Abstract
The hard clam Meretrix meretrix, which has been traditionally used as medicine and seafood, was used in this study to isolate antioxidant peptides. First, a peptide-rich extract was tested for its protective effect against paraquat-induced oxidative stress using the nematode model Caenorhabditis elegans. Then, three novel antioxidant peptides; MmP4 (LSDRLEETGGASS), MmP11 (KEGCREPETEKGHR) and MmP19 (IVTNWDDMEK), were identified and were found to increase the resistance of nematodes against paraquat. Circular dichroism spectroscopy revealed that MmP4 was predominantly in beta-sheet conformation, while MmP11 and MmP19 were primarily in random coil conformation. Using transgenic nematode models, the peptides were shown to promote nuclear translocation of the DAF-16/FOXO transcription factor, a pivotal regulator of stress response and lifespan, and induce the expression of superoxide dismutase 3 (SOD-3), an antioxidant enzyme. Analysis of DAF-16 target genes by real-time PCR reveals that sod-3 was up-regulated by MmP4, MmP11 and MmP19 while ctl-1 and ctl-2 were also up-regulated by MmP4. Further examination of daf-16 using RNA interference suggests that the peptide-increased resistance of C. elegans to oxidative stress was DAF-16 dependent. Taken together, these data demonstrate the antioxidant activity of M. meretrix peptides, which are associated with activation of the stress response factor DAF-16 and regulation of the antioxidant enzyme genes.
1. Introduction
In aging, oxidative stress occurs when the equilibrium between oxidant and antioxidant is disturbed, tipping the redox balance toward an oxidative status. This often causes oxidative modification of cellular constituents such as proteins, nucleotides and lipids, leading to the disruption of cellular metabolism and signaling pathways [1,2]. It has been well established that the accumulation of oxidized biomolecules and associated cell damages are implicated in the pathogenesis of many diseases, including metabolic, cardiovascular and neurodegenerative diseases, most of which are age-related [3,4]. Therefore, protecting normal cells from harmful assaults of either endogenous or exogenous oxidants is an important direction for the prevention and treatment of age-associated diseases.
Reactive oxygen species (ROS) are continually generated as by-products in a number of cellular processes and are also produced when cells are exposed to a variety of external stimuli, such as ultraviolet light, ionizing radiation and other environmental stresses [5,6]. While a homeostatic level of ROS is necessary for normal functions in cells, including intracellular signaling and cellular communication [7], excessive ROS can cause detrimental damage. When an oxidative stress is sensed, the endogenous antioxidants of cells provide a first line of defense against the potentially deleterious effects by reducing the levels of the oxidants. Among the key endogenous antioxidants are superoxide dismutases (SOD), catalases (CAT) and glutathione S-transferases (GST), which work closely to protect cells from ROS-mediated damage [8]. Although cells and organisms can respond to stress signals continuously and attempt to maintain normal physiological balance, it may be necessary in the face of persistent and severe stress to readjust the signal network by exogenous bioactives to restore the homeostasis, otherwise diseases may occur [9].
Bioactive natural products, including peptides, polysaccharides and polyphenols, are increasingly drawing attention to their physiological and pharmacological activities. For example, natural antioxidants are shown to help alleviate oxidative stress, delay aging and alleviate the progression of age-related diseases [10,11,12,13,14,15,16]. Antioxidant peptides, for instance, have been obtained from a variety of natural sources and used as bioactive ingredients, and their bioactivities as well as mechanisms of action are studied in both in vitro and in vivo systems [16,17,18,19]. The Asiatic hard clam Meretrix meretrix Linnaeus is an economically important bivalve clam species in China, where it has been used as a traditional medicine and also as an enticing seafood. According to Essential Prescriptions from the Golden Cabinet (Jin-Gui-Yao-Lue), a classic traditional Chinese medicine book by Zhang Zhongjing (150–219) of the Eastern Han dynasty, M. meretrix was used to treat polydipsia, which is a condition of excessive thirst and one of the initial symptoms of diabetes in modern medicine. In Compendium of Materia Medica (Ben-Cao-Gang-Mu) compiled by Li Shizhen (1518–1593) in the Ming dynasty, it was used to treat furuncle and detoxify alcohol. Recent studies have shown that M. meretrix extracts have a range of biological activities, including antioxidant, antimicrobial, anti-inflammatory and antitumor properties [20,21,22,23]. In particular, a peptide-rich extract of M. meretrix was found to have antioxidant activity in non-alcoholic fatty liver disease mice by dietary supplementation [24,25]. Nevertheless, sequence information and regulation mechanisms of bioactive peptides in M. meretrix are lacking. Here we first examined the in vivo antioxidant properties of a peptide-rich extract of M. meretrix using Caenorhabditis elegans, a powerful model animal widely used in biomedical studies [26]. After separation of the antioxidant peptide fractions, we then identified the peptide sequences and synthesized the peptides for bioactivity studies. Using the synthetic counterparts, we further investigated the bioactivities and mechanisms of action of the identified M. meretrix peptides, including the activation of the transcription factor DAF-16/FOXO and the regulation of stress response genes.
2. Results
2.1. Separation of Antioxidant Peptide Fractions from M. meretrix Peptide-Rich Extract
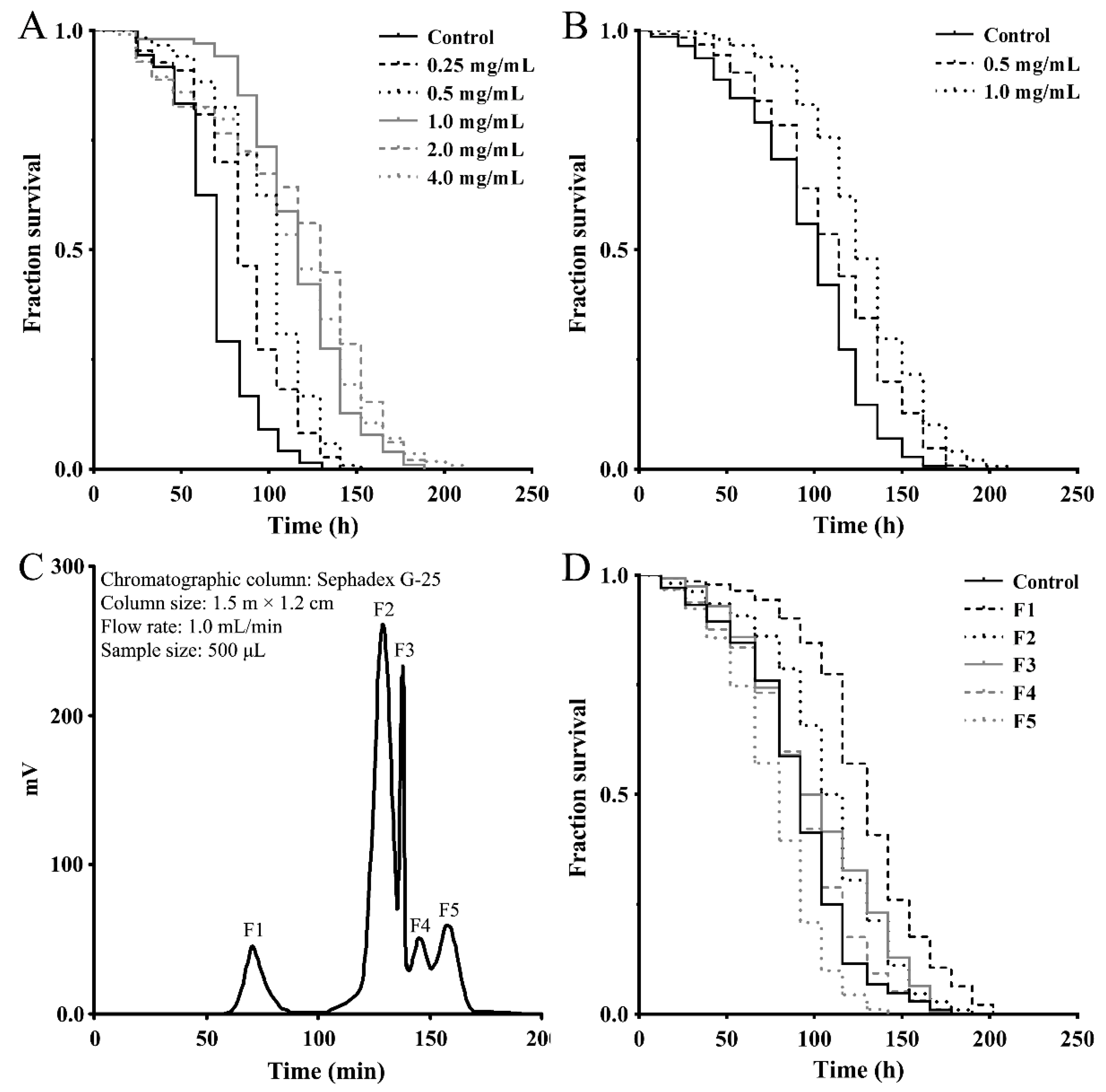
To assess the effect of a M. meretrix peptide-rich extract on stress-related response, we first examined its antioxidant activity using a paraquat resistance assay in wild-type C. elegans. As shown in Figure 1A, the survival rate of the nematodes that were pretreated with the indicated concentrations of extract prior to paraquat exposure (50 mM) was consistently higher than that of the control nematodes exposed to paraquat alone, demonstrating the antioxidant capacity of the peptide-rich extract. To obtain stress-resistant peptide fractions, the M. meretrix peptide-rich extract was separated into <3 kDa and >3 kDa fractions using ultrafiltration tubes with a 3 kDa molecular weight cut off (MWCO) and both fractions were subjected to an antioxidant test as above using the paraquat resistance assay. As shown in Figure 1B, the <3 kDa peptide fraction has better antioxidant activity and can significantly increase the survival rate of nematodes (>3 kDa not shown). The peptide fraction (<3 kDa) was then further separated by gel-filtration chromatography on a Sephadex G-25 column, and five subfractions (F1–F5) were obtained (Figure 1C). These subfractions were tested for their antioxidant ability as above using the paraquat resistance assay in the nematodes. As shown in Figure 2D, pretreatment with 1.0 mg/mL of the subfractions F1, F2 and F3 was able to increase the survival rate of the paraquat-exposed nematodes, demonstrating the antioxidant capacity of these subfractions. As subfraction F1 showed a better effect, this fraction was used for further experiments.
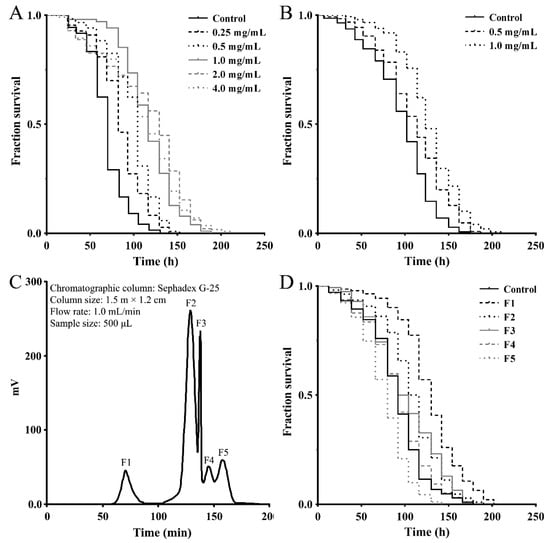
Figure 1.
Antioxidant activities of the peptide-rich extract and the separeted peptide fractions from M. meretrix. (A) Paraquat resistance assay of the peptide-rich extract. (B) Paraquat resistance assay of the <3 kDa peptide fraction. (C) Gel chromatography separation of the <3 kDa peptide fraction by Sephadex G-25. (D) Paraquat resistance assay of the F1, F2, F3, F4 and F5 subfractions (1 mg/mL) from (C). The young adult nematodes were treated with the samples at indicated concentrations for 24 h prior to exposure to 50 mM of paraquat. Survival rates were scored every 12 h and data are shown as representative Kaplan-Meier survival curves.
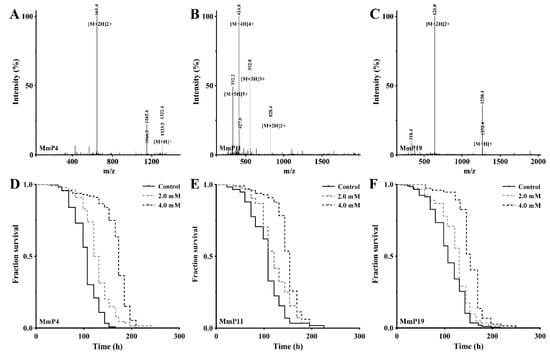
Figure 2.
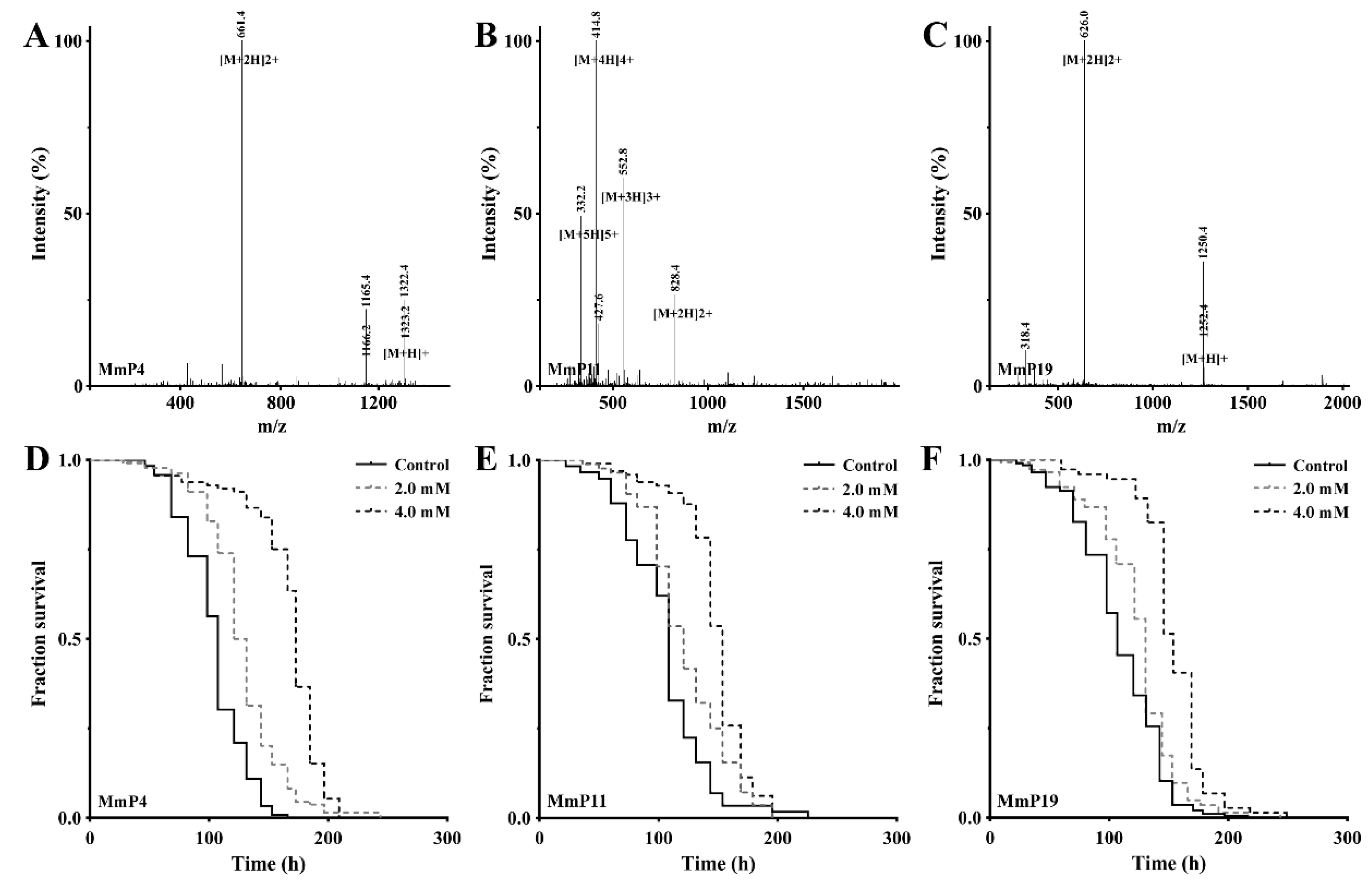
Mass spectra and antioxidant activities of synthetic peptides originated from M. meretrix peptides. The mass spectra of MmP4 (LSDRLEETGGASS), MmP11 (KEGCREPETEKGHR and MmP19 (IVTNWDDMEK) were shown in (A–C), respectively. The paraquat resistance assay was performed as in Figure 1, and representative Kaplan-Meier survival curves were shown for the nematodes treated with the peptides (D) MmP4, (E) MmP11 and (F) MmP19 at indicated concentrations.
2.2. Identification, Synthesis and Characterization of Antioxidant M. meretrix Peptides
The peptide components of subfraction F1 were identified by RP-nano-LC-MS/MS analysis coupled with database-assisted peptide sequencing. In total, 25 peptides were found, which were named MmP followed by a number (Table 1). To determine which peptides contributed to the general antioxidant activity of subfraction F1, all 25 peptides were synthesized individually with a purity of >95% (Table 1) and used for antioxidant screening as shown above in the nematodes. The effective concentrations of the synthetic peptides were shown to be >1.0 mM as tested preliminarily and thus all the peptides were subsequently screened for antioxidant activity at 2.0 and 4.0 mM. As shown in Figure 2, the M. meretrix peptides MmP4, MmP11 and MmP19 were found to increase the survival rate of the nematodes after paraquat exposure, demonstrating the antioxidant activity of these peptides.

Table 1.
Information on HPLC-MS/MS identification and solid-phase synthesis of M. meretrix peptides.
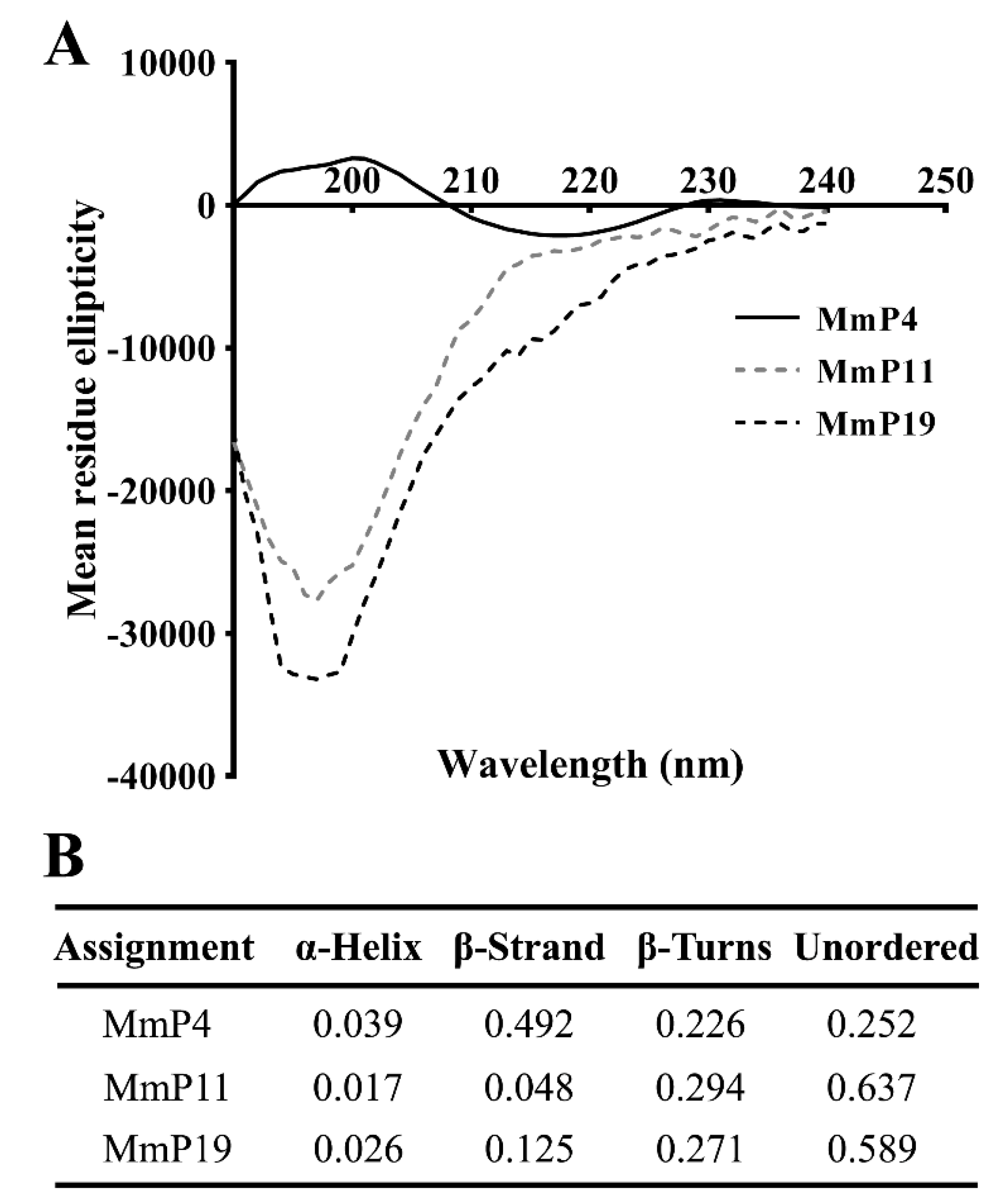
To characterize the structural features of the antioxidant peptides, individual peptides in deionized water were analyzed by circular dichroism (CD) spectroscopy, which is a primary tool for rapid determination of the secondary structure and folding properties of proteins [27,28]. As shown in Figure 3, the CD spectra of MmP4 showed a positive peak at around 200 nm and a negative peak at around 218 nm, which is similar to that generally found for the β-sheet secondary structures [28,29]. Interestingly, the CD spectra of MmP11 and MmP19 showed a strong negative peak at around 195 nm, indicating a random coil in the peptides [28,29]. These data suggest that peptides with different secondary structures may have similar antioxidant activities.
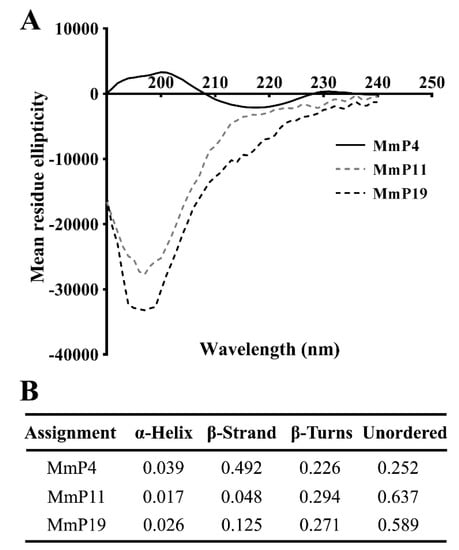
Figure 3.
CD spectra and proportions of secondary structures of antioxidant M. meretrix peptides. CD spectra of synthetic peptides (A) were determined in deionized water at a concentration of 0.15 mg/mL, and the proportions of secondary structures (B) were calculated from the spectra.
2.3. Effects of Antioxidant M. meretrix Peptides on Subcellular Localization of the Transcription Factor DAF-16
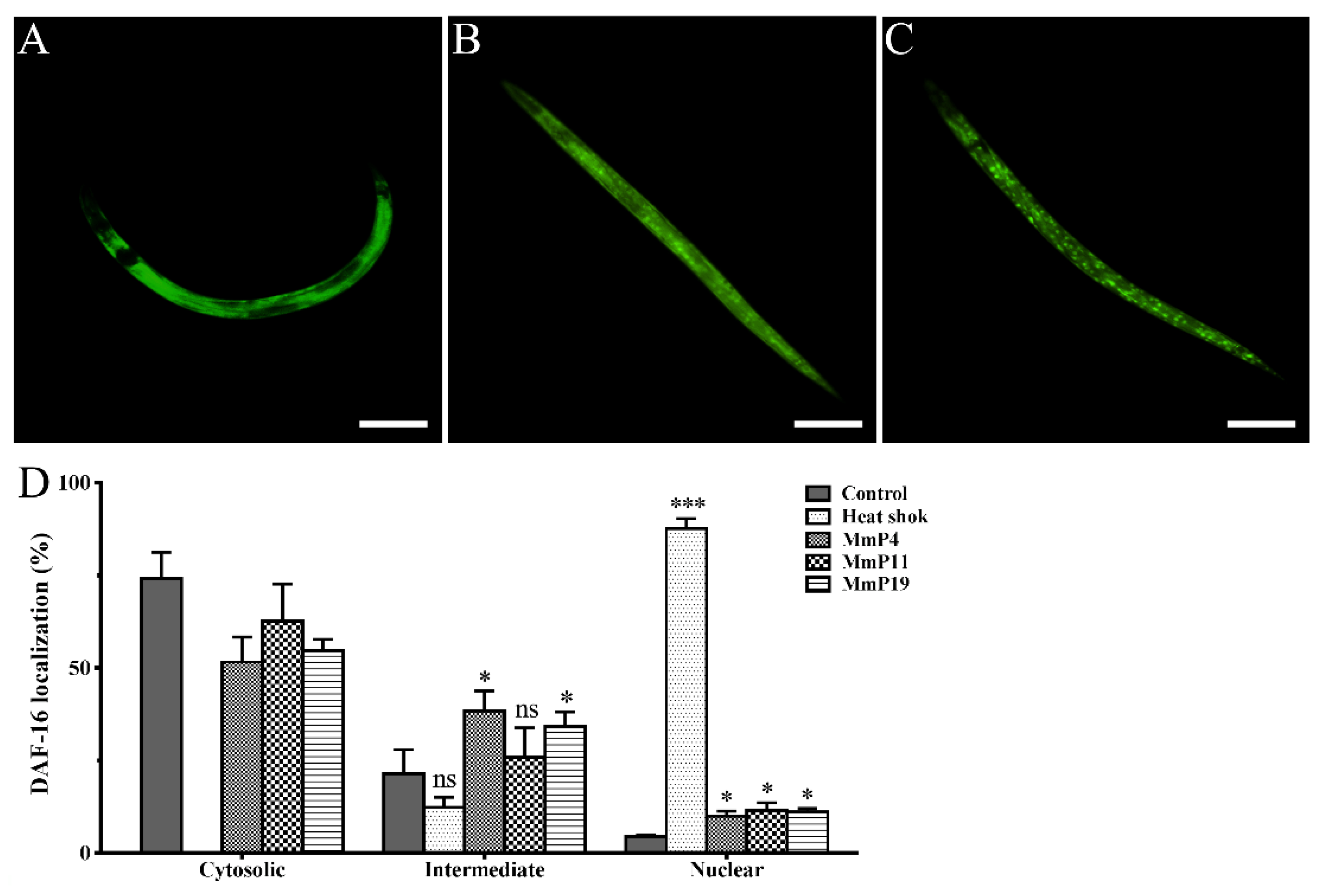
DAF-16 is a C. elegans orthologue to the mammalian FOXO proteins, which play important roles in stress resistance and longevity [30,31,32]. Therefore, we used the transgenic C. elegans strain GR1352, which has been previously used to quantify DAF-16 nuclear localization and monitor relevant signaling activity [33,34], in order to determine the effects of M. meretrix peptides on DAF-16::GFP localization. Synchronized GR1352 larvae were first incubated at 20 °C for 24 h and then treated with or without the peptides at 20 °C for 24 h, while a portion of the adult nematodes without peptide treatment were heat-shocked at 35 °C for 1 h and used as a positive control of DAF-16 nuclear translocation. As shown in Figure 4, the majority of the control nematodes (~75%), which were incubated at normal conditions without peptide treatment, showed a cytosolic DAF-16::GFP localization with only <5% of the nematodes displaying a nuclear DAF-16::GFP localization. On the other hand, the majority of the heat-shocked nematodes (>85%) showed a nuclear DAF-16::GFP localization. Interestingly, when the nematodes were treated with 4.0 mM of M. meretrix peptides at normal conditions, the percentage of nematodes with detectable nuclear DAF-16::GFP localization was noticeably increased as compared to the control, indicating a partial translocation of DAF-16 from cytoplasm to nuclei by the peptides.
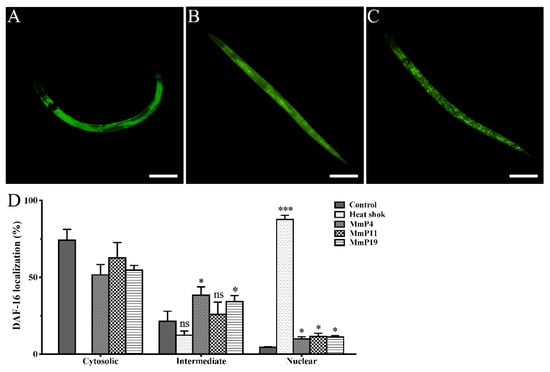
Figure 4.
Effect of antioxidant M. meretrix peptides on cellular localization of the transcription factor DAF-16 in C. elegans. Synchronized L1 stage GR1352 nematodes were incubated for 24 h at 20 °C and treated with the peptides for 24 h; a portion of the adult nematodes without peptide treatment were heat-shocked at 35 °C for 1 h and used as a positive control. Cytosolic (A), intermediate (B) and nuclear (C) categories of DAF-16 localization were scored in approximately 30 animals per condition, and the data are presented as percentages of subcellular categories (D). Three independent biological replicates were performed. * P < 0.05; ** P < 0.01; *** P < 0.001. Scale bar: 200 μm.
2.4. Effects of Antioxidant M. meretrix Peptides on the Expression of Oxidative Stress Related Genes
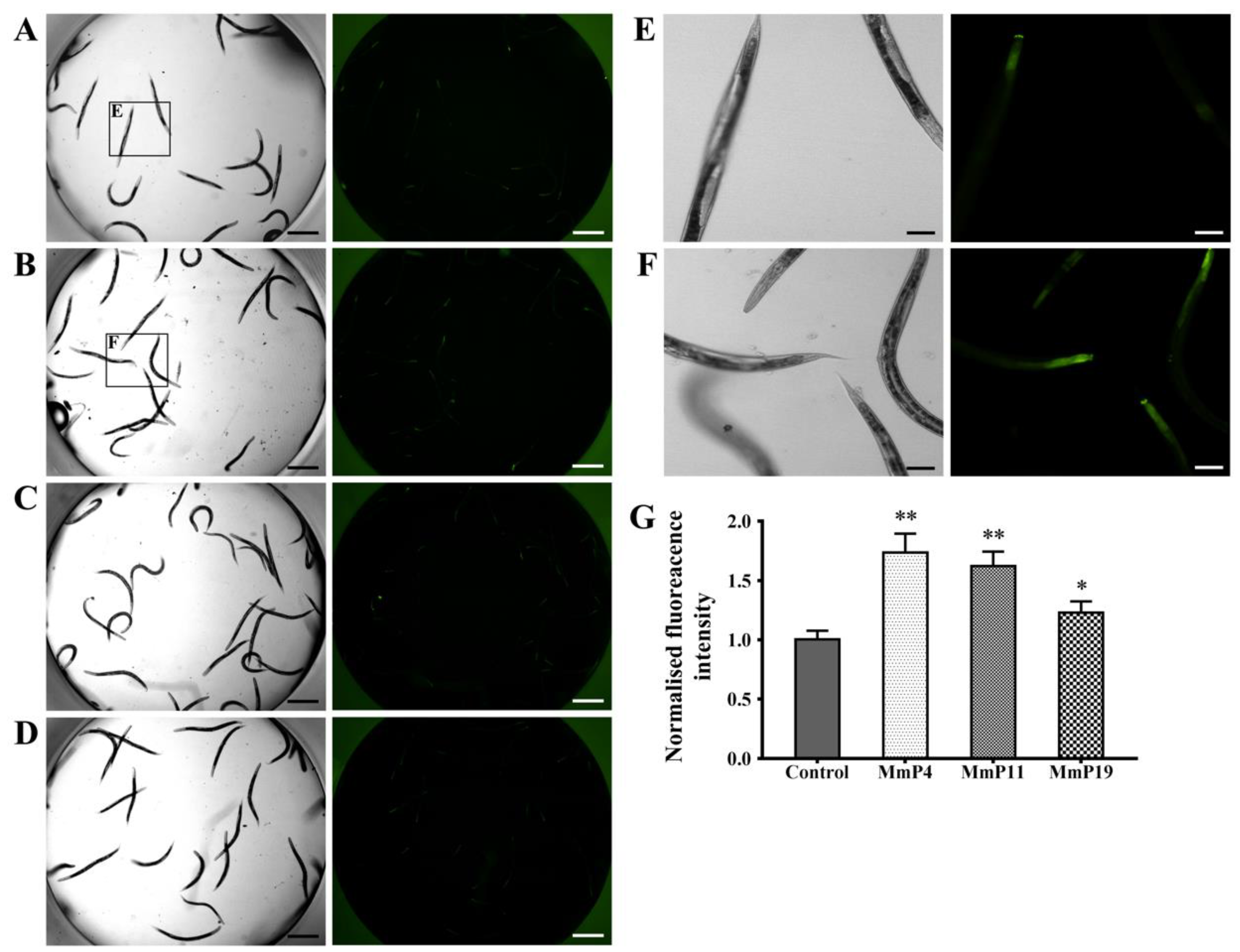
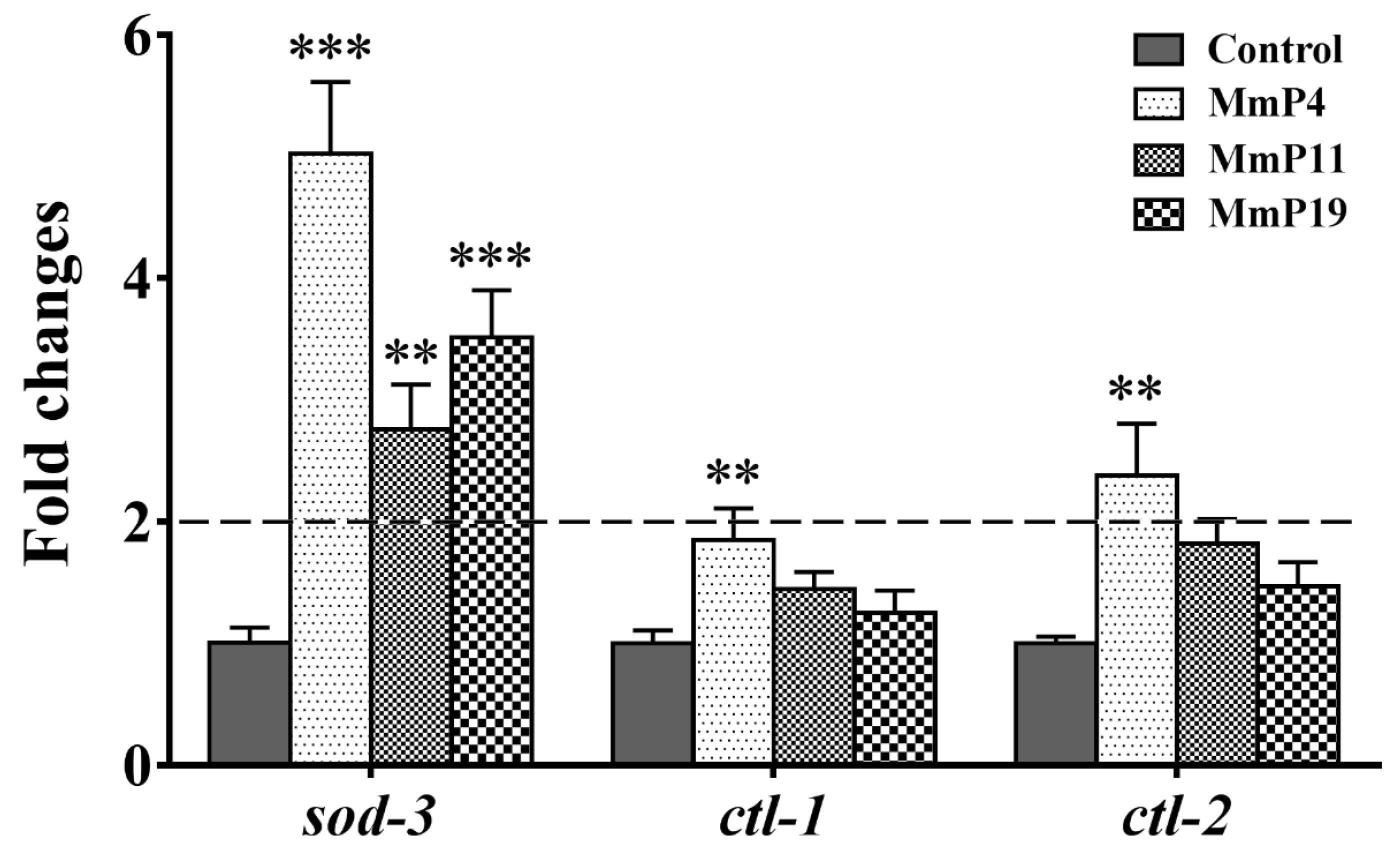
It is known that DAF-16 plays a critical role in regulating the transcription of several antioxidant genes [35]. Since M. meretrix peptides were shown as above to have antioxidant and anti-aging activities, we used the transgenic strain C. elegans CF1553, which expresses a SOD-3::GFP reporter, to detect the effects of the peptides on SOD-3 expression [36]. After treatment with the peptides for 24 h, the SOD-3::GFP fluorescence level in the nematodes treated with MmP4, MmP11 and MmP19 was increased as compared to the control, suggesting the expression of the antioxidant enzyme SOD-3 was up-regulated by these peptides (Figure 5). To further investigate the effects of M. meretrix peptides on oxidative stress-related genes, we determined the expression of the antioxidant enzyme genes sod-3, ctl-1 and ctl-2 in wild-type C. elegans by real-time PCR. As shown in Figure 6, the sod-3 transcript level was increased (>2 folds) in nematodes treated with 4.0 mM of MmP4, MmP11 and MmP19, while the ctl-1 and ctl-2 transcript levels were also increased (~2 folds) by the peptide MmP4.
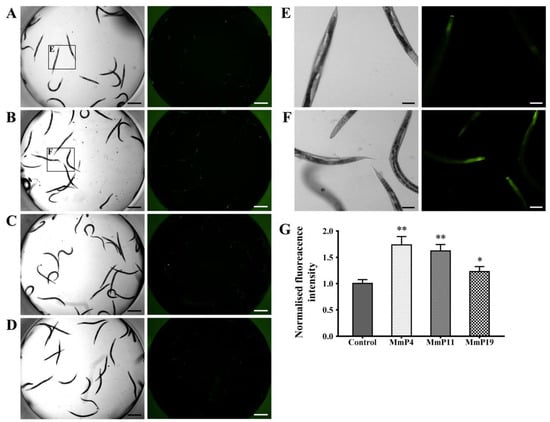
Figure 5.
Effect of antioxidant M. meretrix peptides on the expression of SOD-3 in C. elegans. Synchronized CF1553 nematodes expressing the SOD-3::GFP reporter were incubated for 24 h at 20 °C and then treated with 4.0 mM of the peptides for another 24 h. The nematodes were collected for fluorescence detection of the control (A), MmP4 (B), MmP11 (C) and MmP19 (D). Scale bar: 0.5 mm. Image magnifications of the control (A) and MmP4 treatment (B) were also presented in (E) and (F), respectively. Scale bar: 0.1 mm. SOD-3::GFP fluorescence intensity was calculated from the images and shown in (G). Columns represent pooled normalized values of three independent biological replicates. * P < 0.05; ** P < 0.01.
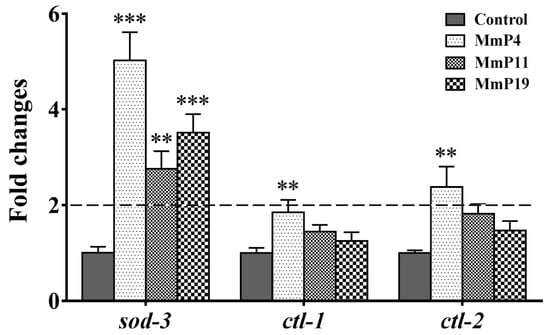
Figure 6.
Effect of antioxidant M. meretrix peptides on the expression of sod-3, ctl-1 and ctl-2 in wild-type C. elegans. Synchronized N2 nematode larvae were incubated for 24 h at 20 °C and then treated with the peptides for 24 h. The expression of sod-3, ctl-1 and ctl-2 was analyzed by real-time PCR with three independent biological replicates. ** P < 0.01; *** P < 0.001.
2.5. Dependency of Antioxidant Activity of M. meretrix Peptides on Transcription Factor DAF-16
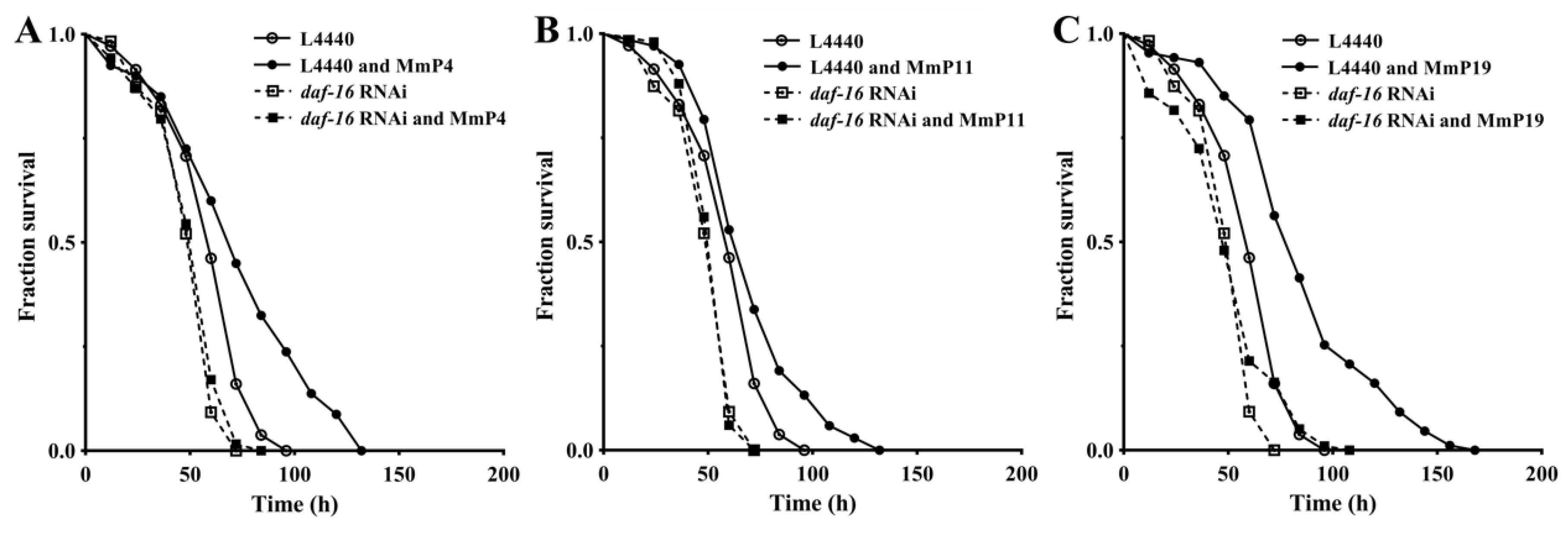
As shown above, activation of the transcription factor DAF-16 may have an important role in promoting oxidative stress resistance by the M. meretrix peptides, as well as in regulating antioxidant enzyme genes. Therefore, we further determined the antioxidant activity of M. meretrix peptides in C. elegans after daf-16 RNA interference (RNAi) to assess whether their antioxidant capacity was DAF-16-dependent. As shown in Figure 7, the oxidative stress resistance of C. elegans with the vector L4440 and without daf-16 RNAi was significantly increased by the tested M. meretrix peptides (“L4440” vs. “L4440 and peptides”), but the increase was abolished under daf-16 RNAi conditions (“daf-16 RNAi” vs. “daf-16 RNAi and peptides”), demonstrating that the increased resistance of the nematodes to oxidative stress by M. meretrix peptides was dependent on the transcriptional regulator DAF-16.
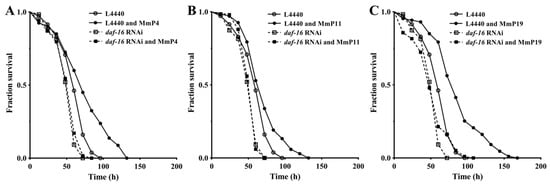
Figure 7.
Antioxidant activity of M. meretrix peptides in wild-type C. elegans after daf-16 RNA interface (RNAi). The nematodes with or without daf-16 suppression by RNAi were treated with 4.0 mM of the peptides MmP4 (A), MmP11 (B) and MmP19 (C) and then subjected to paraquat resistance assay as in Figure 1.
3. Discussion
ROS generated from both endogenous and exogenous sources is increasingly recognized as a pivotal mediator of many stress responses, and an imbalance between ROS production and elimination is considered a risk factor for a number of age-related diseases [37]. Peptides derived from a variety of food sources have shown antioxidant, anti-inflammatory, antitumor and other bioactive properties and thus may have beneficial potential in promoting health and preventing diseases [38]. Several peptides isolated from M. meretrix, a hard clam with high nutritional and medicinal values, have recently been shown to have apoptosis-inducing and antitumor activities [20,21,22]. Using the animal model C. elegans, we demonstrate in this study the in vivo antioxidant activities of a M. meretrix peptide extract and its peptide components, which were identified by RP-nano-LC-MS/MS and individually synthesized for bioactivity studies. We then reveal that the antioxidant M. meretrix peptides were able to regulate the expression of oxidative stress-related genes and that their antioxidant activity was dependent on the transcription factor DAF-16/FOXO, an evolutionarily conserved stress response and lifespan regulator [39,40].
The biological activities of a protein are, in theory, the functional manifestations of the amino acids it contains, and some short peptide segments may have specific roles in the overall function of the protein but not all segments are equally important [41]. It is noteworthy that the amino or carboxyl terminals of some of the identified M. meretrix peptides in this study, e.g., MmP4, are branched-chain amino acids (leucine, isoleucine and valine), which may have potential antioxidant activities [42]. CD spectroscopy is a valuable method for the analysis of peptide secondary structures, including α-helix, β-sheet, β-turn and random coil [27,28]. CD spectra of the antioxidant M. meretrix peptides revealed that MmP4 was able to form a secondary structure of β-sheet, but MmP11 and MmP19 were primarily in random coil conformation, suggesting that the antioxidant activity peptides are not necessarily related to a specific secondary structure and that more detailed structure-activity studies of peptides are needed.
It is established that high concentrations of paraquat can cause significant oxidative stress and trigger the occurrence of a pathological state [43,44]. In this study, we show that treatment with M. meretrix peptides significantly increased the resistance of C. elegans to paraquat-induced oxidative stress. Interestingly, other antioxidants such as C-phycocyanin and vitamin C can also be used in treatment against paraquat injuries [45,46]. The main mechanisms involved in the protective effects of these antioxidants include the reduction of both oxidative stress and inflammation, and induction of antioxidant defenses [44]. In agreement with this, the present study demonstrates that the antioxidant M. meretrix peptides were capable of up-regulating the expression of the antioxidant enzyme SOD-3. Further studies using daf-16 RNAi reveal that the enhanced resistance to oxidative stress by the peptides was dependent on the transcriptional regulator DAF-16.
The transcription factor DAF-16 has been shown to play a variety of roles in regulating biological processes, including stress response and longevity [30,31,32]. For example, the exposure of nematodes to low concentrations of paraquat causes nuclear localization of DAF-16 and increases the expression of the DAF-16 transcriptional target gene sod-3 [47]. Interestingly, the daf-16 mutant is more sensitive to paraquat and heat stresses as compared to wild-type nematodes [48]. In the present study, the antioxidant M. meretrix peptides were shown to activate DAF-16 and increase expression of the antioxidant enzyme genes sod-3, ctl-1 and ctl-2 in different degrees. A number of studies have shown that decreased expression of sod-3, ctl-1 or ctl-2 can shorten the lifespan of daf-2 nematodes, suggesting that these genes may contribute to the longevity of daf-2 mutants [8,31,32,33,34,35]. Therefore, these results suggest that the antioxidant M. meretrix peptides may have dietary health benefits, including stress resistance, disease prevention and aging delay. Future work is needed to elucidate the absorption and distribution patterns of the peptides in nematodes after feeding and to investigate the effectiveness of the peptides in mouse models and humans.
4. Materials and Methods
4.1. Chemicals and Materials
Sephadex G-10 and G-25 gels (medium) were obtained from GE Healthcare (Uppsala, Sweden). 1,1′-Dimethyl-4,4′-bipyridinium dichloride (paraquat), 5-fluoro-2′-deoxyuridine (5-FUdR), 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2′,7′-dichlorofluorescein diacetate (DCFH-DA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The TRIzol reagent and RNeasy Mini Kit were obtained from Invitrogen (Carlsbad, CA, USA) and Qiagen (Hilden, Germany), respectively. DNase I, PrimeScript RT and SYBR Premix Ex Taq kits were purchased from Takara (Dalian, China). The bicinchoninic acid (BCA) protein assay kit was purchased from Beyotime (Shanghai, China). Other chemicals used in this study were of analytical grade and obtained from Guangzhou Chemical Reagent Co. (Guangzhou, China). The ultrafiltration centrifuge tube with a molecular weight cut off (MWCO) of 3 kDa was purchased from Millipore (Bedford, MA, USA). The Asiatic hard clam Meretrix meretrix was purchased from Huangsha Aquatic Market Co. (Guangzhou, China).
4.2. Nematode and Bacterial Strains
The following C. elegans strains were used in this study: Bristol N2 (wild-type), GR1352 {xrIs87 [daf-16(alpha)::GFP::daf-16B + rol-6(su1006)]} and CF1553 {muIs84 [(pAD76) sod-3p::GFP + rol-6 (su1006)]}. Escherichia coli strains OP50, NA22 and HT115 were used as food sources for the nematodes as appropriate. All C. elegans and E. coli strains were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA). The nematodes were maintained under standard conditions at 20 °C unless otherwise stated, and synchronization was performed using the standard alkaline hypochlorite method.
4.3. Preparation of Natural Peptides
Fresh M. meretrix was used to collect the clam meat, which was mixed with deionized water at a ratio of 1:2 (W/V) and homogenized three times for 15 s at 4 °C on a Polytron homogenizer (CH-6010, Luzern, Switzerland). The homogenate was centrifuged at 6000 × g for 30 min at 4 °C, and the supernatant was used for isolation of natural peptides with ethanol as described [49]. After adding ethanol to a final concentration at 60% (v/v), the mixture was placed at 4 °C for 12 h and centrifuged at 6000 × g for 30 min at 4 °C to collect the supernatant. Ethanol was removed from the supernatant by evaporation under reduced pressure in a rotary evaporator (Yarong, Shanghai, China) at 30 °C, and the aqueous solution was freeze-dried with a recovery rate of 9.8% (dry weight). The sample was redissolved in deionized water (10 mg/mL), filtered with a 0.22 μm Millipore filter and desalted by gel filtration chromatography on a Sephadex G-10 column (20 × 1.5 cm). The elution was monitored at 280 nm with a UV detector (Jinda, Shanghai, China), and the tubes containing proteins were combined and freeze-dried as peptide-rich extracts. The protein content of the extract was >70% as determined by the BCA protein assay kit, and the recovery rate from the original sample was 5.7%. The extract was redissolved in deionized water (20 mg/mL) and separated into <3 kDa and >3 kDa fractions using the ultrafiltration tubes with an MWCO of 3 kDa. Antioxidant activity of the fractions was determined by the paraquat resistance assay as described below. The active fractions (0.5 mL, 10 mg/mL) were further separated on a Sephadex G-25 column (150 × 1.2 cm), which was eluted with deionized water at a flow rate of 1 mL/min and monitored at 280 nm with the UV detector. The tubes were pooled according to the UV detection and freeze-dried as M. meretrix peptide subfractions for further bioactivity tests.
4.4. Paraquat Resistance Assay
Antioxidant activity was tested by the paraquat resistance assay using C. elegans as previously described [18]. Briefly, synchronized L1 larvae of N2 nematodes were incubated in S medium containing E. coli NA22 at 20 °C for 42 h, and 75 μg/mL of 5-FUdR (final concentration) was added to the young adults. The nematodes were then transferred to 96-well plates (~20 individuals per well, approximately 100 nematodes for each group) containing E. coli NA22 as food and peptide samples at the indicated concentrations. After incubation at 20 °C for another 24 h, the nematodes were exposed to 50 mM of paraquat and the survival of nematodes was scored microscopically based on their movement every 12 h until all were dead.
4.5. Identification and Synthesis of Peptides
Peptide sequences in the peptide sample were identified by liquid chromatography-tandem mass spectrometry using a reverse-phase nanocolumn (RP-nano-LC-MS/MS) as previously described [18]. Briefly, peptide samples were dissolved in solvent A (water/acetonitrile/formic acid, 98:2:0.1, v/v/v) and loaded onto a ChromXP C18 (3 μm, 120 Å) nanoLC trap column. The desalting procedure was carried out at 2 μL/min for 10 min with 100% solvent A. Then, a linear gradient of 5–35% solvent B (water/acetonitrile/formic acid, 2:98:0.1, v/v/v) over 50 min was used on an analytical column (75 μm × 15 cm C18, 3 μm, 120 Å, ChromXP Eksigent). LC-MS/MS analysis was performed with a TripleTOF 5600 System (AB SCIEX, Concord, Ontario, Canada) fitted with a Nanospray III source (AB SCIEX, Concord, Ontario, Canada). All raw data files (*.wiff) were collectively searched with ProteinPilot Software v. 4.5 (AB SCIEX, Foster City, CA, USA) against a translated protein database of M. meretrix nucleotide sequences from the National Center for Biotechnology Information (NCBI). A threshold of confidence above 95% and a local false discovery rate (FDR) of less than 1% were used for peptide identification. Peptides identified in the bioactive peptide subfractions were synthesized by Shanghai Top-peptide Biotechnology Co., Ltd. (Shanghai, China), using the solid-phase synthesis method with a purity of >95%. The synthetic peptides were subjected to antioxidant activity screening using the paraquat resistance assay.
The synthetic peptides with purity >95% were subjected to antioxidant activity screening using the paraquat resistance assay.
4.6. Circular Dichroism Spectroscopy
Circular dichroism (CD) spectra of synthetic peptides were obtained and analyzed with a J-815 CD spectrophotometer (Jasco, Tokyo, Japan) as described previously [27]. In brief, the samples were dissolved at a concentration of 0.15 mg/mL in deionized water and phosphate buffered saline (PBS, pH 7.4), respectively, and CD spectra were generated in the far-ultraviolet region from 190 to 240 nm using a quartz cuvette with a path length of 0.1 cm. The DichroWeb online analysis system (http://dichroweb.cryst.bbk.ac.uk) was used to calculate the secondary structure composition through the peptide CD spectra [28].
4.7. Nuclear Localization Assay of Transcription Factor DAF-16
The nuclear localization of transcription factor DAF-16 was conducted using transgenic C. elegans GR1352 as described [33]. Synchronized GR1352 larvae were first incubated at 20 °C in S medium containing E. coli NA22 for 24 h. The nematodes were then treated at 20 °C for 24 h with or without peptide samples. The nematodes were collected and washed three times with a M9 buffer, and then placed on microscope slides coated with 2% agarose, anesthetized with 1% sodium azide and capped with coverslips. A portion of the nematodes treated at 20 °C for 24 h without peptide samples were heat-shocked and used as a positive control. The subcellular localizations of DAF-16::GFP were classified into cytosolic, intermediate and nuclear categories, which were scored in approximately 30 animals per condition with an Olympus BX51 fluorescent microscope (Olympus, Tokyo, Japan). At least 3 independent experiments were performed and data were presented as a ratio of a sub-localization phenotype to the whole population.
4.8. Determination of SOD-3 Expression Levels
The transgenic C. elegans CF1553 expressing SOD-3::GFP reporter was used to detect SOD-3 expression [50]. In brief, synchronized CF1553 larvae were first incubated in S medium containing E. coli NA22 for 24 h at 20 °C and then treated with peptide samples for another 24 h at 20 °C as above in 24-well plates. To visually observe SOD-3::GFP expression, some of the nematodes were transferred to 384-well plates with 40 μL of M9 buffer containing 1% sodium azide prior to acquiring the fluorescence images with an ImageXpress Micro System (Molecular Devices, Sunnyvale, CA, USA). To determine SOD-3::GFP expression levels, the rest of the nematodes were collected and washed three times with M9 buffer and then homogenized in PBS with 0.1% Tween 20. The lysates were centrifuged at 10,000 × g for 5 min at 4 °C and protein content was determined with the BCA protein assay kit as described above. Fluorescence intensity was measured by the Fluoroskan Ascent FL plate reader at an excitation level of 485 nm and an emission level of 530 nm.
4.9. Real-time PCR Analysis
Relative quantification of gene expression by real-time PCR was performed essentially as described [39]. Synchronized N2 nematode larvae were incubated in S medium containing E. coli NA22 for 24 h at 20 °C and then incubated with peptide samples for another 24 h. The total RNA was extracted from the nematodes with TRIzol reagent according to the manufacturer’s instruction. After reverse transcription, the real-time PCR was performed on a StepOne Plus Real-Time PCR Detection System (Applied Biosystems Inc. Foster, CA, USA) using the SYBR Premix Ex Taq reagent kit. The gene expression levels were analyzed by using the following gene-specific primers: β-actin (F: 5′-CCACGAGACTTCTTACAACTCCATC-3′, R: 5′-CTTCATGGTTGATGGGGCAAGAG -3′), sod-3 (F: 5′-GAGCTGATGGACACTATTAAGCG-3′, R: 5′-GCACAGGTGGCGATCTTCAAG-3′), ctl-1 (F: 5′-TCTACTCGGATCGTGGAATTCCT-3′, R: 5′-TTGGAACCTTGAGCAGGCTTG-3′), and ctl-2 (F: 5′-TCCTGTTTTCCGATCGAGGACTC-3′, R: 5′-CTTCACTCCTTGAGTTGGCTTG-3′).
4.10. RNA Interference Analysis
RNA interference of daf-16 was carried out using the feeding method [51]. RNAi plasmids were constructed by cloning a portion of the daf-16 cDNA into the RNAi vector (pL4440). Primer sequences for the daf-16 gene amplification are shown as following with the Xba I (TCTAGA) and Kpn I (GGTACC) sites, respectively; daf-16 (F: 5′-TGCTCTAGACTCGATCCGTCACAATCTGTC-3′, R: 5′-CGGGGTACCAGCTGGAGAAACACGAGACGA-3′). The RNAi bacteria E. coli HT115 were induced with 1 mM IPTG, collected and added to 96-well plates. Then, synchronized N2 nematode larvae were transferred to the 96-well plates (~20 individuals per well and ~100 nematodes for each group) and incubated for 42 h at 20 °C. After adding 75 μg/mL of 5-FUdR and peptide samples, the plates were incubated for another 24 h. The nematodes were then exposed to 50 mM of paraquat for the oxidative resistance assay as described above.
4.11. Statistical Analysis
GraphPad Prism version 7.0 for Microsoft Windows (GraphPad Software, San Diego, CA, USA) was used to generate graphs and perform statistical analysis. C. elegans survival and lifespan curves were analyzed by the Kaplan-Meier method and the log-rank test. Probability values of p < 0.05 were considered as statistically significant.
5. Conclusions
In summary, antioxidant peptides were identified from peptide-rich extract of the Asiatic hard clam Meretrix meretrix and were shown to extend the lifespan of C. elegans. Further studies indicated that the antioxidant peptides were able to promote nuclear translocation of the transcription factor DAF-16 and to enhance expression of genes in the antioxidant defense system, including sod-3, ctl-1 and ctl-2. Using RNAi technology, DAF-16 was found to be required for the peptides to increase the resistance of nematodes to paraquat-induced oxidative stress. Taken together, these results suggest that the antioxidant M. meretrix peptides may have dietary health benefits, including stress resistance and age-related disease prevention. Future work is needed to elucidate the absorption and distribution patterns of the peptides in nematodes after feeding and to investigate the effectiveness of the peptides in mouse models and humans.
Author Contributions
W.J. and Z.H. conceived and designed the research. W.J., Q.P., L.S. and Y.Z. performed the experiments. X.Y. (Xuesong Yu), X.Y. (Xiquan Yin) and M.L. contributed reagents/materials/analytical tools. W.J., Q.P., C.W.M. and Z.H. analyzed the data and wrote/revised the manuscript. All authors have read and approved the final version.
Funding
This research was funded by the Special Funds of the Central Finance to Support the Development of Local Universities and Colleges, Guangdong Province Department of Education (grant number 2015KGJHZ022), Guangdong Provincial Medical Science Foundation (grant number A2016232), and Special Funds “Climbing Program” of Guangdong Province Students for Scientific and Technological Innovation (grant number 138981).
Conflicts of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Mima, A. Inflammation and oxidative stress in diabetic nephropathy: New insights on its inhibition as new therapeutic targets. J. Diabetes Res. 2013, 6, 248563. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Abramov, A.Y. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic. Biol. Med. 2016, 100, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Viktorinova, A.; Svitekova, K.; Stecova, A.; Krizko, M. Relationship between selected oxidative stress markers and lipid risk factors for cardiovascular disease in middle-aged adults and its possible clinical relevance. Clin. Biochem. 2016, 9, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, F.; Pupo, G.; Giraldo, E.; Badìa, M.C.; Monllor, P.; Lloret, A.; Schininà, M.E.; Giorgi, A.; Cini, C.; Tramutola, A.; et al. Oxidative signature of cerebrospinal fluid from mild cognitive impairment and Alzheimer disease patients. Free Radic. Biol. Med. 2016, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Godic, A.; Poljšak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell. Longev. 2014, 860479. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, D.M.; Asaithamby, A.; Bailey, S.M.; Costes, S.V.; Doetsch, P.W.; Dynan, W.S.; Kronenberg, A.; Rithidech, K.N.; Saha, J.; Snijders, A.M.; Werner, E.; et al. Understanding cancer development processes after HZE-particle exposure: Roles of ROS, DNA damage repair and inflammation. Radiat. Res. 2015, 183, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Görlach, A.; Dimova, E.Y.; Petry, A.; Martínez-Ruiz, A.; Hernansanz-Agustín, P.; Rolo, A.P.; Palmeira, C.M.; Kietzmann, T. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol. 2015, 6, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, J.M.; Hekimi, S. Reactive oxygen species and aging in Caenorhabditis elegans: Causal or casual relationship? Antioxid. Redox Signal. 2010, 13, 1911–1953. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Tunnacliffe, A. Cryptobiosis, aging and cancer: Yin-yang balancing of signaling networks. Rejuv. Res. 2006, 9, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Himaya, S.W.; Ryu, B.; Ngo, D.H.; Kim, S.K. Peptide isolated from Japanese flounder skin gelatin protects against cellular oxidative damage. J. Agric. Food Chem. 2012, 60, 9112–9119. [Google Scholar] [CrossRef] [PubMed]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O.; Mawson, A.J.; He, R.; Girgih, A.; Aluko, R.E. Antioxidant properties of Australian canola meal protein hydrolysates. Food Chem. 2014, 146, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Ngoungoure, V.L.; Schluesener, J.; Moundipa, P.F.; Schluesener, H. Natural polyphenols binding to amyloid: A broad class of compounds to treat different human amyloid diseases. Mol. Nutr. Food Res. 2015, 59, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Feng, L.; Bao, W.R.; Ma, D.L.; Leung, C.H.; Nie, S.P.; Han, Q.B. Structure characterization and immunomodulating effects of polysaccharides isolated from Dendrobium officinale. J. Agric. Food Chem. 2016, 64, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A. review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Li, H.; Ding, F.; Xiao, L.; Shi, R.; Wang, H.; Han, W.; Huang, Z. Food-derived antioxidant polysaccharides and their pharmacological potential in neurodegenerative diseases. Nutrients 2017, 9, 778. [Google Scholar] [CrossRef] [PubMed]
- Girgih, A.T.; He, R.; Malomo, S.A.; Offengenden, M.; Wu, J.; Aluko, R.E. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Fan, X.; Bai, L.; Zhu, L.; Yang, L.; Zhang, X. Marine algae-derived bioactive peptides for human nutrition and health. J. Agric. Food Chem. 2014, 62, 9211–9222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, Y.; Qin, C.; Liang, M.; Mao, X.; Li, S.; Zou, Y.; Jia, W.; Li, H.; Ma, C.W.; et al. Bioactive peptides from Angelica sinensis protein hydrolyzate delay senescence in Caenorhabditis elegans through antioxidant activities. Oxid. Med. Cell. Longev. 2016, 2016, 8956981. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cheng, Q.; Peng, Q.; Yu, X.; Yin, X.; Liang, M.; Ma, C.W.; Huang, Z.; Jia, W. Antioxidant peptides derived from the hydrolyzate of purple sea urchin (Strongylocentrotus nudus) gonad alleviate oxidative stress in Caenorhabditis elegans. J. Funct. Foods 2018, 48, 594–604. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Cheng, L.; Wei, J.; Wu, N.; Zheng, L.; Lin, X. A novel polypeptide from Meretrix meretrix Linnaeus inhibits the growth of human lung adenocarcinoma. Exp. Biol. Med. 2012, 237, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, J.; Wu, N.; Liu, M.; Wang, C.; Zhang, Y.; Wang, F.; Liu, H.; Lin, X. Mere15, a novel polypeptide from Meretrix meretrix, inhibits adhesion, migration and invasion of human lung cancer A549 cells via down-regulating MMPs. Pharm. Biol. 2013, 51, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, X.; Zhao, J.; Xiao, L.; Liu, H.; Wang, C.; Cheng, L.; Wu, N.; Lin, X. Induction of apoptosis, G0/G1 phase arrest and microtubule disassembly in K562 leukemia cells by Mere15, a novel polypeptide from Meretrix meretrix Linnaeus. Mar. Drugs 2012, 10, 2596–2607. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, H.; Qian, J.; He, Y.; Zheng, J.; Lu, Z.; Xu, Z.; Shi, J. Structural and immunological activity characterization of a polysaccharide isolated from Meretrix meretrix Linnaeus. Mar. Drugs 2015, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhao, S.; Yu, F.; Yang, Z.; Ding, G. Protective effects and mechanism of Meretrix meretrix oligopeptides against nonalcoholic fatty liver disease. Mar. Drugs 2017, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wang, J.; Yu, F.; Tang, Y.; Ding, G.; Yang, Z.; Sun, Y. Protective effect of Meretrix meretrix oligopeptides on high-fat-diet-induced non-alcoholic fatty liver disease in mice. Mar. Drugs 2018, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, F.; Guo, W.; Zhang, J.; Xiao, L.; Li, H.; Jia, W.; Huang, Z. Caenorhabditis elegans in Chinese medicinal studies: Making the case for aging and neurodegeneration. Rejuv. Res. 2014, 17, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Hong, R.; He, S.; Liu, G.; Huang, Z.; Zheng, Y. Polyproline II structure is critical for the enzyme protective function of soybean Em (LEA1) conserved domains. Biotechnol. Lett. 2011, 33, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Biggs, W.H.; Tieu, D.; Boyer, A.D.; Varki, N.M.; Cavenee, W.K.; Arden, K.C. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc. Natl. Acad. Sci. USA 2004, 101, 2975–2980. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Mukhopadhyay, A.; Dixit, B.L.; Raha, T.; Green, M.R.; Tissenbaum, H.A. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat. Genet. 2006, 38, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Shi, Y.C.; Chang, C.H.; Huang, C.W.; Hsiu-Chuan Liao, V. Selenite protects Caenorhabditis elegans from oxidative stress via DAF-16 and TRXR-1. Mol. Nutr. Food Res. 2014, 58, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Evason, K.; Collins, J.J.; Huang, C.; Hughes, S.; Kornfeld, K. Valproic acid extends Caenorhabditis elegans lifespan. Aging Cell 2008, 7, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, R.; Li, H.; Xiang, Y.; Xiao, L.; Hu, M.; Ma, F.; Ma, C.W.; Huang, Z. Antioxidant and neuroprotective effects of Dictyophora indusiata polysaccharide in Caenorhabditis elegans. J. Ethnopharmacol. 2016, 192, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Libina, N.; Berman, J.R.; Kenyon, C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 2003, 115, 489–502. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Idelchik, M.D.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, N.; Xiong, S.; Zou, S.; Li, H.; Xiao, L.; Cao, Z.; Tunnacliffe, A.; Huang, Z. Inhibition of polyglutamine-mediated proteotoxicity by Astragalus membranaceus polysaccharide through DAF-16/FOXO transcription factor in Caenorhabditis elegans. Biochem. J. 2012, 441, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Murphy, C.T.; Kenyon, C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.M.; Vural, S.; King, B.R.; Guda, C. Mining for class-specificmotifs in protein sequence classification. BMC Bioinform. 2013, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Lee, J.H.; Kim, D.H.; Kim, K.T.; Lee, G.W.; Choi, S.J.; Chang, P.S.; Paik, H.D. Antioxidative and nitric oxide scavenging activity of branched-chain amino acids. Food Sci. Biotechnol. 2015, 24, 1555–1558. [Google Scholar] [CrossRef]
- Dai, D.F.; Chiao, Y.A.; Marcinek, D.J.; Szeto, H.H.; Rabinovitch, P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan 2014, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Ayala, T.; Andérica-Romero, A.C.; Pedraza-Chaverri, J. New insights into antioxidant strategies against paraquat toxicity. Free Radic. Res. 2014, 48, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.; Yan, Y.; Chi, M.; Chen, W.; Sun, P.; Qin, S. The protective effect of C-phycocyanin on paraquat-induced acute lung injury in rats. Environ. Toxicol. Pharmacol. 2011, 32, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.B.; Lin, C.C.; Chiou, J.F.; Mau, S.Y.; Liu, T.Z.; Chen, C.H. Treatment of acute paraquat intoxication using recommended megadose of vitamin C: A reappraisal. Free Radic. Res. 2013, 47, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.I.; Pincus, Z.; Slack, F.J. Longevity and stress in Caenorhabditis elegans. Aging 2011, 3, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Yanase, S.; Yasuda, K.; Ishii, N. Adaptive responses to oxidative damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span. Mech. Ageing Dev. 2002, 123, 1579–1587. [Google Scholar] [CrossRef]
- Wang, B.; Gong, Y.; Li, Z.; Yu, D.; Chi, C.; Ma, J. Isolation and characterisation of five novel antioxidant peptides from ethanol-soluble proteins hydrolysate of spotless smoothhound (Mustelus griseus) muscle. J. Funct. Foods 2014, 6, 176–185. [Google Scholar] [CrossRef]
- Zhang, L.; Jie, G.; Zhang, J.; Zhao, B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic. Biol. Med. 2009, 46, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Kamath, R.S.; Martinez-Campos, M.; Zipperlen, P.; Fraser, A.G.; Ahringer, J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2000, 2, research0002-1. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).