Characterization of a Novel Alginate Lyase from Marine Bacterium Vibrio furnissii H1
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation of Alginate-Degrading Microorganism
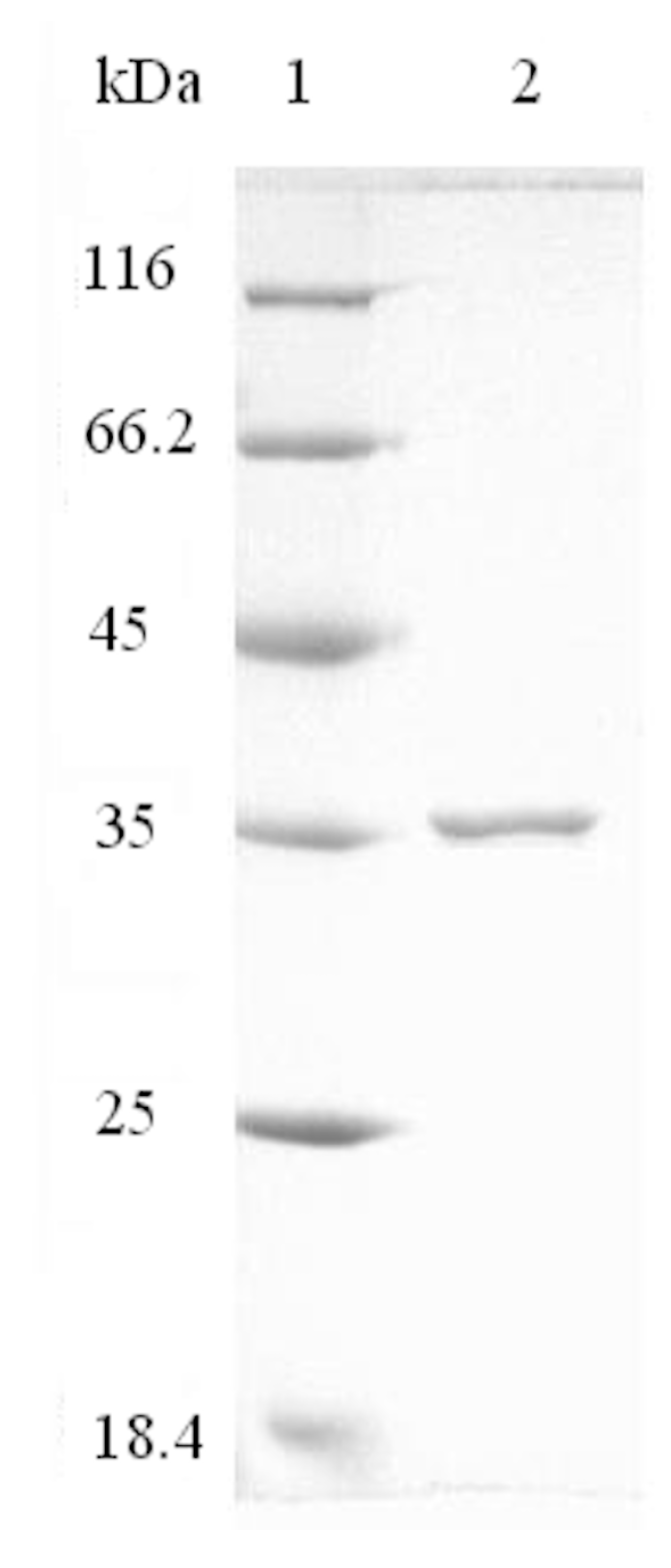
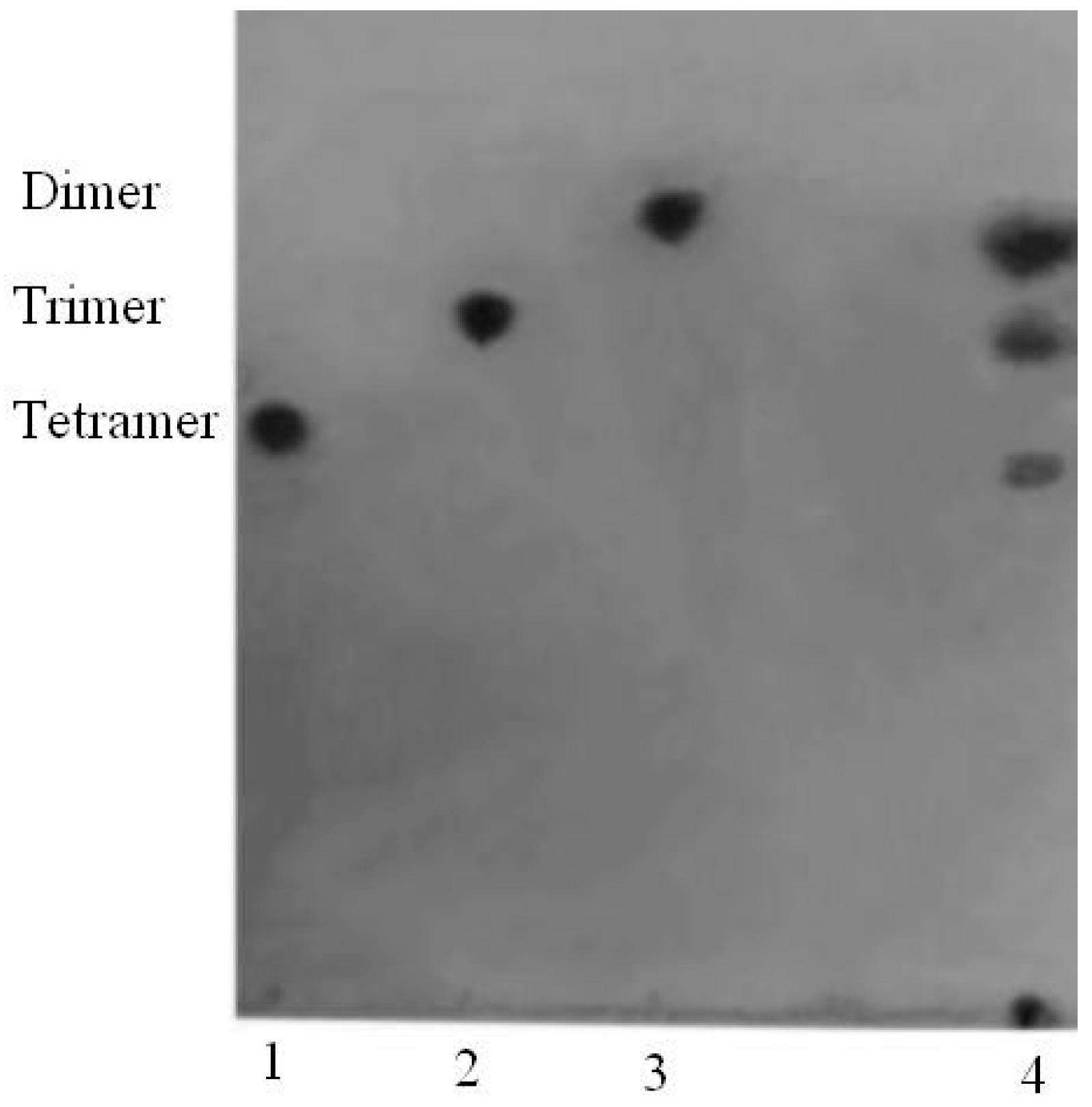
2.2. Purification of AlyH1
2.3. Characterization of AlyH1
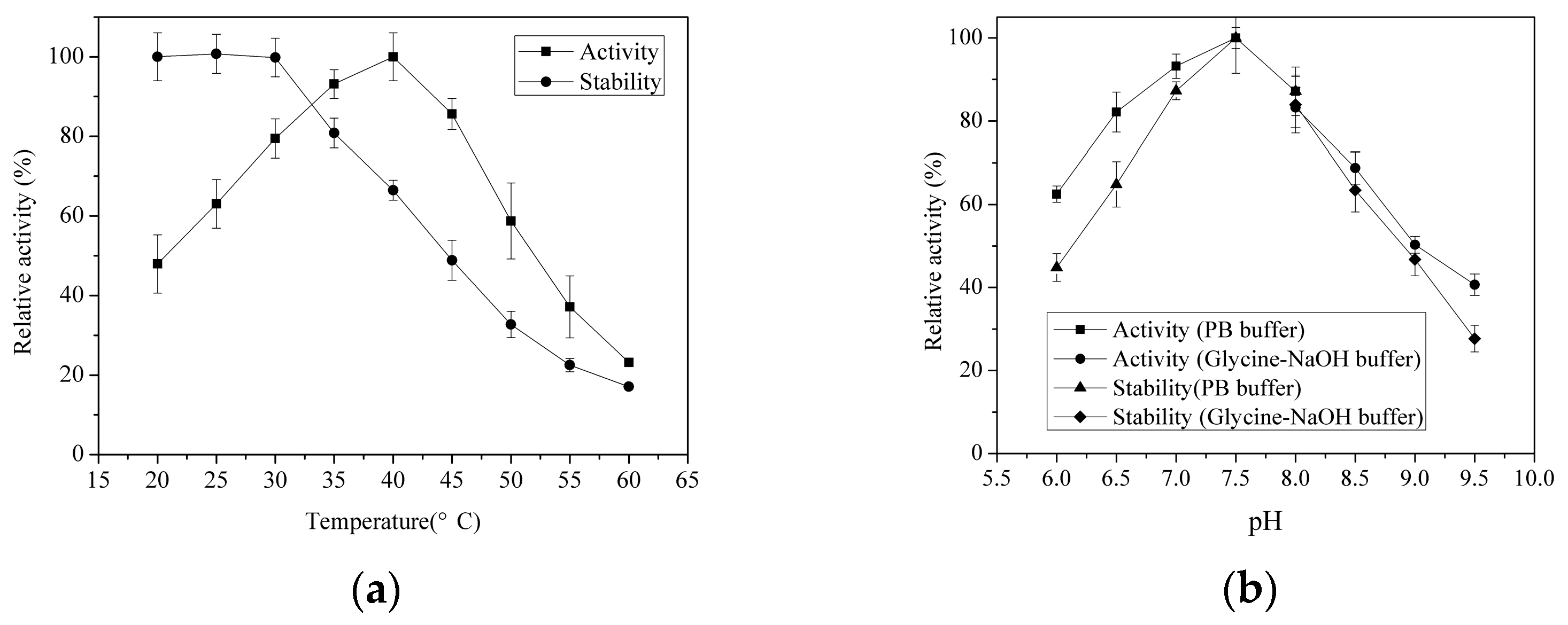
2.3.1. Effects of Temperature and pH on AlyH1 Activity and Stability
2.3.2. Effects of NaCl Concentration and Metal Ions on AlyH1 Activity
2.3.3. Kinetic Parameters
2.3.4. Substrate Specificity
2.3.5. Thin-Layer Chromatography Analysis of the Degradation Products
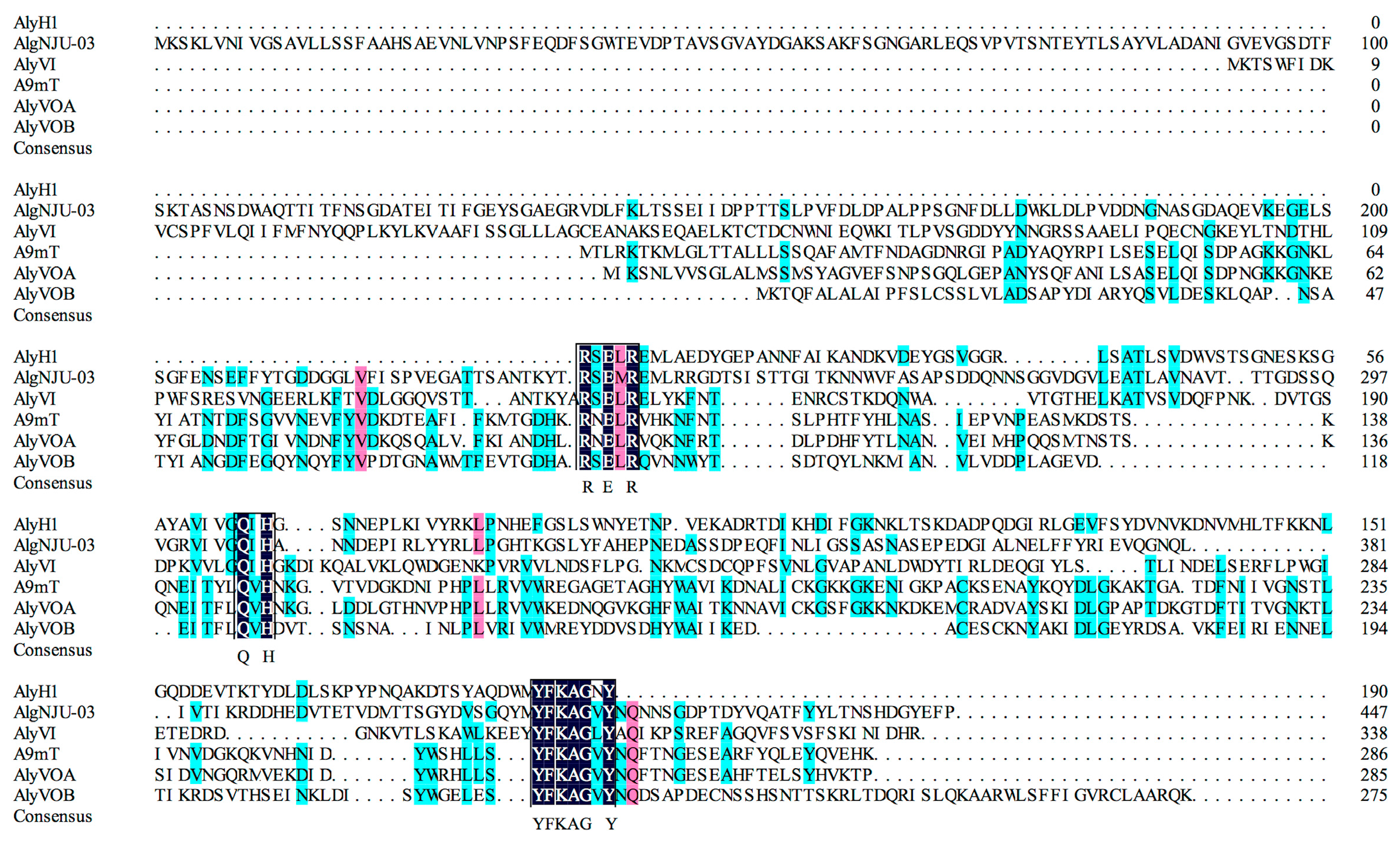
2.3.6. Determination of Partial Amino Acid Sequences of AlyH1
3. Materials and Methods
3.1. Microorganism, Media, and Culture Conditions
3.2. Strain Isolation and Identification
3.3. Purification of Alginate Lyase
3.4. Alginate Lyase Assay
3.5. Characterization of AlyH1
3.5.1. Effects of Temperature and pH on AlyH1 Activity and Stability
3.5.2. Effects of NaCl Concentration and Metal Ions on AlyH1 Activity
3.5.3. Kinetic Parameters
3.5.4. Substrate Specificity
3.5.5. TLC Analysis of the Degradation Products
3.5.6. Determination of Partial Amino Acid Sequences of AlyH1
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ravanal, M.C.; Sharma, S.; Gimpel, J.; Reveco-Urzua, F.E.; Øverland, M.; Horn, S.J.; Lienqueo, M.E. The role of alginate lyases in the enzymatic saccharification of brown macroalgae, Macrocystis pyrifera and Saccharina latissima. Algal Res. 2017, 26, 287–293. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.G.; Lee, E.Y. Alginate lyase: Structure, property, and application. Biotechnol. Bioprocess Eng. 2011, 16, 843–851. [Google Scholar] [CrossRef]
- Wargacki, A.J.; Leonard, E.; Win, M.N.; Regitsky, D.D.; Santos, C.N.; Kim, P.B.; Cooper, S.R.; Raisner, R.M.; Herman, A.; Sivitz, A.B.; et al. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 2012, 335, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Boucelkha, A.; Petit, E.; Elboutachfaiti, R.; Molinié, R.; Amari, S.; Yahaoui, R.Z. Production of guluronate oligosaccharide of alginate from brown algae Stypocaulon scoparium using an alginate lyase. J. Appl. Phycol. 2016, 29, 509–519. [Google Scholar] [CrossRef]
- Zhu, B.; Yin, H. Alginate lyase: Review of major sources and classification, properties, structure-function analysis and applications. Bioengineered 2015, 6, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Wei, D.; Li, H.; Li, H.; Rahman, M.M.; Shi, J.; Xu, Z.; Ma, Y. Purification and characterisation of a bifunctional alginate lyase from novel Isoptericola halotolerans CGMCC 5336. Carbohydr. Polym. 2013, 98, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Araki, R.; Iriyama, K.I.; Oda, T.; Fukuda, H.; Hayashida, S.; Muramatsu, T. Purification and characterization of bifunctional alginate lyase from Alteromonas sp. strain No. 272 and its action on saturated oligomeric substrates. Biosci. Biotechnol. Biochem. 2001, 65, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Bernard, T.; Rancurel, C.; Brumer, H.; Coutinho, P.M.; Henrissat, B. A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem. J. 2010, 432, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, Y.; Zhang, L.; Wang, Y.; Wang, S.; Zhang, Y.; Guo, H.; Ji, D.; Wang, Y. Alginate oligosaccharide DP5 exhibits antitumor effects in osteosarcoma patients following surgery. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Che, J.; Li, X.; Li, J.; Wang, J.; Xu, Y. In vitro non-specific immunostimulatory effect of alginate oligosaccharides with different molecular weights and compositions on sea cucumber (Apostichopus japonicus) coelomocytes. Aquaculture 2014, 434, 434–441. [Google Scholar] [CrossRef]
- Falkeborg, M.; Cheong, L.Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.I.; Matsubara, Y. Purification of alginate oligosaccharides with root growth-promoting activity toward lettuce. Biosci. Biotechnol. Biochem. 2000, 64, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Aarstad, O.A.; Tøndervik, A.; Sletta, H.; Skjåk-Bræk, G. Alginate sequencing: An analysis of block distribution in alginates using specific alginate degrading enzymes. Biomacromolecules 2012, 13, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Mashino, C.; Kodama, T.; Ojima, T. Protoplast preparation from Laminaria japonica with recombinant alginate lyase and cellulase. Mar. Biotechnol. 2011, 13, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Islan, G.A.; Bosio, V.E.; Castro, G.R. Alginate lyase and ciprofloxacin co-immobilization on biopolymeric microspheres for cystic fibrosis treatment. Macromol. Biosci. 2013, 13, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, L.; Chen, Y.; Ni, H.; Xiao, A.; Cai, H. Characterization of an extracellular biofunctional alginate lyase from marine Microbulbifer sp. ALW1 and antioxidant activity of enzymatic hydrolysates. Microbiol. Res. 2016, 182, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Hao, J.; Xing, M.; Sun, J.; Sun, M. Purification and characterization of a new aginate lyase from marine bacterium Vibrio sp. SY08. Mar. Drugs 2017, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Wang, S.; Wu, S.; Wei, J.; Chen, H. Cloning and characterization of an alginate lyase from marine Vibrio. sp. QD-5. Preprints 2017, 2017050055. [Google Scholar] [CrossRef]
- Zhu, B.; Sun, Y.; Ni, F.; Ning, L.; Yao, Z. Characterization of a new endo-type alginate lyase from Vibrio sp. NJU-03. Int. J. Biol. Macromol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Tan, H.; Qin, Y.; Xu, Q.; Du, Y.; Yin, H. Characterization of a new endo-type alginate lyase from Vibrio sp. W13. Int. J. Biol. Macromol. 2015, 75, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Badur, A.H.; Jagtap, S.S.; Yalamanchili, G.; Lee, J.K.; Zhao, H.; Rao, C.V. Alginate lyases from alginate-degrading Vibrio splendidus 12B01 are endolytic. Appl. Environ. Microbiol. 2015, 81, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.S.; Hehemann, J.H.; Polz, M.F.; Lee, J.K.; Zhao, H. Comparative biochemical characterization of three exolytic oligoalginate lyases from Vibrio splendidus reveals complementary substrate scope, temperature, and pH adaptations. Appl. Environ. Microbiol. 2014, 80, 4207–4214. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, K.; Miyazaki, M.; Nogi, Y.; Kobayashi, T.; Horikoshi, K. Cloning and sequencing of alginate lyase genes from deep-sea strains of Vibrio and Agarivorans and characterization of a new Vibrio enzyme. Mar. Biotechnol. 2010, 12, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, E.W.; Yu, W.G.; Han, F. Purification and characterization of a new alginate lyase from a marine bacterium Vibrio sp. Biotechnol. Lett. 2013, 35, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.T.; Lin, H.; Kim, S.M. Purification and characterization of a Na+/K+ dependent alginate lyase from turban shell gut Vibrio sp. YKW-34. Enzym. Microb. Technol. 2007, 41, 828–834. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yu, G.L.; Wang, X.M.; Lv, Z.H.; Zhao, X.; Wu, Z.H.; Ji, W.S. Purification and characterization of alginate lyase from marine Vibrio sp. YWA. Acta Biochim. Biophys. Sin. 2006, 38, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Horibe, A.; Miki, Y.; Kimura, T.; Tanaka, K.; Nakagawa, T.; Kawamukai, M.; Matsuda, H. Cloning and sequencing analysis of alginate lyase genes from the marine bacterium Vibrio sp. O2. Mar. Biotechnol. 2006, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jiang, X.; Hwang, H.M. Purification and Characterization of an Alginate Lyase from Marine Bacterium Vibrio sp. Mutant Strain 510-64. Curr. Microbiol. 2006, 53, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Gong, Q.H.; Song, K.; Li, J.B.; Yu, W.G. Cloning, sequence analysis and expression of gene alyVI encoding alginate lyase from marine bacterium Vibrio sp. QY101. DNA Seq. 2004, 15, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Yamaguchi, K.; Kitamikado, M. Isolation and some properties of alginate lyase from a marine bacterium Vibrio sp. AL-128. Nippon Suisan Gakkaishi 1992, 58, 533–538. [Google Scholar] [CrossRef]
- Tseng, C.H.; Yamaguchi, K.; Kitamikado, M. Two types of alginate lyase from a marine bacterium Vibrio sp. AL-9. Nippon Suisan Gakkaishi 1992, 58, 743–749. [Google Scholar] [CrossRef]
- Gong, J.S.; Liu, X.M.; Zhang, M.J.; Li, H.; Geng, Y.; Li, H.; Li, J.; Lu, Z.M.; Xu, Z.H.; Shi, J.S. Purification and characterization of a high salt-tolerant alginate lyase from Cobetia sp. WG-007. Biotechnol. Appl. Biochem. 2017, 64, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Dong, S.; Song, J.; Li, C.B.; Chen, X.L.; Xie, B.B.; Zhang, Y.Z. Purification and characterization of a bifunctional alginate lyase from Pseudoalteromonas sp. SM0524. Mar. Drugs 2011, 9, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Gupta, V.; Kumari, P.; Kumar, M.; Reddy, C.R.K.; Prasad, K.; Jha, B. Purification and partial characterization of an extracellular alginate lyase from Aspergillus oryzae isolated from brown seaweed. J. Appl. Phycol. 2011, 23, 755–762. [Google Scholar] [CrossRef]
- Kobayashi, T.; Uchimura, K.; Miyazaki, M.; Nogi, Y.; Horikoshi, K. A new high-alkaline alginate lyase from a deep-sea bacterium Agarivorans sp. Extremophiles 2009, 13, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Swift, S.M.; Hudgens, J.W.; Heselpoth, R.D.; Bales, P.M.; Nelson, D.C. Characterization of AlgMsp, an alginate lyase from Microbulbifer sp. 6532A. PLoS ONE 2014, 9, e112939. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.J.; Baik, K.S.; Park, S.C.; Choe, H.N.; Seong, C.N.; Shin, T.S.; Woo, H.C.; Cho, J.Y.; Kim, D. Characterization of alginate lyase gene using a metagenomic library constructed from the gut microflora of abalone. J. Ind. Microbiol. Biotechnol. 2012, 39, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Ogura, K.; Hashimoto, W.; Mikami, B.; Murata, K. A structural basis for depolymerization of alginate by polysaccharide lyase family-7. J. Mol. Biol. 2005, 352, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Ma, L.Y.; Chi, Z.M.; Li, J.; Wu, L.F. Overexpression of alginate lyase of Pseudoalteromonas elyakovii in Escherichia coli, purification, and characterization of the recombinant alginate lyase. World J. Microbiol. Biotechnol. 2008, 24, 89–96. [Google Scholar] [CrossRef]
| Microorganisms | Molecular Mass (kDa) | Optimal pH/Temperature (°C) | Substrate Specificity | Reference |
|---|---|---|---|---|
| V. furnissii H1 | 35.8 | 7.5/40 | Poly-M, poly-G | This study |
| Vibrio sp. SY08 | 33 | 7.6/40 | Poly-M, poly-G | [17] |
| Vibrio sp. QD-5 | 62 | 8.9/35 | poly-G | [18] |
| Vibrio sp. NJU-03 | 48.12 | 7.0/30 | Poly-M, poly-G | [19] |
| Vibrio sp. W13 | 54.12 | 8.0/30 | Poly-M, poly-G | [20] |
| V. splendidus 12B01 | 68.2 | 8.5/25 | Poly-M, poly-G | [21] |
| 59.0 | 7.5/20-25 | Poly-M, poly-G | [21] | |
| 36.5 | 8.0/20 | Poly-M, poly-G | [21] | |
| 35.2 | 7.5/25 | Poly-M, poly-G | [21] | |
| 80 | 6.5/16 | Poly-M, poly-G | [22] | |
| 83 | 7.0/30 | Poly-M, poly-G | [22] | |
| 81 | 7.5/35 | Poly-M | [22] | |
| Vibrio sp. JAM-A9m | 28 | 7.6 and 9/30 | Poly-M | [23] |
| Vibrio sp. QY105 | 37 | 7.0/38 | Poly-M, poly-G | [24] |
| Vibrio sp. YKW-34 | 60.0 | 7.0/40 | Poly-M, poly-G | [25] |
| Vibrio sp.YWA | 62.5 | 7.0/25 | Poly-M, poly-G | [26] |
| Vibrio sp. O2 | 28.4 | - | Poly-M | [27] |
| 25.2 | - | Poly-M | [27] | |
| Vibrio sp. 510-64 | 34.6 | 7.5/35 | Poly-G | [28] |
| Vibrio sp. QY101 | 34 | 7.5/40 | Poly-M, poly-G | [29] |
| Vibrio sp. AL-9 | 25 | 9.0/- | Poly-G | [30] |
| 31 | 8.0/- | Poly-M | [30] | |
| V. harveyi AL-128 | - | 7.8/- | Poly-G | [31] |
| Purification Steps | Total Protein (mg) | Specific Activity (U/mg) | Total Activity (U) | Yield (%) | Purification (Fold) |
|---|---|---|---|---|---|
| Liquid supernatant | 121.84 | 0.13 | 15.84 | 100.00 | 1.00 |
| (NH4)2SO4 fractionation | 79.53 | 0.16 | 12.72 | 80.33 | 1.23 |
| Q-Sepharose chromatography | 6.67 | 0.82 | 5.47 | 34.53 | 6.31 |
| Gel filtration chromatography | 0.61 | 2.40 | 1.47 | 9.28 | 18.46 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Li, X.; Shi, H.; Zhou, J.; Tan, Z.; Yuan, M.; Yao, P.; Liu, X. Characterization of a Novel Alginate Lyase from Marine Bacterium Vibrio furnissii H1. Mar. Drugs 2018, 16, 30. https://doi.org/10.3390/md16010030
Zhu X, Li X, Shi H, Zhou J, Tan Z, Yuan M, Yao P, Liu X. Characterization of a Novel Alginate Lyase from Marine Bacterium Vibrio furnissii H1. Marine Drugs. 2018; 16(1):30. https://doi.org/10.3390/md16010030
Chicago/Turabian StyleZhu, Xiaoyan, Xiangqian Li, Hao Shi, Jia Zhou, Zhongbiao Tan, Mengdi Yuan, Peng Yao, and Xiaoyan Liu. 2018. "Characterization of a Novel Alginate Lyase from Marine Bacterium Vibrio furnissii H1" Marine Drugs 16, no. 1: 30. https://doi.org/10.3390/md16010030
APA StyleZhu, X., Li, X., Shi, H., Zhou, J., Tan, Z., Yuan, M., Yao, P., & Liu, X. (2018). Characterization of a Novel Alginate Lyase from Marine Bacterium Vibrio furnissii H1. Marine Drugs, 16(1), 30. https://doi.org/10.3390/md16010030