Abstract
Alotamide is a cyclic depsipetide isolated from a marine cyanobacterium and possesses a unique activation of calcium influx in murine cerebrocortical neurons (EC50 4.18 µM). Due to its limited source, the three stereocenters (C19, C28, and C30) in its polyketide fragment remain undetermined. In this study, the first asymmetric synthesis of its polyketide fragment was achieved. Four relative possible diastereomers were constructed with a boron-mediated enantioselective aldol reaction and Julia–Kocienski olefination as the key steps. Comparison of 13C NMR spectra revealed the relative structure of fragment C15–C32 of alotamide.
1. Introduction
Recently, several active secondary metabolites have been isolated from marine cyanobacterium and some of these metabolites demonstrate excellent bioactivities such as cytotoxic, antimicrobial, and antiprotozoal properties [1,2]. For example, apratoxins display potent cytotoxicity against several cancer cells at the nanoscale level and have become the new lead compounds in anticancer drug discovery [3,4,5,6].
Alotamide was also isolated from the marine cyanobacterium Lyngbya bouillonii in 2009 [7]. It is a cyclic depsipeptide and structurally has two parts. The northern part is a tripetide that consists of N-Me-Val, Cys-derived thiazolene ring, and Pro and the southern part is a special unsaturated polyketide with three undetermined stereocenters (C19, C28, and C30). Functionally, alotamide is a unique calcium influx activator in murine cerebrocortical neurons (EC50 4.18 µM). Given that calcium overload is involved in physiological processes and may lead to several nervous diseases such as AD and epilepsy, this compound has gained increasing attention as a new neurotoxin from the marine resource [8]. In view of the limited natural source, a concise synthetic strategy of alotamide should be developed. In this study, we described the first asymmetric synthesis of its polyketide fragment C15–C32 and obtained four possible diastereomers. The relative stereochemistry was assigned after the NMR comparison.
2. Results
The chemical structure of alotamide is shown in Figure 1. The southern polyketide with three undetermined stereocenters established eight possible isomers and only four needed to be evaluated for relative stereochemical determination. In this regard, we set C19 as R and listed four diastereomers, which are shown in Figure 2 (1a–1d).
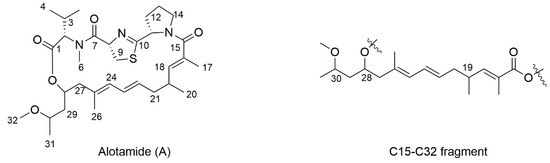
Figure 1.
Structure of alotamide.
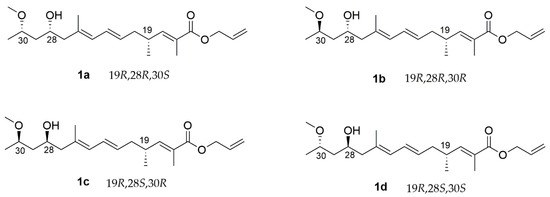
Figure 2.
Structures of four diastereomers 1a–1d.
According to the retrosynthetic analysis (Scheme 1), the dihydroxy unit would arise from an asymmetric aldol reaction and the Julia–Kocienski olefination would be applied to form the diene part. The polyketide fragment would be separated into two subunits, 2 and 3, which both could be prepared from commercial compounds.
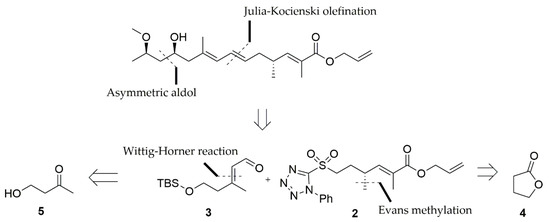
Scheme 1.
Retrosynthetic analysis.
Compound 2 was prepared from commercial lactone 4 and treated with LiOH to open the lactone ring (Scheme 2) [9]. The resulting compound was subjected to TBS protection and condensation with Evan’s protocol 6 [10] in sequence to obtain 7 in 71% yield over three steps. Compound 7 was treated with NaHMDS/MeI at −78 °C to obtain the desired R-methyl 8 in 89% yield (dr = 12:1). After the reduction of 8 by LiBH4 [11], alcohol 9 was oxidized into the corresponding aldehyde, which was immediately refluxed with the Wittig reagent 10 to generate olefin 11. In this reaction, the E/Z selectivity reached 30:1 and the little Z isomer was separated in the following DIBAL-H reduction. The sequential oxidation with IBX and Pinnick reaction generated a free acid and the allyl protection was conducted to acquire 12 in 64% yield for the above four steps. After the deprotection of the TBS group, the Mitsunobu reaction was applied to convert the compound 13 into tetrazole 15 in 94% yield. Then the SPT part was oxidized by H2O2 (20 eq) in the presence of (NH4)6Mo7O24 (0.2 eq) to obtain the desired 2 without the Z isomer [12].
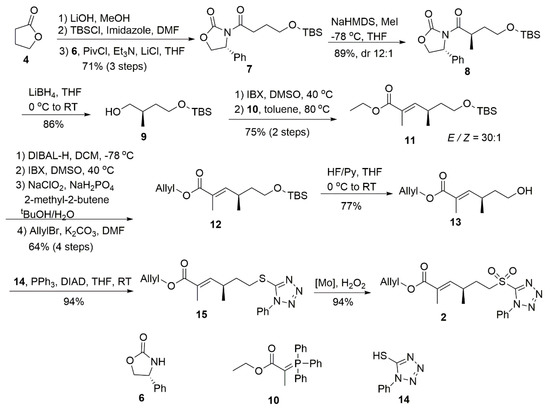
Scheme 2.
Synthesis of subunit 2.
The preparation of subunit 3 was initiated with 4-hydroxy-2-butanone (Scheme 3). The Wittig–Horner reaction was conducted after PMB protection to smoothly obtain the desired product 17 (70% yield, E/Z = 3:1) while a complex mixture appeared when TBS protected alcohol was applied in this HWE reaction [13,14]. After the removal of the PMB group followed by the TBS protection, compound 19 was obtained and then converted into the corresponding aldehyde 3 by sequential steps of DIBAL-H reduction and IBX oxidation prior to the Julia–Kocienski olefination.
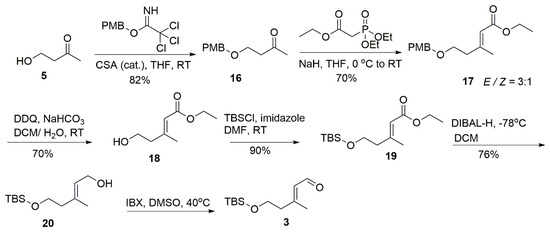
Scheme 3.
Synthesis of subunit 3.
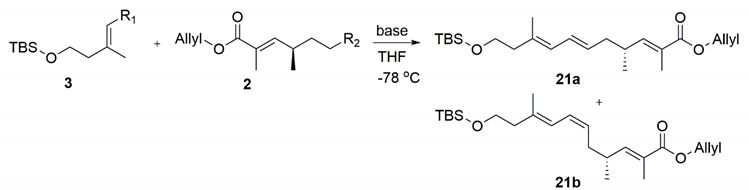
The different bases were tested through olefination (Table 1). When 1 eq KHMDS (entry 1) was applied, the yield was 50% and numerous reactants 2 and 3 remained. The product diene was an E/Z mixture with E/Z selectivity of up to 15:1. When the amount of KHMDS was increased to 1.5 eq, the yield improved and the two reactants remained in small quantities. When 2 eq KHMDS was used, the two reactants were completely consumed and the yield reached 86% while the high selectivity (15:1) was maintained. Other bases such as LiHMDS were also tried, but the resulting E/Z selectivity was low. We also exchanged the aldehyde and SO2PT functional groups and subjected them to olefination (entry 5). The yield of the Z isomer greatly increased and the selectivity was 1.5:1 [15,16].

Table 1.
Optimization of the Julia–Kocienski olefination.
Having completed the construction of diene 21a, we started to prepare the dihydroxy unit (Scheme 4). To our delight, under the condition of the HF/Py complex, we fairly achieved the alcohol 22 and oxidized it to corresponding aldehyde 23 by DMP prior to the aldol reaction (deprotection of PMB group in this step led to a complex mixture).
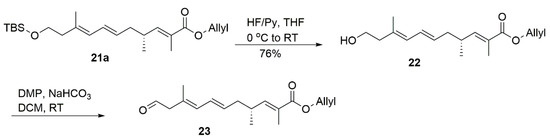
Scheme 4.
Synthesis of compound 23.
IPCBCl-controlled aldol reaction [17,18] was selected to install the C28 stereo-center (Table 2). The application of (−)-IPCBCl at −20 °C successfully generated 24b with the C28 (S) configuration in 59% yield (dr = 99:1). The chiral reactant was changed into (+)-IPCBCl, which afforded the C28 (R) product as expected and maintained the high diastereoselectivity (dr = 98:2). LiHMDS also proceeded and a 1:1 mixture was obtained in this aldol reaction.

Table 2.
Boron-mediated aldol reaction.
After TBS protection followed by the reduction of the combination of BH3-DMS and CBS catalysts, we obtained 26a (dr = 4:1, determined by 1H NMR) and 26b (dr = 17:1, determined by 1H NMR), respectively. Compounds 26c (dr = 3.6:1, determined by 1H NMR) and 26d (dr = 14:1, determined by 1H NMR) were achieved from 25b with the similar dr value (Scheme 5).
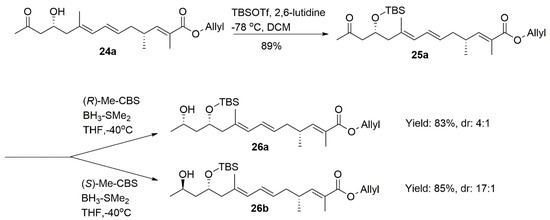
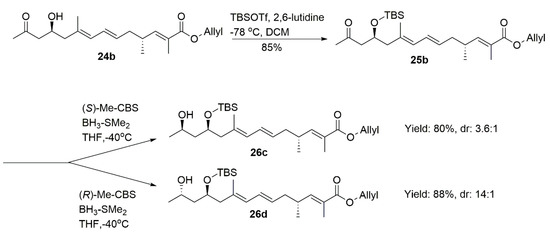
Scheme 5.
Synthesis of 26a–26d.
The stereochemistries of the 1,3-diol part in 26a–26d were confirmed by their 13C NMR chemical shifts of the corresponding acetonides 28a–28d (Scheme 6). Syn-diol acetonide preferred a chair conformation and two ketal methyl groups were significantly different (e.g., 19.94 and 30.46 ppm for 28a). Meanwhile anti-diol acetonide preferred a twist-boat conformation and two similar methyl groups existed (e.g., 25.24 and 25.13 ppm for 28b) [19,20,21].
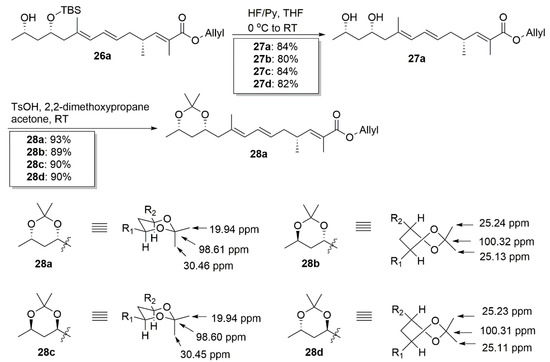
Scheme 6.
Stereo-chemical assignments of 28a–28d.
After the sequential methylation and removal of the TBS group from 26a–26d, we successfully furnished the four desired analogues 1a–1d (Scheme 7). The total yield was 2.5% for 1a from the lactone and 2.7% for 1b, 2.5% for 1c, and 2.6% for 1d.
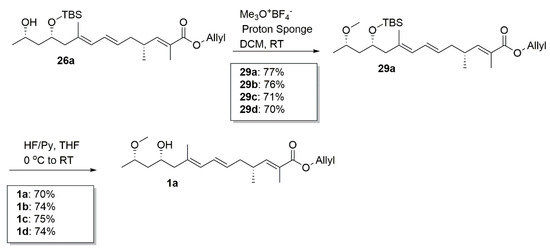
Scheme 7.
Synthesis of 1a–1d.
3. Discussion
A careful comparison between four isomers and alotamide was conducted. The differences in 13C NMR chemical shifts are shown in Figure 3a. From C15 to C28, several significant variations (Δδ > 1 ppm) existed between all four isomers and alotamide possibly because of the difference between the “straight-chain” mode in our analogues and the “ring” mode in the original structure. From C28 to C32, the dihydroxy unit is a linear chain both in our analogues and natural compound. Thus, comparing the data in this portion is suitable for relative stereochemistry determination.
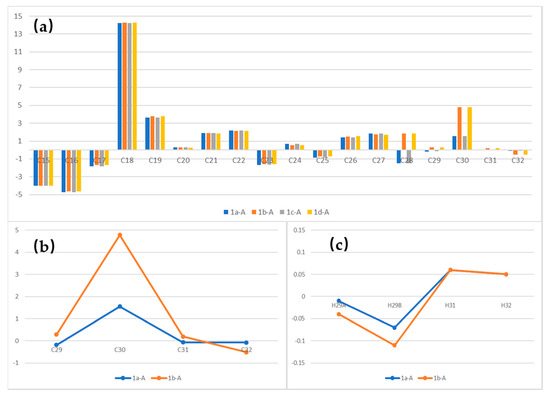
Figure 3.
(a) Δδ 13C (ppm) between alotamide and 1a–1d, (b) Δδ 13C (ppm) between alotamide and 1a and 1b from C29–C32, (c) Δδ 1H (ppm) between alotamide and 1a and 1b in dihydroxy unit.
At the same time, several obvious variations in 13C spectra were observed between 1,3-syn isomers (1a and 1c) and 1,3-anti isomers (1b and 1d) from C29 to C32 (Figure 3a). Therefore, we chose 1a and 1b to represent the syn-analogues and anti-analogues to distinguish syn-configurations and anti-configurations (Figure 3b,c).
A significant variation at C30 position of 1b (Δδ > 4 ppm) and a closer correlation of 1a at C29, C31 and C32 in the 13C spectra revealed the syn configuration in the C28–C30 unit. In addition, smaller variations with alotamide in 1H spectra were noticed for analogue 1a at protons H29A and H29B (differences between alotamide and 1a and 1b at proton H30 were not plotted in Figure 3c because the differences were all greater than 0.4 ppm and insignificant). This finding confirmed the 1,3-syn structure in the dihydroxy unit.
Two 1,3-syn isomers 1a and 1c were also compared. Their 1H spectra were the same and the differences in 13C NMR chemical shifts are listed in Table 3. A closer correlation of 1c was observed especially at the sites C20, C29, and C31. Thus, it appeared that 1c (19R, 28S, 30R) most closely fit the original natural alotamide. The total synthesis of alotamide with fragment 1c and another (19S, 28R, 30S) enantiomer is in progress.

Table 3.
Δδ 13C (ppm) between alotamide and 1a and 1c.
4. Materials and Methods
All anaerobic and moisture-sensitive manipulations were carried out with standard Schlenk techniques under argon. Solvents were dried and distilled by standard procedures. 1H- NMR and 13C-NMR spectra were recorded in CDCl3 on a Bruker Ascend-400 400 MHz or Bruker Ascend-500 500 MHz at room temperature. Chemical shifts (δ) are reported in ppm and are referenced to chloroform (δ 7.26 ppm for 1H, δ 77.16 ppm for 13C). Data for NMR spectra are reported as follows: s = singlet, d = doublet, t = triplet, q = quartet, quint. = quintet, sext. = sextet, m = multiplet, br = broad signal, J = coupling constant in Hz. HRMS were recorded on an Agilent 6530-Q-TOF mass spectrometer equipped with an Agilent 1260- HPLC. Optical rotations were measured on a PerkinElmer 241 MC polarimeter.
4.1. (R)-3-(4-((Tert-Butyldimethylsilyl)Oxy)Butanoyl)-4-Phenyloxazolidin-2-One (7)
To a solution of the lactone (5 g, 58 mmol) in MeOH (50 mL) at room temperature was added LiOH (2.44 g, 58 mmol) and stirred overnight. The solvent was removed from the reaction and the residue was dissolved in dimethylformamide (50 mL) at 0 °C. Imidazole (8 g, 116 mmol) was added to this solution, which was followed by TBSCl (8.7 g, 58 mmol) in two portions over 15 min. The reaction mixture was warmed to room temperature overnight with stirring and then diluted with 1 M HCl and extracted with EtOAc (3 × 100 mL). The combined organic layers were washed with brine and dried over Na2SO4. The organic layer was removed under vacuum and the crude acid was used without further purification.
To a stirred solution of the acid in dry THF (200 mL) at 0 °C under argon, Et3N (20 mL, 145 mmol) and PivCl (7.1 mL, 58 mmol) were added sequentially. After 1 h stirring at 0 °C, LiCl (0.62 g, 14.5 mmol), followed by oxazolidinone 6 (9.4 g, 58 mmol), were added. The reaction was continued for 1 h at 0 °C and another 2 h at room temperature prior to quenching with a saturated NH4Cl solution (50 mL) and extracted with DCM (2 × 200 mL). The combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 9:1) afforded compound 7 (15 g, 71% for three steps) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 7.47–7.21 (m, 5H), 5.43 (dd, J = 8.7, 3.6 Hz, 1H), 4.69 (td, J = 8.8, 1.0 Hz, 1H), 4.28 (ddd, J = 8.9, 3.6, 1.3 Hz, 1H), 3.64 (t, J = 6.3 Hz, 2H), 3.03 (qt, J = 17.7, 7.4 Hz, 2H), 1.92–1.77 (m, 2H), 0.90 (s, 9H), 0.04 (d, J = 1.3 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 172.62, 153.76, 139.27, 129.18, 128.68, 125.93, 70.00, 62.25, 61.91, 57.56, 32.08, 27.09, 25.96, 18.32, −5.34, −5.62. = −42.63, (c 3.38, CHCl3). HRMS (ESI+): calcd. for C19H29NO4Si [M + H]+, 364.1939; found 364.1940.
4.2. (R)-3-((R)-4-((Tert-Butyldimethylsilyl)oxy)-2-Methylbutanoyl)-4-Phenyloxazolidin-2-one (8)
To a stirred solution of compound 7 (15 g, 41.2 mmol) in dry THF (100 mL) at −78 °C under argon, NaHMDS (2.0 M solution in THF, 24.7 mL, 49.4 mmol) was added dropwise. After 1 h, MeI (7.7 mL, 123.5 mmol) was added and the mixture was stirred overnight at the same temperature. The reaction mixture was quenched with a saturated NH4Cl solution (100 mL) and warmed up to room temperature before being extracted with DCM (2 × 100 mL). The combined organic extracts were washed with water and brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 13:1) gave pure 8 (13.8 g, 89%) as a white solid.
1H NMR (400 MHz, CDCl3) δ 7.45–7.23 (m, 5H), 5.44 (dd, J = 8.7, 3.7 Hz, 1H), 4.67 (t, J = 8.8 Hz, 1H), 4.25 (dd, J = 8.9, 3.7 Hz, 1H), 4.02–3.85 (m, 1H), 3.73–3.55 (m, 2H), 2.00 (td, J = 13.9, 6.4 Hz, 1H), 1.59 (dq, J = 12.2, 6.1 Hz, 1H), 1.15 (d, J = 7.0 Hz, 3H), 0.91 (s, 9H), 0.06 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 176.36, 153.35, 139.47, 129.28, 128.70, 125.75, 69.80, 61.02, 57.74, 35.72, 34.71, 26.00, 18.39, 17.83, −5.33. = −79.14, (c 2.34, CHCl3). HRMS (ESI+): calcd. for C20H31NO4Si [M + H]+, 378.2095, found 378.2095.
4.3. (R)-4-((Tert-Butyldimethylsilyl)Oxy)-2-Methylbutan-1-Ol (9)
To an ice-cold solution of compound 8 (13.8 g, 36.6 mmol) in THF (100 mL) moist with a catalytic amount of water, LiBH4 (1.2 g, 54.9 mmol) was added portion wise under argon. After 12 h of stirring at room temperature, the reaction was quenched cautiously with a saturated NH4Cl solution (50 mL) and then distilled under a reduced pressure followed by extraction with DCM. The combined organic solution was dried over Na2SO4 and concentrated in vacuum. Purification by column chromatography (PE/EA = 9:1) provided pure compound 9 (6.86 g, 86%) as a colorless oil [11].
1H NMR (400 MHz, CDCl3) δ 3.81–3.69 (m, 1H), 3.68–3.60 (m, 1H), 3.46 (s, 1H), 3.43–3.33 (m, 1H), 3.19 (s, 1H), 1.86–1.70 (m, 1H), 1.59–1.46 (m, 2H), 0.89 (m, 12H), 0.05 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 68.21, 61.82, 37.53, 34.46, 25.98, 18.34, 17.46, −5.33, −5.36. = −10.98, (c 1.82, CHCl3). HRMS (ESI+): calcd. for C11H26O2Si [M + H]+, 219.1775, found 219.1771.
4.4. Ethyl (R,E)-6-((Tert-Butyldimethylsilyl)Oxy)-2,4-Dimethylhex-2-Enoate (11)
To a stirred solution of 9 (6.86 g, 31.5 mmol) in DMSO (50 mL) IBX (10.6 g, 37.8 mmol) was added. After 1 h stirring at 40 °C, the reaction was quenched with water (50 mL) and extracted with ether (2 × 100 mL). The combined organic layers were washed with brine, dried over Na2SO4, and removed under vacuum. The residue was refluxed with 10 (22 g, 63 mmol) in toluene (100 mL) at 80 °C for 3 h and the solvent was removed under vacuum. Purification by column chromatography (PE/EA = 40:1) provided pure compound 11 (7 g, 75%) as a colorless oil [22].
1H NMR (400 MHz, CDCl3) δ 6.53 (dd, J = 10.1, 1.4 Hz, 1H), 4.17 (q, J = 7.1 Hz, 2H), 3.70–3.41 (m, 2H), 2.80–2.45 (m, 1H), 1.83 (d, J = 1.4 Hz, 3H), 1.66–1.44 (m, 2H), 1.28 (t, J = 7.1 Hz, 3H), 1.00 (d, J = 6.7 Hz, 3H), 0.87 (s, 9H), 0.01 (d, J = 2.1 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 168.57, 147.56, 126.83, 61.02, 60.52, 39.83, 29.78, 26.04, 20.06, 18.37, 14.42, 12.59, −5.23, −5.25. = −2.167, (c 0.1, CHCl3). HRMS (ESI+): calcd. for C16H32O3Si [M + H]+, 301.2193, found 301.2194.
4.5. Allyl (R,E)-6-((Tert-Butyldimethylsilyl)oxy)-2,4-Dimethylhex-2-Enoate (12)
To a stirred solution of compound 11 (7 g, 23.6 mmol) in dry DCM (60 mL) at −78 °C under argon, DIBAL-H (1.5 M solution in toluene, 18.0 mL, 27.0 mmol) was added dropwise. After 1 h, the reaction mixture was quenched with aqueous sodium-potassium tartrate solution (20 mL) and warmed up to room temperature before being extracted with DCM (2 × 100 mL). The combined organic extracts were washed with water and brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 8:1) gave alcohol (4.8 g, 79%) as a colorless oil.
To a stirred solution of above alcohol in DMSO (50 mL), IBX (6.2 g, 22 mmol) was added. After 1 h stirring at 40 °C, the reaction was quenched with water (50 mL) and extracted with ether (2 × 100 mL). The combined organic layers were washed with brine, dried over Na2SO4, and removed under vacuum. The crude anhydride was used without further purification.
To the solution of above crude aldehyde in tBuOH (40 mL), NaH2PO4 (11 g, 93 mmol) in H2O (40 mL), 2-methyl-2-butene (19 mL, 186 mmol), and NaClO2 (3.1 g, 28 mmol, >79.0% purity) were added. After stirring for 2 h at room temperature, the mixture was extracted with EA (50 mL × 3) and the combined organic layers were washed with brine, dried over Na2SO4, filtrated, and concentrated. The crude acid was taken into the next step without future purification.
To a stirred solution of above acid in dry DMF (50 mL) allylBr (3.2 g, 37.2 mmol) and K2CO3 (5.1 g, 37.2 mmol) were added separately. After being stirred for 12 h, the mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with EA (100 mL × 3). The combined organic layers were washed with brine, dried over Na2SO4, filtrated, and concentrated. The residue was purified by column chromatography on silica gel (PE/EA = 40:1) to give 12 (4.7 g, 81%) for three steps as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.58 (dd, J = 10.1, 1.4 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.32 (ddd, J = 17.2, 3.0, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.3 Hz, 1H), 4.64 (dt, J = 5.6, 1.4 Hz, 2H), 3.66–3.42 (m, 2H), 2.82–2.58 (m, 1H), 1.86 (d, J = 1.4 Hz, 3H), 1.68–1.55 (m, 2H), 1.01 (d, J = 6.7 Hz, 3H), 0.88 (s, 10H), 0.02 (d, J = 2.2 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 168.16, 148.14, 132.76, 126.60, 117.84, 65.27, 61.00, 39.80, 29.82, 26.04, 20.02, 18.38, 12.62, −5.22. = −53.365, (c 0.52, CHCl3). HRMS (ESI+): calcd. for C17H32O3Si [M + H]+, 313.2193, found 313.2186.
4.6. Allyl (R,E)-6-Hydroxy-2,4-Dimethylhex-2-Enoate (13)
To a stirred solution of 12 (4.7 g, 15 mmol) in dry THF (10 mL), the HF/Py complex (2 mL) was added at 0 °C. After being stirred for 1 h, the mixture was quenched with a saturated aqueous solution of NaHCO3 and extracted with DCM (20 mL × 3). The combined organic layers were washed with 1 M HCl brine, dried over Na2SO4, filtrated, and concentrated. The residue was purified by column chromatography on silica gel (PE/EA = 6:1) to give 13 (2.3 g, 77%) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.59 (dd, J = 10.1, 1.4 Hz, 1H), 5.97 (ddt, J = 17.1, 10.6, 5.6 Hz, 1H), 5.34 (dq, J = 17.2, 1.5 Hz, 1H), 5.24 (ddd, J = 10.5, 2.6, 1.3 Hz, 1H), 4.65 (dt, J = 5.6, 1.4 Hz, 2H), 3.75–3.44 (m, 2H), 2.78–2.63 (m, 1H), 1.88 (d, J = 1.4 Hz, 3H), 1.75–1.62 (m, 1H), 1.62–1.50 (m, 2H), 1.05 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.07, 147.61, 132.63, 126.88, 117.97, 65.34, 60.94, 39.55, 29.97, 20.09, 12.62. = −55.72, (c 0.79, CHCl3). HRMS (ESI+): calcd. for C11H18O3 [M + H]+, 199.1329, found 199.1328.
4.7. Allyl (R,E)-2,4-Dimethyl-6-((1-Phenyl-1H-Tetrazol-5-yl)Thio)Hex-2-Enoate (15)
To a stirred solution of 13 (2.3 g, 11.6 mmol) in anhydrous dry THF (50 mL) at 0 °C under argon, PPh3 (6.1 g, 23.2 mmol), 14 (2 g, 11.6 mmol), and DIAD (4.6 mL, 23.2 mmol) were added sequentially. The reaction was continued further for 1 h at room temperature prior to quenching with the saturated NH4Cl solution (50 mL) and extracted with DCM (2 × 100 mL). The combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 15:1) afforded compound 15 (3.9 g, 94%) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 7.67–7.46 (m, 5H), 6.55 (dd, J = 10.1, 1.4 Hz, 1H), 5.94 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.42–5.29 (m, 1H), 5.22 (dd, J = 10.4, 1.3 Hz, 1H), 4.62 (dt, J = 5.6, 1.4 Hz, 2H), 3.41–3.13 (m, 2H), 2.77–2.60 (m, 1H), 2.06–1.90 (m, 1H), 1.89–1.74 (m, 4H), 1.05 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 167.69, 154.18, 146.10, 133.74, 132.49, 130.22, 129.89, 127.82, 123.90, 118.07, 65.40, 35.95, 32.63, 31.37, 19.89, 12.76. = −58.87, (c 1.47, CHCl3). HRMS (ESI+): calcd. for C18H22N4O2S [M + H]+, 359.1536, found 359.1532.
4.8. Allyl (R,E)-2,4-Dimethyl-6-((1-Phenyl-1H-Tetrazol-5-yl)sulfonyl)Hex-2-Enoate (2)
To a stirred solution of 15 (3.9 g, 10.1 mmol) in ethanol (100 mL) at 0 °C, (NH4)6Mo7O24 (2.9 g, 2.2 mmol) and H2O2 (20 mL) were added sequentially. The reaction was continued further for 12 h at room temperature prior to quenching with saturated NH4Cl solution (50 mL) and extracted with DCM (2 × 100 mL). The combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 8:1) afforded compound 2 (3.6 g, 94%) as a white solid.
1H NMR (400 MHz, CDCl3) δ 7.76–7.51 (m, 5H), 6.53 (dd, J = 10.1, 1.4 Hz, 1H), 5.97 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.48–5.32 (m, 1H), 5.25 (dd, J = 10.4, 1.3 Hz, 1H), 4.65 (dt, J = 5.6, 1.3 Hz, 2H), 3.77–3.58 (m, 2H), 2.95–2.62 (m, 1H), 2.24–2.04 (m, 1H), 2.01–1.92 (m, 1H), 1.88 (d, J = 1.4 Hz, 3H), 1.11 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 167.47, 153.49, 144.44, 133.12, 132.42, 131.64, 129.88, 128.93, 125.17, 118.32, 65.61, 54.40, 32.31, 28.74, 19.98, 12.86. = −13.87, (c 0.79, CHCl3). HRMS (ESI+): calcd. for C18H22N4O2S2 [M + H]+, 391.1435, found 391.1435.
4.9. 4-((4-Methoxybenzyl)Oxy)Butan-2-One (16)
To a stirred solution of 4-hydroxy-2-butanone (1.1 mL ,12.8 mmol) in dry THF (20 mL) at 0 °C, 4-methoxybenzyl 2,2,2-trichloroacetimidate (2 mL, 10.7 mmol) and triphenylcarbenium tetrafluoroborate (cat.) were added sequentially. The reaction was continued further for 1 h at room temperature prior to quenching with the saturated NH4Cl solution (50 mL) and extracted with DCM (2 × 100 mL). The combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 6:1) afforded compound 16 (1.8 g, 82%) as a colorless oil [14].
1H NMR (400 MHz, CDCl3) δ 7.33–7.18 (m, 2H), 6.94–6.81 (m, 2H), 4.45 (s, 2H), 3.81 (s, 3H), 3.72 (t, J = 6.3 Hz, 2H), 2.71 (t, J = 6.3 Hz, 2H), 2.18 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 207.51, 159.27, 130.16, 129.42, 113.84, 72.91, 64.96, 55.31, 43.81, 30.48. HRMS (ESI+): calcd. for C12H16O3 [M + H]+, 209.1172, found 209.1170.
4.10. Ethyl (E)-5-((4-Methoxybenzyl)Oxy)-3-Methylpent-2-Enoate (17)
To a stirred solution of triethyl phosphonoacetate (3.5 mL, 17.5 mmol) in dry THF (50 mL) at 0 °C, NaH (0.7 g, 17.5 mmol) was added. After stirring at room temperature for 1 h, 16 (1.8 g, 8.8 mmol) in THF (5 mL) was added at 0 °C. The reaction was continued further for 12 h at room temperature prior to quenching with a saturated NH4Cl solution (50 mL) and extracted with DCM (2 × 100 mL). The combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 20:1) afforded compound 17 in the E/Z mixture (1.7 g, 70%) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 7.27 (d, J = 8.6 Hz, 2H), 6.99–6.72 (m, 2H), 5.82–5.63 (m, 1H), 4.47 (d, J = 2.8 Hz, 2H), 4.23–4.08 (m, 2H), 3.82 (d, J = 2.1 Hz, 3H), 3.70–3.51 (m, 2H), 2.98 (t, J = 6.6 Hz, 1H), 2.45 (t, J = 6.4 Hz, 2H), 2.19 (d, J = 1.2 Hz, 2H), 1.96 (d, J = 1.3 Hz, 1H), 1.29 (td, J = 7.1, 5.4 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 166.73, 166.34, 159.33, 159.19, 157.81, 156.72, 130.70, 130.30, 129.38, 129.27, 117.46, 117.10, 113.90, 113.81, 72.76, 72.46, 68.74, 67.59, 59.60, 55.35, 40.89, 33.73, 26.16, 19.00, 14.42. HRMS (ESI+): calcd. for C16H22O4 [M + H]+, 279.1591; found 279.1589.
4.11. Ethyl (E)-5-Hydroxy-3-Methylpent-2-Enoate (18)
To a stirring solution of 17 (1.7 g, 6.1 mmol) in DCM (20 mL) and water (4 mL) DDQ (1.67 g, 7.3 mmol) was added at room temperature. After 1 h, the reaction mixture was quenched with saturated aqueous NaHCO3 (10 mL) and 1 M aqueous NaHSO3 (10 mL) and extracted with DCM (30 mL × 3). The combined organic layers were washed with brine, dried over Na2SO4, and concentrated. The crude product was purified by column chromatography (PE/EA = 3:1) to give the desired compound 18 as a colorless oil (672 mg, 70% yield) [13].
1H NMR (400 MHz, CDCl3) δ 5.71 (d, J = 1.2 Hz, 1H), 4.12 (q, J = 7.1 Hz, 2H), 3.76 (t, J = 6.4 Hz, 2H), 2.47–2.30 (m, 2H), 2.16 (d, J = 1.2 Hz, 3H), 1.92 (s, 1H), 1.25 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 166.67, 156.12, 117.73, 60.22, 59.78, 43.82, 18.82, 14.37. HRMS (ESI+): calcd. for C8H14O3 [M + H]+, 159.1016, found 159.1014.
4.12. Ethyl (E)-5-((Tert-Butyldimethylsilyl)Oxy)-3-Methylpent-2-Enoate (19)
To a stirred solution of 18 (672 mg, 4.3 mmol) in anhydrous DMF (10 mL) at 0 °C, imidazole (7.7 g, 11.4 mmol) and TBSCl (1.1 g, 6.84 mmol) were added sequentially. The reaction was continued further for 12 h at room temperature prior to quenching with saturated NH4Cl solution (20 mL) and extracted with EA (2 × 20 mL). The combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 40:1) created compound 19 (1.05 g, 90%) as a colorless oil [13].
1H NMR (400 MHz, CDCl3) δ 5.66 (d, J = 1.1 Hz, 1H), 4.12 (q, J = 7.1 Hz, 2H), 2.32 (t, J = 6.6 Hz, 2H), 2.15 (d, J = 1.2 Hz, 3H), 1.24 (t, J = 7.1 Hz, 3H), 0.86 (s, 9H), 0.02 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 166.74, 156.92, 117.34, 61.41, 59.53, 44.12, 25.97, 19.21, 18.36, 14.42, −5.29. HRMS (ESI+): calcd. for C14H28O3Si [M + H]+, 273.1880, found 273.1880.
4.13. (E)-5-((Tert-Butyldimethylsilyl)Oxy)-3-Methylpent-2-En-1-Ol (20)
To a stirred solution of compound 19 (1.05 g, 3.87 mmol) in dry DCM (15 mL) at −78 °C under argon, DIBAL-H (1.5 M solution in toluene, 3.9 mL, 5.8 mmol) was added dropwise. After 1 h, the reaction mixture was quenched with the aqueous sodium-potassium tartrate solution (20 mL) and warmed up to room temperature before being extracted with DCM (2 × 20 mL). The combined organic extracts were washed with water and brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 7:1) gave 20 (680 mg, 76%) as a colorless oil [23].
1H NMR (400 MHz, CDCl3) δ 5.45 (td, J = 6.9, 1.2 Hz, 1H), 4.16 (d, J = 6.9 Hz, 2H), 3.71 (t, J = 7.0 Hz, 2H), 2.25 (t, J = 7.0 Hz, 2H), 1.71 (s, 3H), 1.53 (s, 1H), 0.90 (s, 9H), 0.06 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 136.95, 125.38, 62.20, 59.42, 42.91, 26.05, 18.45, 16.80, −5.17. HRMS (ESI+): calcd. for C12H26O2Si ([M + H]+, 231.1775, found 231.1775.
4.14. Allyl (R,2E,6E,8E)-11-((Tert-butyldimethylsilyl)Oxy)-2,4,9-Trimethylundeca-2,6,8-Trienoate (21a)
To a stirred solution of 20 (680 mg, 3.0 mmol) in DMSO (10 mL) IBX (990 mg, 3.5 mmol) was added. After 1 h of stirring at 40 °C, the reaction was quenched with water (10 mL) and extracted with ether (2 × 20 mL). The combined organic layers were washed with brine, dried over Na2SO4, and removed under vacuum. The crude aldehyde 3 was used without further purification.
To a stirred solution of crude 3 and compound 2 (1.3 g, 3.3 mmol) in dry THF (30 mL) at −78 °C under argon, KHMDS (1.0 M solution in THF, 27.0 mL, 27.0 mmol) was added dropwise. After 1 h, the reaction mixture was quenched with the saturated NH4Cl solution (20 mL) and warmed up to room temperature before being extracted with DCM (2 × 100 mL). The combined organic extracts were washed with water and brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 40:1) gave 21a (1.0 g, 86%, 2 steps) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.61 (dd, J = 10.0, 1.4 Hz, 1H), 6.23 (dd, J = 15.0, 10.8 Hz, 1H), 5.97 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.79 (d, J = 10.8 Hz, 1H), 5.48 (dt, J = 14.8, 7.3 Hz, 1H), 5.33 (ddd, J = 17.2, 3.1, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.3 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 3.68 (t, J = 7.0 Hz, 2H), 2.64–2.47 (m, 1H), 2.24 (t, J = 7.0 Hz, 2H), 2.14 (t, J = 7.1 Hz, 2H), 1.85 (d, J = 1.3 Hz, 3H), 1.74 (s, 3H), 1.02 (d, J = 6.7 Hz, 3H), 0.88 (s, 9H), 0.04 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 168.13, 147.84, 134.13, 132.75, 129.61, 128.53, 126.49, 126.40, 117.83, 65.27, 62.39, 43.29, 40.02, 33.96, 26.09, 19.68, 18.47, 17.20, 12.73, −5.14. = −32.72, (c 0.38, CHCl3). HRMS (ESI+): calcd. for C23H40O3Si [M + H]+, 293.2819, found 293.2819.
4.15. Allyl (R,2E,6E,8E)-11-Hydroxy-2,4,9-Trimethylundeca-2,6,8-Trienoate (22)
To a stirred solution of 21a (1.0 g, 2.6 mmol) in dry THF (5 mL), HF/Py complex (1 mL) was added at 0 °C. After being stirred for 1 h, the mixture was quenched with a saturated aqueous solution of NaHCO3 and extracted with DCM (20 mL × 3). The combined organic layers were washed with 1 M HCl, brine, dried over Na2SO4, filtrated, and concentrated. The residue was purified by column chromatography on silica gel (PE/EA = 6:1) to give 22 (550 mg, 76%) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 10.0, 1.4 Hz, 1H), 6.24 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.85 (d, J = 10.8 Hz, 1H), 5.52 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (ddd, J = 17.2, 3.0, 1.5 Hz, 1H), 5.22 (dd, J = 10.4, 1.3 Hz, 1H), 4.63 (d, J = 5.6 Hz, 2H), 3.69 (t, J = 6.3 Hz, 2H), 2.65–2.51 (m, 1H), 2.29 (t, J = 6.3 Hz, 2H), 2.15 (t, J = 7.0 Hz, 2H), 1.84 (d, J = 1.4 Hz, 3H), 1.75 (s, 3H), 1.54 (s, 1H), 1.01 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.09, 147.69, 132.93, 132.70, 130.49, 128.14, 127.38, 126.53, 117.82, 65.26, 60.49, 42.95, 39.97, 33.84, 19.67, 16.50, 12.72. = −38.23, (c 0.96, CHCl3). HRMS (ESI+): calcd. for C17H26O3 [M + H]+, 279.1955, found 279.1954.
4.16. Allyl (2E,4R,6E,8E,11R)-11-Hydroxy-2,4,9-Trimethyl-13-Oxotetradeca-2,6,8-Trienoate (24a)
To the above alcohol 22 (200 mg, 0.72 mmol) in dry DCM (5 mL, 0 °C), DMP (610 mg, 1.44 mmol) and NaHCO3 (240 mg, 2.9 mmol) was added sequentially. After being stirred for 30 min, the mixture was carefully quenched with a solution of saturated aqueous NaHCO3 and Na2S2O3. The resulting mixture was extracted with DCM (20 mL × 3) and the combined organic layers were washed with brine, dried over Na2SO4, and concentrated. The crude aldehyde 23 was used without future purification.
To a stirred solution of (+)-IPCBCl (1.8 M in heptane, 1.08 mmol, 0.6 mL) in dry ether (3 mL) at −0 °C under argon, Et3N (0.2 mL, 1.44 mmol) and acetone (80 uL, 1.08 mmol) were added sequentially. After 1 h stirring at −0 °C, aldehyde 23 in ether (2 mL) was added at −78 °C. The reaction was continued further for 1 h at −78 °C and another 12 h at −20 °C prior to quenching with a mixture of PH 7 buffer (1 mL), methanol (1 mL), and H2O2 (1 mL). The mixture was warmed up to room temperature before being extracted with ether (2 × 10 mL). The combined organic extracts were washed with water and brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 4:1) created 24a (132 mg, 55%) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 10.0, 1.2 Hz, 1H), 6.22 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.83 (d, J = 10.8 Hz, 1H), 5.52 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (dd, J = 17.2, 1.4 Hz, 1H), 5.23 (dd, J = 10.4, 1.1 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.20 (tt, J = 11.6, 5.9 Hz, 1H), 2.79 (s, 1H), 2.66–2.47 (m, 3H), 2.24 (dd, J = 13.4, 7.5 Hz, 1H), 2.19–2.07 (m, 6H), 1.84 (d, J = 1.2 Hz, 3H), 1.75 (s, 3H), 1.02 (d, J = 6.7 Hz, 3H).13C NMR (101 MHz, CDCl3) δ 209.56, 168.10, 147.68, 132.82, 132.72, 130.79, 128.10, 127.99, 126.58, 117.85, 65.91, 65.29, 49.62, 47.13, 40.02, 33.86, 30.98, 19.72, 16.89, 12.75. = −33.92, (c 0.34, CHCl3). HRMS (ESI+): calcd. for C20H30O4 [M + H]+, 335.2217, found 335.2217.
4.17. Allyl (2E,4R,6E,8E,11S)-11-Hydroxy-2,4,9-Trimethyl-13-Oxotetradeca-2,6,8-Trienoate (24b)
To a stirred solution of (−)-IPCBCl (1.7 M in heptane, 1.08 mmol, 0.64 mL) in dry ether (3 mL) at 0 °C under argon, Et3N (0.2 mL, 1.44 mmol) and acetone (80 uL, 1.08 mmol) were added sequentially. After 1 h stirring at 0 °C, aldehyde 23 in ether (2 mL) was added at −78 °C. The reaction was continued further for 1 h at −78 °C and another 12 h at −20 °C prior to quenching with a mixture of PH 7 buffer (1 mL), methanol (1 mL), and H2O2 (1 mL). The mixture was warmed up to room temperature before being extracted with ether (2 × 10 mL). The combined organic extracts were washed with water and brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 4:1) provided 24b (142 mg, 59%) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 10.0, 1.2 Hz, 1H), 6.23 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.83 (d, J = 10.8 Hz, 1H), 5.53 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (dd, J = 17.2, 1.4 Hz, 1H), 5.23 (dd, J = 10.4, 1.1 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.29–4.12 (m, 1H), 2.66–2.51 (m, 3H), 2.23 (dd, J = 13.5, 7.6 Hz, 1H), 2.19–2.08 (m, 6H), 1.84 (d, J = 1.2 Hz, 3H), 1.75 (s, 3H), 1.02 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 209.53, 168.10, 147.67, 132.83, 132.72, 130.76, 128.12, 127.98, 126.57, 117.86, 65.92, 65.29, 49.66, 47.11, 39.97, 33.86, 30.98, 19.69, 16.91, 12.74. = −26.04, (c 0.37, CHCl3). HRMS (ESI+): calcd. for C20H30O4 [M + H]+, 335.2217, found 335.2216.
4.18. Allyl (2E,4R,6E,8E,11R)-11-((Tert-Butyldimethylsilyl)oxy)-2,4,9-Trimethyl-13-Oxotetradeca-2,6,8-Trienoate (25a)
To a stirred solution of compound 24a (132 mg, 0.4 mmol) in dry DCM (3 mL) at −78 °C under argon, 2,6-lutidine (0.23 mL, 2 mmol) and TBSOTf (0.35 mL, 1.5 mmol) were added sequentially. After 1 h, the reaction mixture was quenched with aqueous NaHCO3 (5 mL) and warmed up to room temperature before being extracted with DCM (2 × 10 mL). The combined organic extracts were washed with 1 M HCl and brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 40:1) created 25a (160 mg, 89%) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 10.0, 1.3 Hz, 1H), 6.19 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.75 (d, J = 10.8 Hz, 1H), 5.48 (dt, J = 14.8, 7.3 Hz, 1H), 5.33 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.2 Hz, 1H), 4.64 (d, J = 5.6 Hz, 2H), 4.38–4.20 (m, 1H), 2.67–2.41 (m, 3H), 2.29–2.20 (m, 1H), 2.19–2.00 (m, 6H), 1.84 (d, J = 1.1 Hz, 3H), 1.73 (s, 3H), 1.01 (d, J = 6.7 Hz, 3H), 0.85 (s, 9H), 0.03 (d, J = 8.1 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 208.04, 168.10, 147.75, 133.24, 132.74, 130.28, 128.31, 128.07, 126.53, 117.86, 68.06, 65.29, 50.86, 48.64, 40.00, 33.93, 31.85, 25.96, 19.67, 18.12, 17.30, 12.74, −4.41, −4.71. = −40.90, (c 0.26, CHCl3). HRMS (ESI+): calcd. for C26H44O4Si [M + H]+, 449.3082, found 449.3078.
4.19. Allyl (2E,4R,6E,8E,11S)-11-((Tert-Butyldimethylsilyl)oxy)-2,4,9-Trimethyl-13-Oxotetradeca-2,6,8-Trienoate (25b)
The procedure was identical to 25a. Compound 25b (160 mg, 85%) was obtained as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 10.0, 1.3 Hz, 1H), 6.19 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.74 (d, J = 10.8 Hz, 1H), 5.48 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.3 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.35–4.23 (m, 1H), 2.66–2.42 (m, 3H), 2.23 (dd, J = 13.2, 5.7 Hz, 1H), 2.19–2.05 (m, 6H), 1.84 (d, J = 1.2 Hz, 3H), 1.73 (s, 3H), 1.01 (d, J = 6.7 Hz, 3H), 0.84 (s, 9H), 0.02 (d, J = 7.1 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 208.07, 168.09, 147.76, 133.25, 132.74, 130.27, 128.31, 128.04, 126.52, 117.85, 68.09, 65.28, 50.84, 48.60, 40.04, 33.92, 31.84, 25.95, 19.71, 18.11, 17.33, 12.73, −4.42, −4.74. = −17.13, (c 0.51, CHCl3). HRMS (ESI+): calcd. for C26H44O4Si [M + H]+, 449.3082, found 449.3079.
4.20. Allyl (2E,4R,6E,8E,11R,13S)-11-((Tert-Butyldimethylsilyl)Oxy)-13-Hydroxy-2,4,9-Trimethyltetradeca-2,6,8-Trienoate (26a)
To a solution of (R)-Me-CBS (1 M in toluene, 0.05 mL, 0.05 mmol) in dry THF (5 mL) was slowly added BH3·DMS (12 uL, 0.13 mmol) at −40 °C. After being stirred for 30 min at the same temperature, a solution of 25a (57 mg, 0.13 mmol) in THF (2 mL) was slowly added. After being stirred for 2 h at −40 °C, the mixture was diluted with MeOH. The resulting mixture was concentrated and extracted with DCM (30 mL × 3). The combined organic layers were washed with brine, dried, filtrated, and concentrated. The residue was purified by column chromatography on silica gel (PE/EA = 15:1) to give a mixture of 26a and 26b (4:1, 48 mg, 83%) as a colorless oil (pure major isomer 26a can be obtained by repeating the purification on silica gel).
1H NMR (400 MHz, CDCl3) δ 6.60 (d, J = 9.9 Hz, 1H), 6.18 (dd, J = 14.7, 11.0 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.77 (d, J = 10.8 Hz, 1H), 5.49 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (dd, J = 17.2, 1.4 Hz, 1H), 5.23 (dd, J = 10.5, 1.0 Hz, 1H), 4.63 (d, J = 5.5 Hz, 2H), 4.27–4.06 (m, 2H), 2.66–2.47 (m, 1H), 2.35 (dd, J = 13.4, 6.3 Hz, 1H), 2.23 (dd, J = 13.3, 7.6 Hz, 1H), 2.16 (dd, J = 20.8, 13.7 Hz, 2H), 1.84 (s, 3H), 1.71 (s, 3H), 1.61 (ddd, J = 14.0, 10.0, 3.7 Hz, 1H), 1.48 (ddd, J = 14.5, 9.4, 3.7 Hz, 1H), 1.14 (d, J = 6.2 Hz, 3H), 0.99 (d, J = 11.9 Hz, 3H), 0.88 (s, 9H), 0.07 (d, J = 18.8 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 168.12, 147.74, 133.01, 132.72, 130.26, 128.29, 127.95, 126.52, 117.86, 70.36, 65.29, 64.58, 46.98, 43.26, 40.03, 33.91, 25.95, 23.98, 19.68, 18.06, 17.15, 12.76, −4.51, −4.70. = −17.67, (c 0.1, CHCl3). HRMS (ESI+): calcd. for C26H46O4Si [M + H]+, 451.3238, found 451.3241.
4.21. Allyl (2E,4R,6E,8E,11R,13R)-11-((Tert-Butyldimethylsilyl)Oxy)-13-Hydroxy-2,4,9-Trimethyltetradeca-2,6,8-Trienoate (26b)
To a solution of (S)-Me-CBS (1 M in toluene, 0.1 mL, 0.1 mmol) in dry THF (5 mL), BH3·DMS (24 uL, 0.25 mmol) was slowly added at −40 °C. After being stirred for 30 min at the same temperature, a solution of 25a (110 mg, 0.24 mmol) in THF (2 mL) was slowly added. After being stirred for 2 h at −40 °C, the mixture was diluted with MeOH. The resulting mixture was concentrated and extracted with DCM (30 mL × 3). The combined organic layers were washed with brine, dried, filtrated, and concentrated. The residue was purified by column chromatography on silica gel (PE/EA = 15:1) to create 26b (92 mg, 85%) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 10.0, 1.3 Hz, 1H), 6.19 (dd, J = 15.0, 10.8 Hz, 1H), 6.04–5.92 (m, 1H), 5.77 (d, J = 10.8 Hz, 1H), 5.51 (dd, J = 14.9, 7.4 Hz, 1H), 5.33 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.3 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.01 (dq, J = 13.0, 4.3 Hz, 1H), 3.96–3.83 (m, 1H), 3.18 (s, 1H), 2.66–2.50 (m, 1H), 2.32 (dd, J = 13.1, 4.6 Hz, 1H), 2.12 (dt, J = 13.2, 7.9 Hz, 3H), 1.84 (d, J = 1.1 Hz, 3H), 1.72 (s, 3H), 1.59–1.52 (m, 1H), 1.47–1.37 (m, 1H), 1.14 (d, J = 6.2 Hz, 3H), 1.02 (d, J = 6.7 Hz, 3H), 0.89 (s, 9H), 0.12 (d, J = 8.4 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 168.12, 147.73, 132.79, 132.72, 130.34, 128.23, 127.93, 126.54, 117.86, 72.22, 67.43, 65.29, 49.26, 44.82, 40.01, 33.91, 25.95, 23.70, 19.70, 18.03, 17.28, 12.75, −3.77, −4.55. = −40.33, (c 0.35, CHCl3). HRMS (ESI+): calcd. for C26H46O4Si [M + H]+, 451.3238, found 451.3241.
4.22. Allyl (2E,4R,6E,8E,11S,13R)-11-((Tert-Butyldimethylsilyl)Oxy)-13-Hydroxy-2,4,9-Trimethyltetradeca-2,6,8-Trienoate (26c)
To a solution of (S)-Me-CBS (1 M in toluene, 0.05 mL, 0.05 mmol) in dry THF (5 mL), BH3·DMS (12 uL, 0.13 mmol) was slowly added at −40 °C. After being stirred for 30 min at the same temperature, a solution of 25b (55 mg, 0.12 mmol) in THF (2 mL) was slowly added. After being stirred for 2 h at −40 °C, the mixture was diluted with MeOH. The resulting mixture was concentrated and extracted with DCM (30 mL × 3). The combined organic layers were washed with brine, dried, filtrated, and concentrated. The residue was purified by column chromatography on silica gel (PE/EA = 15:1) to give a mixture of 26c and 26d (3.6:1, 44 mg, 80%) as a colorless oil (pure major isomer 26c can be obtained by repeating the purification on silica gel).
1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 10.0, 1.3 Hz, 1H), 6.19 (dd, J = 15.0, 10.9 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.77 (d, J = 10.8 Hz, 1H), 5.49 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.2 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.23–4.07 (m, 2H), 3.36 (s, 1H), 2.67–2.49 (m, 1H), 2.35 (dd, J = 13.3, 6.2 Hz, 1H), 2.29–2.21 (m, 1H), 2.14 (t, J = 7.0 Hz, 2H), 1.84 (d, J = 1.1 Hz, 3H), 1.71 (s, 3H), 1.64–1.56 (m, 1H), 1.49 (ddd, J = 14.5, 9.5, 3.7 Hz, 1H), 1.14 (d, J = 6.2 Hz, 3H), 1.01 (d, J = 6.6 Hz, 3H), 0.88 (s, 9H), 0.06 (d, J = 20.4 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 168.09, 147.74, 133.01, 132.71, 130.27, 128.27, 127.94, 126.52, 117.86, 70.43, 65.29, 64.58, 46.92, 43.25, 40.04, 33.93, 25.95, 23.98, 19.73, 18.06, 17.21, 12.73, −4.52, −4.71. = −14.83, (c 0.1, CHCl3). HRMS (ESI+): calcd. for C26H46O4Si [M + H]+, 451.3238, found 451.3240.
4.23. Allyl (2E,4R,6E,8E,11S,13S)-11-((Tert-Butyldimethylsilyl)Oxy)-13-Hydroxy-2,4,9-Trimethyltetradeca-2,6,8-Trienoate (26d)
To a solution of (R)-Me-CBS (1 M in toluene, 0.1 mL, 0.1 mmol) in dry THF (5 mL), BH3·DMS (24 uL, 0.25 mmol) was slowly added at −40 °C. After being stirred for 30 min at the same temperature, a solution of 25b (113 mg, 0.25 mmol) in THF (2 mL) was slowly added. After being stirred for 2 h at −40 °C, the mixture was diluted with MeOH. The resulting mixture was concentrated and extracted with DCM (30 mL × 3). The combined organic layers were washed with brine, dried, filtrated, and concentrated. The residue was purified by column chromatography on silica gel (PE/EA = 15:1) to provide 26d (100 mg, 88%) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 10.0, 1.3 Hz, 1H), 6.19 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.76 (d, J = 10.8 Hz, 1H), 5.50 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.3 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.11–3.98 (m, 1H), 3.95–3.82 (m, 1H), 3.12 (s, 1H), 2.67–2.51 (m, 1H), 2.32 (dd, J = 13.2, 4.7 Hz, 1H), 2.19–2.06 (m, 3H), 1.84 (d, J = 1.2 Hz, 3H), 1.71 (s, 3H), 1.56 (dt, J = 14.4, 3.2 Hz, 1H), 1.46–1.38 (m, 1H), 1.14 (d, J = 6.2 Hz, 3H), 1.02 (d, J = 6.7 Hz, 3H), 0.89 (s, 9H), 0.11 (d, J = 8.9 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 168.10, 147.74, 132.78, 132.71, 130.32, 128.22, 127.91, 126.53, 117.84, 72.26, 67.41, 65.28, 49.21, 44.81, 40.03, 33.90, 25.95, 23.70, 19.72, 18.02, 17.33, 12.74, −3.78, −4.56. = −15.29, (c 0.63, CHCl3). HRMS (ESI+): calcd. for C26H46O4Si [M + H]+, 451.3238, found 451.3228.
4.24. Synthetic Procedure of 27a–27d
To a stirred solution of 26a (35 mg) in dry THF (2 mL), the HF/Py complex (0.7 mL) was added at 0 °C. After being stirred for 1 h, the mixture was quenched with a saturated aqueous solution of NaHCO3 and extracted with DCM (10 mL × 3). The combined organic layers were washed with 1 M HCl, brine, dried over Na2SO4, filtrated, and concentrated. The residue was purified by a column chromatography on silica gel (PE/EA = 2:1) to create 27a (22 mg, 84%) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.64–6.56 (m, 1H), 6.23 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.84 (d, J = 10.8 Hz, 1H), 5.54 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (dd, J = 17.2, 1.4 Hz, 1H), 5.23 (dd, J = 10.5, 0.9 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.13–4.02 (m, 1H), 4.02–3.92 (m, 1H), 2.65–2.51 (m, 1H), 2.24–2.10 (m, 4H), 1.84 (d, J = 1.3 Hz, 3H), 1.75 (s, 3H), 1.61–1.55 (m, 1H), 1.55–1.47 (m, 1H), 1.20 (d, J = 6.2 Hz, 3H), 1.02 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.11, 147.65, 132.71, 132.58, 130.99, 128.31, 128.00, 126.60, 117.87, 70.45, 68.91, 65.30, 48.84, 44.80, 40.00, 33.83, 24.07, 19.72, 16.88, 12.75. = −29.76, (c 0.34, CHCl3). HRMS (ESI+): calcd. for C20H32O4 [M + Na]+,337.2373, found 337.2375.
Compound 27b (20.9 mg, 80%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 10.0, 1.3 Hz, 1H), 6.23 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.85 (d, J = 10.7 Hz, 1H), 5.54 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.2 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.21–4.12 (m, 1H), 4.08 (ddd, J = 12.4, 7.8, 5.3 Hz, 1H), 2.65–2.51 (m, 1H), 2.22–2.18 (m, 2H), 2.16 (d, J = 7.2 Hz, 2H), 1.84 (d, J = 1.3 Hz, 3H), 1.76 (s, 3H), 1.67–1.54 (m, 2H), 1.23 (d, J = 6.3 Hz, 3H), 1.02 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.11, 147.65, 133.00, 132.71, 130.92, 128.15, 128.03, 126.59, 117.86, 66.73, 65.47, 65.30, 48.14, 44.09, 40.01, 33.84, 23.69, 19.72, 16.81, 12.75. = −14.00, (c 0.05, CHCl3). HRMS (ESI+): calcd. for C20H32O4 [M + Na]+,337.2373, found 337.2377.
Compound 27c (22 mg, 84%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 10.0, 1.3 Hz, 1H), 6.23 (dd, J = 14.9, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.84 (d, J = 10.8 Hz, 1H), 5.54 (dt, J = 14.9, 7.3 Hz, 1H), 5.33 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.2 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.10–4.02 (m, 1H), 4.01–3.94 (m, 1H), 2.62–2.52 (m, 1H), 2.20–2.11 (m, 4H), 1.84 (d, J = 1.3 Hz, 3H), 1.75 (s, 3H), 1.61–1.55 (m, 1H), 1.54–1.46 (m, 1H), 1.20 (d, J = 6.2 Hz, 3H), 1.02 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.12, 147.64, 132.71, 132.57, 130.99, 128.32, 128.01, 126.60, 117.87, 70.45, 68.91, 65.31, 48.84, 44.81, 39.99, 33.85, 24.07, 19.70, 16.88, 12.75. = −29.37, (c 0.32, CHCl3). HRMS (ESI+): calcd. for C20H32O4 [M + Na]+,337.2373, found 337.2374.
Compound 27d (21.4 mg, 82%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 6.23 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.86 (d, J = 10.8 Hz, 1H), 5.54 (dt, J = 14.8, 7.3 Hz, 1H), 5.33 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.3 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.21–4.11 (m, 1H), 4.12–4.02 (m, 1H), 2.65–2.51 (m, 1H), 2.23–2.18 (m, 2H), 2.15 (t, J = 7.2 Hz, 2H), 1.84 (d, J = 1.3 Hz, 3H), 1.76 (s, 3H), 1.65–1.56 (m, 2H), 1.23 (d, J = 6.3 Hz, 3H), 1.02 (d, J = 6.6 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.12, 147.64, 132.99, 132.72, 130.95, 128.18, 128.04, 126.60, 117.87, 66.72, 65.48, 65.31, 48.14, 44.10, 39.99, 33.86, 23.69, 19.70, 16.82, 12.76. = −17.62, (c 0.32, CHCl3). HRMS (ESI+): calcd. for C20H32O4 [M + Na]+, 337.2373, found 337.2374.
4.25. Synthetic Procedure of 28a–28d
To a stirred solution of compound 27a (22 mg, 0.065 mmol) in dry acetone (2 mL) under argon, PTSA· H2O (1.14 mg, 0.006 mmol) and 2,2-dimethylpropane (0.08 mL, 0.65 mmol) were added sequentially. After 1 h, the reaction mixture was quenched with a saturated aqueous solution of NaHCO3 and extracted with DCM (2 × 10 mL). The combined organic extracts were washed with water and brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 20:1) provided 28a (23 mg, 93%) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.61 (dd, J = 9.9, 1.3 Hz, 1H), 6.23 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.80 (d, J = 10.8 Hz, 1H), 5.50 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.3 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.04–3.88 (m, 2H), 2.65–2.50 (m, 1H), 2.30 (dd, J = 13.6, 5.9 Hz, 1H), 2.15 (t, J = 7.1 Hz, 2H), 2.06 (dd, J = 13.6, 7.0 Hz, 1H), 1.84 (d, J = 1.1 Hz, 3H), 1.74 (s, 3H), 1.49–1.42 (m, 4H), 1.41–1.34 (m, 4H), 1.15 (d, J = 6.1 Hz, 3H), 1.02 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.11, 147.77, 133.12, 132.72, 129.98, 128.39, 127.15, 126.52, 117.83, 98.61, 67.97, 65.34, 65.27, 46.85, 40.00, 38.60, 33.90, 30.46, 22.38, 19.94, 19.69, 17.34, 12.74. = −30.22, (c 0.26, CHCl3). HRMS (ESI+): calcd. for C23H36O4 [M + Na]+,377.2686, found 377.2689.
Compound 28b (20.8 mg, 89%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 6.61 (dd, J = 10.0, 1.3 Hz, 1H), 6.23 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.81 (d, J = 10.7 Hz, 1H), 5.49 (dt, J = 14.8, 7.3 Hz, 1H), 5.33 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.3 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.04–3.88 (m, 2H), 2.64–2.51 (m, 1H), 2.31 (dd, J = 14.1, 6.9 Hz, 1H), 2.22–2.07 (m, 3H), 1.84 (d, J = 1.3 Hz, 3H), 1.74 (s, 3H), 1.63–1.57 (m, 2H), 1.35 (s, 6H), 1.18 (d, J = 6.3 Hz, 3H), 1.02 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.13, 147.79, 133.39, 132.74, 129.93, 128.43, 126.83, 126.52, 117.84, 100.32, 65.40, 65.28, 62.94, 46.16, 40.00, 39.83, 33.93, 25.24, 25.13, 21.86, 19.70, 17.07, 12.75. = −47.32, (c 0.11, CHCl3). HRMS (ESI+): calcd. for C23H36O4 [M + Na]+, 377.2686, found 377.2684.
Compound 28c (22 mg, 90%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 6.61 (dd, J = 9.9, 1.3 Hz, 1H), 6.23 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.80 (d, J = 10.8 Hz, 1H), 5.50 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.2 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.02–3.88 (m, 2H), 2.66–2.50 (m, 1H), 2.30 (dd, J = 13.6, 5.9 Hz, 1H), 2.15 (t, J = 7.1 Hz, 2H), 2.06 (dd, J = 13.7, 7.0 Hz, 1H), 1.84 (d, J = 1.2 Hz, 3H), 1.74 (s, 3H), 1.49–1.42 (m, 4H), 1.41–1.34 (m, 4H), 1.15 (d, J = 6.1 Hz, 3H), 1.02 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.10, 147.76, 133.12, 132.72, 129.96, 128.39, 127.11, 126.51, 117.83, 98.60, 67.97, 65.34, 65.27, 46.81, 40.00, 38.60, 33.89, 30.45, 22.38, 19.94, 19.70, 17.35, 12.73. = −25.76, (c 0.69, CHCl3). HRMS (ESI+): calcd. for C23H36O4 [M + Na]+, 377.2686, found 377.2685.
Compound 28d (21.5 mg, 90%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 6.61 (dd, J = 10.0, 1.3 Hz, 1H), 6.23 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.49 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.3 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 4.04–3.86 (m, 2H), 2.65–2.48 (m, 1H), 2.31 (dd, J = 14.1, 7.0 Hz, 1H), 2.20–2.06 (m, 3H), 1.84 (d, J = 1.3 Hz, 3H), 1.74 (s, 3H), 1.68–1.57 (m, 2H), 1.35 (s, 6H), 1.18 (d, J = 6.3 Hz, 3H), 1.02 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.11, 147.78, 133.38, 132.73, 129.90, 128.42, 126.79, 126.51, 117.83, 100.31, 65.38, 65.27, 62.93, 46.13, 40.00, 39.84, 33.92, 25.23, 25.11, 21.85, 19.70, 17.07, 12.74. = −17.71, (c 0.23, CHCl3). HRMS (ESI+): calcd. for C23H36O4 [M + Na]+, 377.2686, found 377.2692.
4.26. Synthetic Procedure of 29a–29d
To a stirred solution of compound 26a (48 mg, 0.11 mmol) in dry DCM (3 mL) and 4A molecular sieve under argon, the Proton Sponge (107 mg, 0.5 mmol) and trimethyloxonium tetrafluoroborate (60 mg, 0.4 mmol) were added sequentially. After 1 h, the reaction mixture was quenched with 1 M HCl (10 mL) and extracted with DCM (2 × 10 mL). The combined organic extracts were washed with water and brine, dried over Na2SO4, and concentrated in vacuum. Purification by column chromatography (PE/EA = 40:1) created 29a (38 mg, 77%) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.60 (d, J = 9.9 Hz, 1H), 6.20 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.75 (d, J = 10.8 Hz, 1H), 5.47 (dt, J = 14.8, 7.3 Hz, 1H), 5.28 (ddd, J = 13.8, 11.6, 1.3 Hz, 2H), 4.64 (d, J = 5.6 Hz, 2H), 4.00 (tdd, J = 8.7, 5.4, 3.2 Hz, 1H), 3.54–3.37 (m, 1H), 3.28 (d, J = 2.2 Hz, 3H), 2.71–2.44 (m, 1H), 2.36–2.21 (m, 1H), 2.21–2.01 (m, 3H), 1.84 (d, J = 1.2 Hz, 3H), 1.72 (s, 3H), 1.62–1.54 (m, 1H), 1.37–1.28 (m, 1H), 1.11 (d, J = 6.1 Hz, 3H), 1.01 (d, J = 6.6 Hz, 3H), 0.88 (s, 9H), 0.05 (d, J = 6.1 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 168.12, 147.85, 133.80, 132.75, 129.65, 128.52, 127.56, 126.47, 117.85, 73.13, 67.86, 65.27, 55.62, 49.18, 44.92, 39.99, 33.96, 26.08, 19.64, 19.38, 18.20, 17.36, 12.72, −3.96, −4.53. = −38.67, (c 0.05, CHCl3). HRMS (ESI+): calcd. for C27H48O4Si [M + H]+, 465.3395, found 465.3388.
Compound 29b (72 mg, 76%) was obtained as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 6.61 (dd, J = 9.9, 1.1 Hz, 1H), 6.20 (dd, J = 14.9, 10.9 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.77 (d, J = 10.8 Hz, 1H), 5.46 (dt, J = 14.8, 7.3 Hz, 1H), 5.33 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.2 Hz, 1H), 4.64 (dd, J = 5.5, 1.1 Hz, 2H), 3.93–3.83 (m, 1H), 3.41 (dd, J = 12.5, 6.3 Hz, 1H), 3.29 (s, 3H), 2.64–2.47 (m, 1H), 2.28–2.05 (m, 4H), 1.84 (d, J = 1.3 Hz, 3H), 1.72 (s, 4H), 1.48–1.39 (m, 1H), 1.11 (d, J = 6.1 Hz, 3H), 1.01 (d, J = 6.7 Hz, 3H), 0.86 (s, 9H), 0.02 (d, J = 9.8 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 168.14, 147.87, 133.66, 132.73, 129.73, 128.47, 127.73, 126.45, 117.85, 74.24, 68.48, 65.28, 55.97, 48.38, 44.21, 40.01, 33.97, 26.01, 19.64, 19.43, 18.16, 17.33, 12.74, −4.15, −4.47. = −34.83, (c 0.38, CHCl3). HRMS (ESI+): calcd. for C27H48O4Si [M + H]+, 465.3395, found 465.3391.
Compound 29c (32 mg, 71%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 6.59 (d, J = 9.7 Hz, 1H), 6.18 (dd, J = 15.0, 10.9 Hz, 1H), 5.94 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.73 (d, J = 10.7 Hz, 1H), 5.45 (dt, J = 14.7, 7.2 Hz, 1H), 5.31 (dd, J = 17.2, 1.1 Hz, 1H), 5.21 (d, J = 10.5 Hz, 1H), 4.62 (d, J = 5.5 Hz, 2H), 3.98 (dt, J = 16.1, 6.0 Hz, 1H), 3.51–3.35 (m, 1H), 3.26 (s, 3H), 2.62–2.44 (m, 1H), 2.27 (ddd, J = 19.7, 13.8, 9.4 Hz, 1H), 2.18–2.01 (m, 3H), 1.82 (s, 3H), 1.70 (s, 3H), 1.60–1.52 (m, 1H), 1.37–1.25 (m, 1H), 1.09 (d, J = 6.1 Hz, 3H), 0.99 (d, J = 6.6 Hz, 3H), 0.86 (s, 9H), 0.03 (d, J = 6.8 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 168.11, 147.86, 133.79, 132.74, 129.66, 128.51, 127.55, 126.46, 117.84, 73.13, 67.86, 65.27, 55.63, 49.18, 44.91, 40.05, 33.97, 26.08, 19.71, 19.38, 18.20, 17.39, 12.72, −3.96, −4.54. = −26.17, (c 0.1, CHCl3). HRMS (ESI+): calcd. for C27H48O4Si [M + H]+, 465.3395, found 465.3389.
Compound 29d (72 mg, 70%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 6.61 (dd, J = 10.0, 1.2 Hz, 1H), 6.20 (dd, J = 15.0, 10.9 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.77 (d, J = 10.8 Hz, 1H), 5.46 (dt, J = 14.7, 7.2 Hz, 1H), 5.33 (dd, J = 17.2, 1.5 Hz, 1H), 5.23 (dd, J = 10.4, 1.2 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 3.94–3.79 (m, 1H), 3.51–3.36 (m, 1H), 3.29 (s, 3H), 2.70–2.50 (m, 1H), 2.25–2.07 (m, 4H), 1.85 (d, J = 1.3 Hz, 3H), 1.79–1.67 (m, 4H), 1.45 (m, 1H), 1.12 (d, J = 6.1 Hz, 3H), 1.01 (d, J = 6.6 Hz, 3H), 0.86 (s, 9H), 0.01 (d, J = 9.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 168.12, 147.87, 133.67, 132.75, 129.73, 128.47, 127.72, 126.47, 117.84, 74.25, 68.54, 65.28, 55.97, 48.39, 44.20, 40.07, 33.96, 26.02, 19.70, 19.44, 18.17, 17.37, 12.74, −4.15, −4.47. = −16.46, (c 0.41, CHCl3). HRMS (ESI+): calcd. for C27H48O4Si [M + H]+, 465.3395, found 465.3389.
4.27. Synthetic Procedure of 1a–1d
To a stirred solution of 29a in dry THF (2 mL), the HF/Py complex (0.4 mL) was added at 0 °C. After being stirred for 1 h, the mixture was quenched with a saturated aqueous solution of NaHCO3 and extracted with DCM (10 mL × 3). The combined organic layers were washed with 1 M HCl, brine, dried over Na2SO4, filtrated, and concentrated. The residue was purified by column chromatography on silica gel (PE/EA = 4:1) to create 1a (20 mg, 70%) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 9.9, 1.3 Hz, 1H), 6.23 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.83 (d, J = 10.8 Hz, 1H), 5.59–5.42 (m, 1H), 5.32 (dd, J = 17.2, 1.5 Hz, 1H), 5.22 (dd, J = 10.4, 1.2 Hz, 1H), 4.63 (d, J = 5.5 Hz, 2H), 4.10–3.98 (m, 1H), 3.69–3.61 (m, 1H), 3.34 (s, 3H), 2.57 (m, 1H), 2.17 (m, 4H), 1.84 (d, J = 1.3 Hz, 3H), 1.75 (s, 3H), 1.59–1.55 (m, 2H), 1.17 (d, J = 6.2 Hz, 3H), 1.01 (d, J = 6.6 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.11, 147.75, 133.68, 132.71, 130.36, 128.23, 127.58, 126.52, 117.85, 74.75, 66.38, 65.28, 56.34, 48.36, 42.82, 40.00, 33.89, 19.70, 18.94, 16.91, 12.73. = −25.83, (c 0.46, CHCl3). HRMS (ESI+): calcd. for C21H34O4 [M + Na]+, 373.2349, found 373.2346.
Compound 1b (40 mg, 74%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 9.9, 1.1 Hz, 1H), 6.23 (dd, J = 15.0, 10.8 Hz, 1H), 5.95 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.82 (d, J = 10.8 Hz, 1H), 5.50 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (dd, J = 17.2, 1.5 Hz, 1H), 5.22 (dd, J = 10.4, 1.2 Hz, 1H), 4.63 (d, J = 5.5 Hz, 2H), 3.98–3.87 (m, 1H), 3.61–3.49 (m, 1H), 3.33 (s, 3H), 2.65–2.47 (m, 1H), 2.26–2.05 (m, 4H), 1.83 (s, 3H), 1.75 (s, 3H), 1.59–1.50 (m, 2H), 1.17 (d, J = 6.0 Hz, 3H), 1.01 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.10, 147.78, 133.68, 132.70, 130.13, 128.30, 127.42, 126.48, 117.82, 77.98, 69.74, 65.25, 55.89, 48.24, 43.29, 40.00, 33.88, 19.68, 19.20, 17.00, 12.71. = −29.17, (c 0.80, CHCl3). HRMS (ESI+): calcd. for C21H34O4 [M + Na]+, 373.2349, found 373.2348.
Compound 1c (18 mg, 75%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 9.9, 1.3 Hz, 1H), 6.23 (dd, J = 15.0, 10.8 Hz, 1H), 5.96 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.83 (d, J = 10.8 Hz, 1H), 5.59–5.42 (m, 1H), 5.32 (dd, J = 17.2, 1.5 Hz, 1H), 5.22 (dd, J = 10.4, 1.2 Hz, 1H), 4.63 (d, J = 5.5 Hz, 2H), 4.10–3.98 (m, 1H), 3.69–3.61 (m, 1H), 3.34 (s, 3H), 2.57 (m, 1H), 2.17 (m, 4H), 1.84 (d, J = 1.3 Hz, 3H), 1.75 (s, 3H), 1.59–1.55 (m, 2H), 1.17 (d, J = 6.2 Hz, 3H), 1.01 (d, J = 6.6 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.11, 147.74, 133.68, 132.71, 130.34, 128.24, 127.58, 126.52, 117.85, 74.74, 66.38, 65.28, 56.34, 48.36, 42.87, 39.98, 33.89, 19.68, 18.96, 16.91, 12.73. = −21.89, (c 0.52, CHCl3). HRMS (ESI+): calcd. for C21H34O4 [M + Na]+, 373.2349, found 373.2349.
Compound 1d (40 mg, 74%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 6.60 (dd, J = 9.9, 1.1 Hz, 1H), 6.23 (dd, J = 15.0, 10.8 Hz, 1H), 5.95 (ddt, J = 17.1, 10.5, 5.6 Hz, 1H), 5.82 (d, J = 10.8 Hz, 1H), 5.50 (dt, J = 14.8, 7.3 Hz, 1H), 5.32 (dd, J = 17.2, 1.5 Hz, 1H), 5.22 (dd, J = 10.4, 1.2 Hz, 1H), 4.63 (d, J = 5.5 Hz, 2H), 3.98–3.87 (m, 1H), 3.61–3.49 (m, 1H), 3.33 (s, 3H), 2.65–2.47 (m, 1H), 2.26–2.05 (m, 4H), 1.83 (s, 3H), 1.75 (s, 3H), 1.59–1.50 (m, 2H), 1.17 (d, J = 6.0 Hz, 3H), 1.01 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.10, 147.78, 133.69, 132.70, 130.11, 128.31, 127.41, 126.48, 117.83, 77.96, 69.75, 65.26, 55.89, 48.22, 43.30, 39.96, 33.88, 19.66, 19.20, 17.04, 12.71. = −38.68, (c 0.76, CHCl3). HRMS (ESI+): calcd. for C21H34O4 [M + Na]+, 373.2349, found 373.2350. Detailed NMR data tables of 1a–1d are in the Supplementary Material.
5. Conclusions
Asymmetric synthesis of the alotamdie fragment C15–C32 was established and four diastereomers were achieved concisely. Boron-mediated enantioselective aldol reaction led to a good diastereoselectivity and Julia-Kocienski olefination constructed the diene part in excellent E/Z selectivity and yield. A careful NMR comparison between four isomers and natural alotamide suggested the relative structure.
Supplementary Materials
A supplementary file is available online at http://www.mdpi.com/1660-3397/16/11/414/s1. Supplementary Information shows the NMR spectra of the synthetic compounds and the NMR data tables of 1a–1d.
Author Contributions
Y.G. and C.-g.H. designed the experiments. H.-y.S., Y.X., P.H., Z.-Q.G., and Y.-h.L. performed the experiments. H.-y.S. and C.-g.H. wrote the paper.
Funding
We acknowledge financial support from the National Natural Science Foundation of China for Young Scientists [no. 81602998] and the National Science and Technology Major Project of the Ministry of Science and Technology of China (No. 2018ZX09731016-003 and No. 2018ZX09201001-001-008).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nagarajan, M.; Maruthanayagam, V.; Sundararaman, M. A review of pharmacological and toxicological potentials of marine cyanobacterial metabolites. J. Appl. Toxicol. 2012, 32, 153–185. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Corbett, T.H. Total Structure Determination of Apratoxin A, a Potent Novel Cytotoxin from the Marine CyanobacteriumLyngbyamajuscula. J. Am. Chem Soc. 2001, 123, 5418–5423. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Suyama, T.L.; Engene, N.; Wingerd, J.S.; Matainaho, T.; Gerwick, W.H. Apratoxin D, a potent cytotoxic cyclodepsipeptide from papua new guinea collections of the marine cyanobacteria Lyngbya majuscula and Lyngbya sordida. J. Nat. Prod. 2008, 71, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Matthew, S.; Schupp, P.J.; Luesch, H. Apratoxin E, a cytotoxic peptolide from a guamanian collection of the marine cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2008, 71, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Suzuki, J.; Onda, Y.; Fujino, Y.; Yoshida, M.; Doi, T. Total synthesis and conformational analysis of apratoxin C. J. Org. Chem. 2014, 79, 8000–8009. [Google Scholar] [CrossRef] [PubMed]
- Soria-Mercado, I.E.; Pereira, A.; Cao, Z.; Murray, T.F.; Gerwick, W.H. Alotamide A, a novel neuropharmacological agent from the marine cyanobacterium Lyngbya bouillonii. Org. Lett. 2009, 11, 4704–4707. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Rein, K.S. New Peptides Isolated from Lyngbya Species: A Review. Mar. Drugs 2010, 8, 1817–1837. [Google Scholar] [CrossRef] [PubMed]
- Matinkhoo, K.; Pryyma, A.; Todorovic, M.; Patrick, B.O.; Perrin, D.M. Synthesis of the Death-Cap Mushroom Toxin alpha-Amanitin. J. Am. Chem. Soc. 2018, 140, 6513–6517. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, F.; Xu, Z.; Liu, Q.; Cui, Y.; Jia, Y. Stereocontrolled and efficient total synthesis of (−)-stephanotic acid methyl ester and (−)-celogentin C. Org. Lett. 2010, 12, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.-G.; Li, M.; Si, C.-M.; Mao, Z.-Y.; Wei, B.-G. Studies toward asymmetric synthesis of leiodelide A. Tetrahedron Lett. 2014, 55, 6903–6906. [Google Scholar] [CrossRef]
- Bosch, L.; Mola, L.; Petit, E.; Saladrigas, M.; Esteban, J.; Costa, A.M.; Vilarrasa, J. Formal Total Synthesis of Amphidinolide E. J. Org. Chem. 2017, 82, 11021–11034. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Romano, C.; Mazet, C. Palladium-Catalyzed Long-Range Deconjugative Isomerization of Highly Substituted alpha,beta-Unsaturated Carbonyl Compounds. J. Am. Chem Soc. 2016, 138, 10344–10350. [Google Scholar] [CrossRef] [PubMed]
- Menche, D.; Hassfeld, J.; Li, J.; Mayer, K.; Rudolph, S. Modular total synthesis of archazolid A and B. J. Org. Chem. 2009, 74, 7220–7229. [Google Scholar] [CrossRef] [PubMed]
- Takano, D.; Nagamitsu, T.; Ui, H.; Shiomi, K.; Yamaguchi, Y.; Masuma, R.; Kuwajima, I.; Ōmura, S. Total Synthesis of Nafuredin, a Selective NADH-fumarate Reductase Inhibitor. Org. Lett. 2001, 3, 2289–2291. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xu, Z.; Ye, T. Total synthesis of amphidinins E, F and epi-amphidinin F. Org. Chem. Front. 2018, 5, 629–632. [Google Scholar] [CrossRef]
- ElMarrouni, A.; Joolakanti, S.R.; Colon, A.; Heras, M.; Arseniyadis, S.; Cossy, J. Two concise total syntheses of (−)-bitungolide F. Org. Lett. 2010, 12, 4074–4077. [Google Scholar] [CrossRef] [PubMed]
- Brun, E.; Bellosta, V.; Cossy, J. Synthesis of the Acyclic Carbon Skeleton of Filipin III. J. Org. Chem. 2016, 81, 8206–8221. [Google Scholar] [CrossRef] [PubMed]
- Rychnovsky, S.D.; Rogers, B.N.; Richardson, T.I. Configurational Assignment of Polyene Macrolide Antibiotics Using the [13C]Acetonide Analysis. Acc. Chem. Res. 1998, 31, 9–17. [Google Scholar] [CrossRef]
- Huang, W.; Ren, R.G.; Dong, H.Q.; Wei, B.G.; Lin, G.Q. Diverse synthesis of marine cyclic depsipeptide lagunamide A and its analogues. J. Org. Chem. 2013, 78, 10747–10762. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, M.; Sueyoshi, K.; Teruya, T.; Ohno, H.; Fujii, N.; Oishi, S. Total synthesis of odoamide, a novel cyclic depsipeptide, from an Okinawan marine cyanobacterium. Org. Biomol. Chem. 2016, 14, 9093–9104. [Google Scholar] [CrossRef] [PubMed]
- Babu, V.S.; Zhou, Y.; Kishi, Y. Design, synthesis, and cytotoxicity of stabilized mycolactone analogs. Bioorg. Med. Chem. Lett. 2017, 27, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, G.; Black, M. A Synthesis of the C3-C15 Fragment of the Archazolids. Synlett 2009, 2010, 107–110. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).