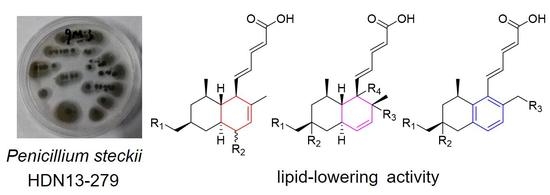
Lipid-Lowering Polyketides from the Fungus Penicillium Steckii HDN13-279
Abstract
:1. Introduction
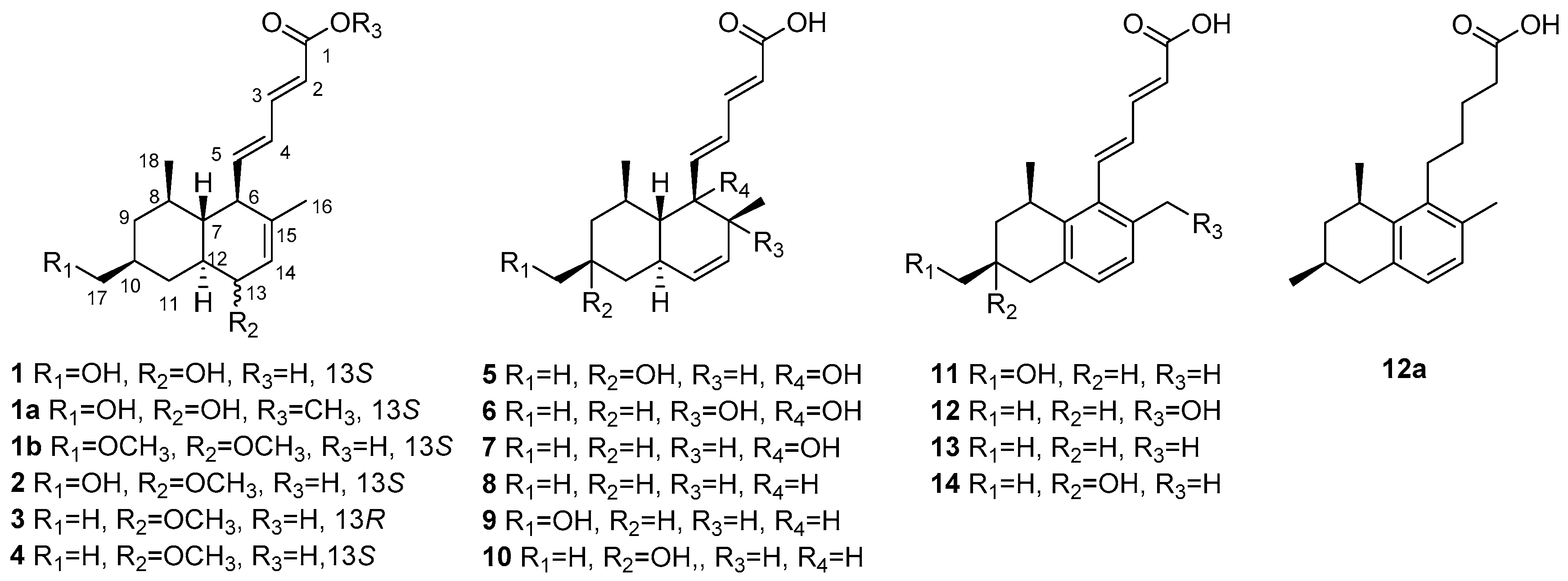
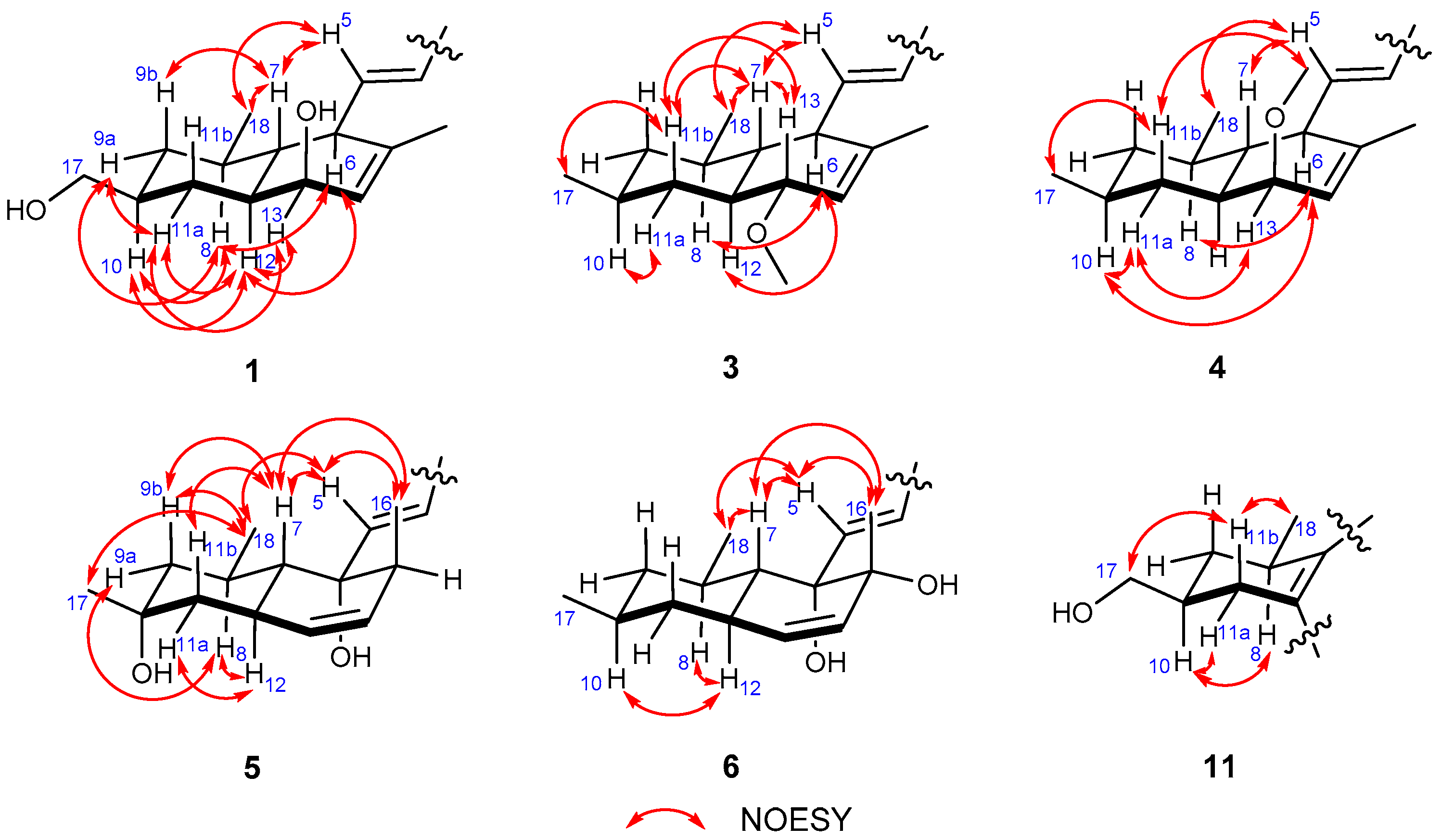
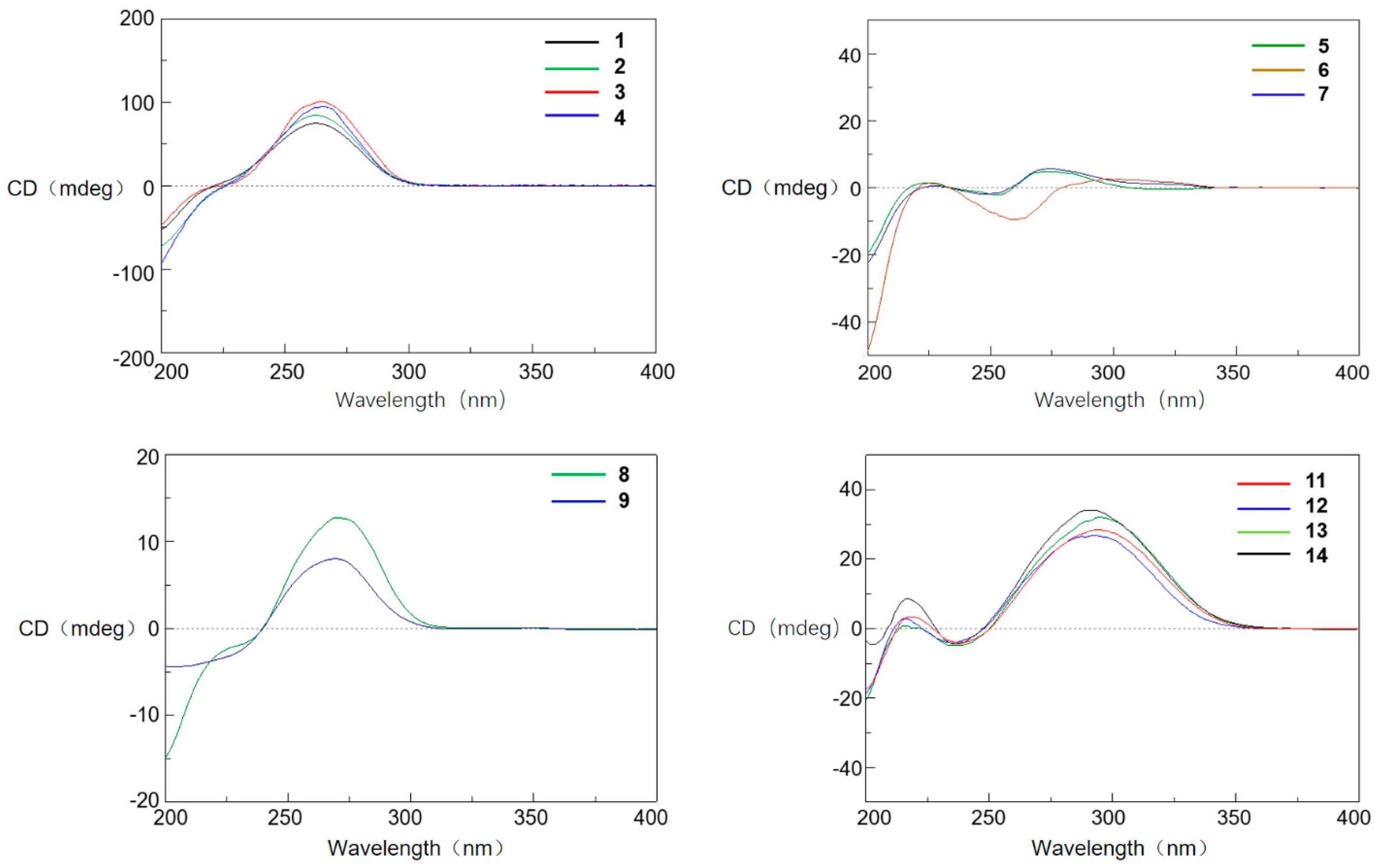
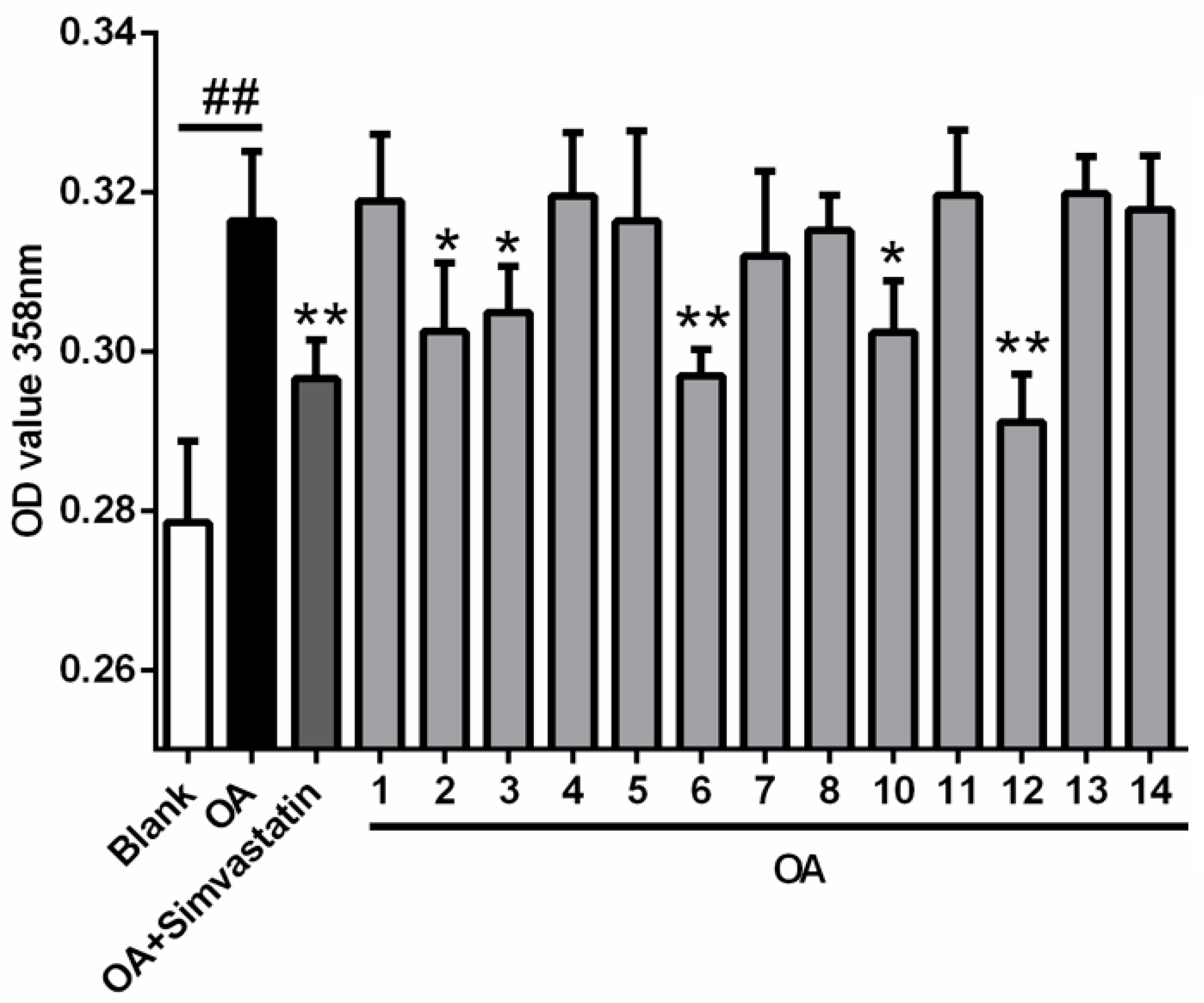
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Isolation
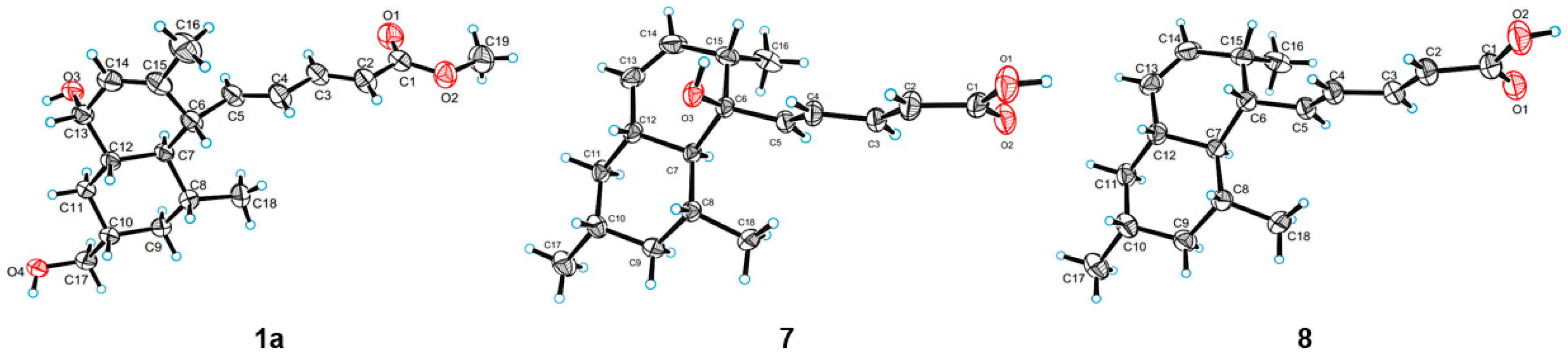
3.5. Crystal Data for 1a
3.6. Crystal Data for 7
3.7. Crystal Data for 8
3.8. X-ray Crystallographic Analysis of Compound 1a, 7 and 8
3.9. Esterification of 1
3.10. Methylation of 1 and 2
3.11. Reduction of 12 and 13
3.12. Cell-Based Lipid Accumulation Assay
3.13. Assay of Cytotoxicity, Antiviral Activity and NF-κB Inhibitory Activity
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, W.; Lu, H.; Yang, J.; Xiang, H.; Peng, H. Sphingosine 1-phosphate in metabolic syndrome. Int. J. Mol. Med. 2016, 38, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, F.M.; de Sousa, F.R.; Barbosa, A.L.; Martins, S.C.; Araújo, R.L.; Soares, R.; Abreu, C. Metabolic syndrome and risk of cancer: Which link? Metabolism 2015, 64, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; White, H.D. Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 2173–2195. [Google Scholar] [CrossRef] [PubMed]
- Wald, N.J.; Law, M.R. Serum cholesterol and ischemic heart disease. Atherosclerosis 1995, 118, S1–S5. [Google Scholar] [CrossRef]
- Studer, M.; Briel, M.; Leimenstoll, B.; Glass, T.R.; Bucher, H.C. Effect of different antilipidemic agents and diets on mortality: A systematic review. Arch. Intern. Med. 2005, 165, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Brugts, J.J.; Yetgin, T.; Hoeks, S.E.; Gotto, A.M.; Shepherd, J.; Westendorp, R.G.J.; de Craen, A.J.M.; Knopp, R.H.; Nakamura, H.; Ridker, P.; et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: Meta-analysis of randomised controlled trials. Br. Med. J. 2009, 338. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.; Foote, C.; Lv, J.; Neal, B.; Patel, A.; Nicholls, S.J.; Grobbee, D.E.; Cass, A.; Chalmers, J.; Perkovic, V. Effects of fibrates on cardiovascular outcomes: A systematic review and meta-analysis. Lancet 2010, 375, 1875–1884. [Google Scholar] [CrossRef]
- Last, A.R.; Ference, J.D.; Falleroni, J. Pharmacologic treatment of hyperlipidemia. Am. Fam. Physician 2011, 84, 551–558. [Google Scholar] [PubMed]
- Upendra, R.S.; Khandelwal, P.; Amiri, Z.R.; Shwetha, L.; Mohammed, A.S. Screening and molecular characterization of natural fungal isolates producing lovastatin. J. Microb. Biochem. Technol. 2013, 5. [Google Scholar] [CrossRef]
- Phainuphong, P.; Rukachaisirikul, V.; Saithong, S.; Phongpaichit, S.; Bowornwiriyapan, K.; Muanprasat, C.; Srimaroeng, C.; Duangjai, A.; Sakayaroj, J. Lovastatin Analogues from the Soil-Derived Fungus Aspergillus sclerotiorum PSU-RSPG178. J. Nat. Prod. 2016, 79, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.H.; Zhou, G.L.; Zhu, M.L.; Wang, W.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Neosartoryadins A and B, Fumiquinazoline Alkaloids from a Mangrove-Derived Fungus Neosartorya udagawae HDN13-313. Org. Lett. 2016, 18, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.W.; Yu, G.H.; Kurtán, T.; Mándi, A.; Peng, J.X.; Mo, X.M.; Liu, M.; Li, H.; Sun, X.H.; Li, J.; et al. Versixanthones A-F, Cytotoxic Xanthone-Chromanone Dimers from the Marine-Derived Fungus Aspergillus versicolor HDN1009. J. Nat. Prod. 2015, 78, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Malmstrøm, J.; Christophersen, C.; Frisvad, J.C. Secondary metabolites characteristic of Penicillium citrinum, Penicillium steckii and related species. Phytochemistry 2000, 54, 301–309. [Google Scholar] [CrossRef]
- Sabat, J.; Gupta, N. Nutritional factors affecting the antifungal activity of Penicillium steckii of mangrove origin. Afr. J. Microbiol. Res. 2010, 4, 126–135. [Google Scholar]
- Cox, R.H.; Hernandez, O.; Dorner, J.W.; Cole, R.J.; Fennell, D.I. A new isochroman mycotoxin isolated from penicillium steckii. J. Agric. Food Chem. 1979, 27, 999–1001. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Pil, G.B.; Heo, S.J.; Lee, H.S.; Lee, J.S.; Lee, Y.J.; Lee, J.; Won, H.S. Anti-inflammatory activity of tanzawaic acid derivatives from a marine-derived fungus Penicillium steckii 108YD142. Mar. Drugs 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, M.; Yamada, K.; Shikano, M.; Yazawa, K.; Arimoto, H.; Okamura, T.; Uemura, D. Tanzawaic acid A, B, C, and D: Inhibitors of superoxide anion production from Penicillium citrinum. Chem. Lett. 1997, 26, 885–886. [Google Scholar] [CrossRef]
- Cardoso-Martínez, F.; de la Rosa, J.M.; Díaz-Marrero, A.R.; Darias, J.; Cerella, C.; Diederich, M.; Cueto, M. Tanzawaic acids isolated from a marine-derived fungus of the genus Penicillium with cytotoxic activities. Org. Biomol. Chem. 2015, 13, 7248–7256. [Google Scholar] [CrossRef] [PubMed]
- Quang, T.H.; Ngan, N.T.T.; Ko, W.; Kim, D.C.; Yoon, C.S.; Sohn, J.H.; Yim, J.H.; Kim, Y.; Oh, H. Tanzawaic acid derivatives from a marine isolate of Penicillium sp. (SF-6013) with anti-inflammatory and PTP1B inhibitory activities. Bioorg. Med. Chem. Lett. 2014, 24, 5787–5791. [Google Scholar] [CrossRef] [PubMed]
- Masuma, R.; Tabata, N.; Tomoda, H.; Haneda, K.; Iwai, Y.; Omura, S. Arohynapenes A and B, new anticoccidial agents produced by Penicillium sp. Taxonomy, fermentation, and structure elucidation. J. Antibiot. 1994, 47, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Tabata, N.; Tomoda, H.; Masuma, R.; Haneda, K.; Iwai, Y.; Omura, S. Hynapenes A, B and C, new anticoccidial agents produced by Penicillium sp. I. Production, isolation and physico-chemical and biological properties. J. Antibiot. 1993, 46, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Ondeyka, J.G.; Giacobbe, R.A.; Bills, G.F.; Cuadrillero, C.; Schmatz, D.; Goetz, M.A.; Zink, D.L.; Singh, S.B. Coprophilin: An anticoccidial agent produced by a dung inhabiting fungus. Bioorg. Med. Chem. Lett. 1998, 8, 3439–3442. [Google Scholar] [CrossRef]
- El-Neketi, M.; Ebrahim, W.; Lin, W.; Gedara, S.; Badria, F.; Saad, H.E.A.; Lai, D.; Proksch, P. Alkaloids and Polyketides from Penicillium citrinum, an Endophyte Isolated from the Moroccan Plant Ceratonia siliqua. J. Nat. Prod. 2013, 76, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, H.; Nishimura, K.; Kuramoto, M.; Uemura, D. Synthesis and absolute stereochemistry of tanzawaic acid (GS-1302). Tetrahedron Lett. 1998, 39, 9513–9516. [Google Scholar] [CrossRef]
- Wu, G.W.; Ma, H.Y.; Zhu, T.J.; Li, J.; Gu, Q.Q.; Li, D.H. Penilactones A and B, two novel polyketides from Antarctic deep-sea derived fungus Penicillium crustosum PRB-2. Tetrahedron 2012, 68, 9745–9749. [Google Scholar] [CrossRef]
- Peng, J.X.; Zhang, X.M.; Du, L.; Wang, W.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Sorbicatechols A and B, antiviral sorbicillinoids from the marine-derived fungus Penicillium chrysogenum PJX-17. J. Nat. Prod. 2014, 77, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Access Structures. Available online: http://www.ccdc.cam.ac.uk/data_request/cif (accessed on 13 March 2017).
- Zhang, X.P.; Wu, C.M.; Wu, H.F.; Sheng, L.H.; Su, Y.; Zhang, X.; Luan, H.; Sun, G.; Sun, X.B.; Tian, Y.; et al. Anti-hyperlipidemic effects and potential mechanisms of action of the caffeoylquinic acid-rich Pandanus tectorius fruit extract in hamsters fed a high fat-diet. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhu, T.J.; Liu, H.B.; Fang, Y.C.; Zhu, W.M.; Gu, Q.Q. Cytotoxic polyketides from a marine-derived fungus, Aspergillus glaucus. J. Nat. Prod. 2008, 71, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.X.; Jiao, J.Y.; Li, J.; Wang, W.; Gu, Q.Q.; Zhu, T.J.; Li, D.H. Pyronepolyene C-glucosides with NF-κB inhibitory and anti-influenza A viral (H1N1) activities from the sponge-associated fungus Epicoccum sp. JJY40. Bioorg. Med. Chem. Lett. 2012, 22, 3188–3190. [Google Scholar] [CrossRef] [PubMed]


| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 170.1 | - | 169.3 | - |
| 2 | 120.3 | 5.82 overlap | 119.4 | 5.80 d (15.3) |
| 3 | 144.6 | 7.22 dd (11.1, 15.3) | 145.1 | 7.23 dd (11.0, 15.3) |
| 4 | 129.8 | 6.32 dd (10.6, 15.3) | 129.4 | 6.29 dd (11.0, 15.3) |
| 5 | 149.1 | 6.00 dd (9.4, 15.3) | 149.3 | 5.96 overlap |
| 6 | 50.3 | 2.61 t (8.0) | 50.1 | 2.61 dd (4.7, 9.0) |
| 7 | 43.7 | 1.33 m | 44.5 | 1.32 overlap |
| 8 | 39.6 | 1.37 m | 39.7 | 1.32 overlap |
| 9 | 39.6 | 1.69 overlap | 39.5 | 1.69 overlap |
| 0.74 q (12.2) | 0.72 q (11.8) | |||
| 10 | 39.9 | 1.61 overlap | 39.9 | 1.57 m |
| 11 | 32.6 | 1.66 overlap | 32.7 | 1.68 overlap |
| 1.13 q (12.1) | 1.17 q (12.2) | |||
| 12 | 42.6 | 1.29 m | 42.4 | 1.32 overlap |
| 13 | 67.0 | 3.83 dd (2.3, 6.4) | 76.3 | 3.46 d (6.1) |
| 14 | 125.5 | 5.83 overlap | 123.7 | 5.96 overlap |
| 15 | 138.2 | - | 139.7 | - |
| 16 | 21.1 | 1.61 s | 21.1 | 1.63 s |
| 17 | 67.3 | 3.37 d (6.2) | 67.3 | 3.35 d (6.6) |
| 18 | 21.5 | 0.97 d (6.1) | 21.1 | 0.95 d (5.8) |
| 13-OCH3 | - | - | 55.5 | 3.33 s |
| No. | 3 | 4 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 172.0 | - | 172.3 | - |
| 2 | 118.5 | 5.80 d (15.4) | 118.5 | 5.79 d (15.3) |
| 3 | 147.0 | 7.33 dd (10.9, 15.1) | 147.2 | 7.33 dd (11.0, 15.3) |
| 4 | 129.0 | 6.19 dd (11.0, 15.2) | 129.0 | 6.21 dd (11.2, 15.3) |
| 5 | 150.7 | 5.92 dd (9.4, 15.2) | 151.0 | 6.04 dd (9.4, 15.3) |
| 6 | 49.9 | 2.57 t (8.4) | 50.3 | 2.53 d (5.9, 8.1) |
| 7 | 48.7 | 0.94 m | 44.1 | 1.30 overlap |
| 8 | 39.7 | 1.38 m | 40.1 | 1.30 overlap |
| 9 | 45.7 | 1.62 overlap | 45.2 | 1.55 overlap |
| 0.75 q (12.3) | 0.77 q (11.9) | |||
| 10 | 31.5 | 1.44 m | 32.3 | 1.46 m |
| 11 | 38.2 | 2.19 d (3.2, 12.6) | 38.5 | 1.57 overlap |
| 0.61 q (12.2) | 1.16 q (12.0) | |||
| 12 | 43.9 | 1.23 m | 42.8 | 1.30 overlap |
| 13 | 80.6 | 3.32 d (10.2) | 76.4 | 3.41 d (6.2) |
| 14 | 126.0 | 5.66 s | 124.3 | 5.92 d (5.9) |
| 15 | 134.7 | - | 139.5 | - |
| 16 | 21.8 | 1.58 s | 22.5 | 1.61 s |
| 17 | 22.5 | 0.89 overlap | 22.4 | 0.88 overlap |
| 18 | 22.4 | 0.89 overlap | 21.8 | 0.88 overlap |
| 13-OCH3 | 56.0 | 3.37 s | 56.7 | 3.35 s |
| No. | 5 a | 6 b | 11 b | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 169.8 | - | 171.5 | - | 171.6 | - |
| 2 | 120.0 | 5.84 d (15.3) | 120.0 | 5.90 d (15.6) | 119.8 | 5.94 d (15.3) |
| 3 | 144.9 | 7.34 dd (10.0, 15.4) | 145.9 | 7.42 dd (11.6, 14.4) | 147.1 | 7.58 dd (11.0, 15.2) |
| 4 | 122.3 | 6.47 dd (9.9, 15.4) | 125.4 | 6.51 dd (11.5, 14.4) | 131.2 | 6.43 dd (11.0, 15.8) |
| 5 | 153.5 | 6.52 d (15.3) | 149.0 | 6.40 d (15.2) | 141.4 | 7.12 d (15.9) |
| 6 | 75.8 | - | 77.9 | - | 135.0 | - |
| 7 | 49.1 | 1.18 overlap | 51.9 | 1.35 t (9.8) | 140.8 | - |
| 8 | 29.0 | 2.00 m | 33.7 | 1.69 m | 30.4 | 3.26 m |
| 9 | 50.1 | 1.62 dt (3.2, 14.1) | 47.1 | 1.66 overlap | 35.0 | 2.18 m |
| 1.23 overlap | 0.81 overlap | 1.19 m | ||||
| 10 | 69.1 | - | 32.6 | 1.56 m | 37.3 | 1.85 m |
| 11 | 45.3 | 1.72 dt (3.0, 13.4) | 41.4 | 1.78 d (12.2) | 33.6 | 2.77 dt (3.05,14.5) |
| 1.25 m | 0.83 overlap | 2.46 dd (11.9, 15.1) | ||||
| 12 | 33.4 | 2.58 m | 38.3 | 2.21 t (10.7) | 135.2 | - |
| 13 | 130.1 | 5.36 d (9.8) | 132.5 | 5.49 d (9.9) | 128.7 | 6.98 overlap |
| 14 | 129.1 | 5.50 m | 130.1 | 5.40 d (9.9) | 127.8 | 6.98 overlap |
| 15 | 45.7 | - | 75.0 | - | 134.1 | - |
| 16 | 18.3 | 0.97 d (7.1) | 27.4 | 1.27 s | 21.1 | 2.28 s |
| 17 | 30.1 | 1.18 s | 22.2 | 0.88 d (6.5) | 68.0 | 3.66 overlap |
| 3.65 overlap | ||||||
| 18 | 22.0 | 0.91 d (6.6) | 23.5 | 0.95 d (6.1) | 24.0 | 1.16 d (7.0) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Wang, S.; Wang, L.; Che, Q.; Zhu, T.; Zhang, G.; Gu, Q.; Guo, P.; Li, D. Lipid-Lowering Polyketides from the Fungus Penicillium Steckii HDN13-279. Mar. Drugs 2018, 16, 25. https://doi.org/10.3390/md16010025
Yu G, Wang S, Wang L, Che Q, Zhu T, Zhang G, Gu Q, Guo P, Li D. Lipid-Lowering Polyketides from the Fungus Penicillium Steckii HDN13-279. Marine Drugs. 2018; 16(1):25. https://doi.org/10.3390/md16010025
Chicago/Turabian StyleYu, Guihong, Shuai Wang, Lu Wang, Qian Che, Tianjiao Zhu, Guojian Zhang, Qianqun Gu, Peng Guo, and Dehai Li. 2018. "Lipid-Lowering Polyketides from the Fungus Penicillium Steckii HDN13-279" Marine Drugs 16, no. 1: 25. https://doi.org/10.3390/md16010025
APA StyleYu, G., Wang, S., Wang, L., Che, Q., Zhu, T., Zhang, G., Gu, Q., Guo, P., & Li, D. (2018). Lipid-Lowering Polyketides from the Fungus Penicillium Steckii HDN13-279. Marine Drugs, 16(1), 25. https://doi.org/10.3390/md16010025