberici, L.C. Protective action of Omega-3 on paraquat intoxication in Drosophila melanogaster. J. Toxicol. Environ. Health Part A 2017, 80, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Léveillé, P.; Chouinard-Watkins, R.; Windust, A.; Lawrence, P.; Cunnane, S.C.; Brenna, J.T.; Plourde, M. Metabolism of uniformly labeled 13C-eicosapentaenoic acid and 13C-arachidonic acid in young and old men. Am. J. Clin. Nutr. 2017, 106, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Fortin, S. Polyunsaturated Fatty Acid Monoglycerides, Derivatives, and Uses Thereof. U.S. Patent 8,119,690 B2, 11 December 2012. [Google Scholar]
- Simard, C.J.; Pelletier, G.; Boudreau, L.H.; Hebert-Chatelain, E.; Pichaud, N. Measurement of Mitochondrial Oxygen Consumption in Permeabilized Fibers of Drosophila Using Minimal Amounts of Tissue. J. Vis. Exp. 2018, e57376. [Google Scholar] [CrossRef] [PubMed]
- Pichaud, N.; Ballard, J.W.O.; Tanguay, R.M.; Blier, P.U. Thermal sensitivity of mitochondrial functions in permeabilized muscle fibers from two populations of Drosophila simulans with divergent mitotypes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R48–R59. [Google Scholar] [CrossRef] [PubMed]
- Pichaud, N.; Messmer, M.; Correa, C.C.; Ballard, J.W.O. Diet influences the intake target and mitochondrial functions of Drosophila melanogaster males. Mitochondrion 2013, 13, 817–822. [Google Scholar] [CrossRef] [PubMed]
| Denominator df | Dietary Treatment df = 2 | Age df = 4 | Dietary Treatment * Age df = 8 | |
|---|---|---|---|---|
| Respiration Rates | ||||
| CI-LEAK | 73 | 30.68 *** | 8.57 *** | 4.00 *** |
| CI-OXPHOS | 73 | 16.58 *** | 12.88 *** | 2.88 ** |
| CI+ProDH-OXPHOS | 73 | 8.56 *** | 10.52 *** | 2.51 * |
| CI+ProDH+CII-OXPHOS | 73 | 5.47 ** | 7.88 *** | 2.31 * |
| CI+ProDH+CII+mG3PDH-OXPHOS | 73 | 2.24 | 12.52 *** | 4.67 *** |
| ETS | 73 | 1.90 | 9.83 *** | 6.53 *** |
| Complex IV | 73 | 0.009 | 11.94 *** | 0.55 |
| P/L for Complex I | 73 | 40.71 *** | 10.98 *** | 2.11 * |
| Citrate Synthase Activity | 75 | 13.65 *** | 8.78 *** | 0.64 |
| Oxidative Stress Markers | ||||
| Superoxide dismutase activity | 75 | 4.34 * | 26.03 *** | 4.57 *** |
| Malondialdehyde concentration | 75 | 0.29 | 44.07 *** | 12.11 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Champigny, C.M.; Cormier, R.P.J.; Simard, C.J.; St-Coeur, P.-D.; Fortin, S.; Pichaud, N. Omega-3 Monoacylglyceride Effects on Longevity, Mitochondrial Metabolism and Oxidative Stress: Insights from Drosophila melanogaster. Mar. Drugs 2018, 16, 453. https://doi.org/10.3390/md16110453
Champigny CM, Cormier RPJ, Simard CJ, St-Coeur P-D, Fortin S, Pichaud N. Omega-3 Monoacylglyceride Effects on Longevity, Mitochondrial Metabolism and Oxidative Stress: Insights from Drosophila melanogaster. Marine Drugs. 2018; 16(11):453. https://doi.org/10.3390/md16110453
Chicago/Turabian StyleChampigny, Camille M., Robert P. J. Cormier, Chloé J. Simard, Patrick-Denis St-Coeur, Samuel Fortin, and Nicolas Pichaud. 2018. "Omega-3 Monoacylglyceride Effects on Longevity, Mitochondrial Metabolism and Oxidative Stress: Insights from Drosophila melanogaster" Marine Drugs 16, no. 11: 453. https://doi.org/10.3390/md16110453
APA StyleChampigny, C. M., Cormier, R. P. J., Simard, C. J., St-Coeur, P.-D., Fortin, S., & Pichaud, N. (2018). Omega-3 Monoacylglyceride Effects on Longevity, Mitochondrial Metabolism and Oxidative Stress: Insights from Drosophila melanogaster. Marine Drugs, 16(11), 453. https://doi.org/10.3390/md16110453