Bioactive Steroids with Methyl Ester Group in the Side Chain from a Reef Soft Coral Sinularia brassica Cultured in a Tank
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Cytotoxicity Assay
3.5. Human Neutrophil Superoxide Anion Generation and Elastase Release
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294, (and previous articles in this series). [Google Scholar] [CrossRef] [PubMed]
- Chitturi, B.R.; Tatipamula, V.B.; Dokuburra, C.B.; Mangamuri, U.K.; Tuniki, V.R.; Kalivendi, S.V.; Bunce, R.A.; Yenamandra, V. Pambanolides A–C from the South Indian soft coral Sinularia inelegans. Tetrahedron 2016, 72, 1933–1940. [Google Scholar] [CrossRef]
- Huang, C.Y.; Tseng, Y.J.; Chokkalingam, U.; Hwang, T.L.; Hsu, C.H.; Dai, C.F.; Sung, P.J.; Sheu, J.H. Bioactive isoprenoid-derived natural products from a Dongsha Atoll soft coral Sinularia erecta. J. Nat. Prod. 2016, 79, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, H.; Taira, J.; Ueda, K. Hydrogen peroxide derived from marine peroxy sesquiterpenoids induces apoptosis in HCT116 human colon cancer cells. Bioorg. Med. Chem. Lett. 2016, 26, 4641–4644. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.J.; Yang, Y.C.; Wang, S.K.; Duh, C.Y. Numerosol A–D, new cembranoid diterpenes from the soft coral Sinularia numerosa. Mar. Drugs 2014, 12, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Nielson, J.L.; Tapiolas, D.M.; Liptrot, C.H.; Motti, C.A. A Great Barrier Reef Sinularia sp. yields two new cytotoxic diterpenes. Mar. Drugs 2012, 10, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Arepalli, S.K.; Sridhar, V.; Rao, J.V.; Kennady, P.K.; Venkateswarlu, Y. Furano-sesquiterpene from soft coral, Sinularia kavarittiensis: Induces apoptosis via the mitochondrial-mediated caspase-dependent pathway in THP-1, leukemia cell line. Apoptosis 2009, 14, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.N.; Ferreira, D.; Garcia-Fernandez, L.F.; Slattery, M. Cytotoxic diterpenoids from the Hybrid soft coral Sinularia maxima × Sinularia polydactyla. J. Nat. Prod. 2007, 70, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.W.; Geng, Z.F.; Deng, Z.W.; van Ofwegen, L.; Proksch, P.; Lin, W.H. Cembranoids from the soft coral Sinularia rigida with antifouling activities. J. Agric. Food Chem. 2013, 61, 4585–4592. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Ashimine, R.; Miyazato, H.; Taira, J.; Ueda, K. Endoperoxy and hydroperoxy cadinane-type sesquiterpenoids from an Okinawan soft coral, Sinularia sp. Arch. Pharm. Res. 2016, 39, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.C.; Chen, H.Y.; Sheu, J.H.; Chiang, M.Y.; Wen, Z.H.; Dai, C.F.; Su, J.H. Structural elucidation and structure–Anti-inflammatory activity relationships of cembranoids from cultured soft corals Sinularia sandensis and Sinularia flexibilis. J. Agric. Food. Chem. 2015, 63, 7211–7218. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Huang, T.Z.; Wu, C.Y.; Chen, B.W.; Huang, C.Y.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. Steroidal and α-tocopherylhydroquinone glycosides from two soft corals Cladiella hirsuta and Sinularia nanolobata. RSC Adv. 2015, 5, 74256–74262. [Google Scholar] [CrossRef]
- Lillsunde, K.E.; Festa, C.; Adel, H.; De Marino, S.; Lombardi, V.; Tilvi, S.; Nawrot, D.A.; Zampella, A.; D’Souza, L.; D’Auria, M.V.; et al. Bioactive cembrane derivatives from the Indian Ocean soft coral, Sinularia kavarattiensis. Mar. Drugs 2014, 12, 4045–4068. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Nam, N.H.; Cuong, N.X.; Quang, T.H.; Tung, P.T.; Dat, L.D.; Chae, D.; Kim, S.; Koh, Y.S.; Kiem, P.V.; et al. Anti-inflammatory norditerpenoids from the soft coral Sinularia maxima. Bioorg. Med. Chem. Lett. 2013, 23, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Putra, M.Y.; Ianaro, A.; Panza, E.; Bavestrello, G.; Cerrano, C.; Fattorusso, E.; Taglialatela-Scafati, O. Sinularioside, a triacetylated glycolipid from the Indonesian soft coral Sinularia sp. is an inhibitor of NO release. Bioorg. Med. Chem. Lett. 2012, 22, 2723–2725. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.J.; Tseng, Y.J.; Huang, C.Y.; Wen, Z.H.; Dai, C.F.; Sheu, J.H. Cytotoxic and anti-inflammatory diterpenoids from the Dongsha Atoll soft coral Sinularia flexibilis. Tetrahedron 2012, 68, 244–249. [Google Scholar] [CrossRef]
- Chao, C.H.; Chou, K.J.; Huang, C.Y.; Wen, Z.H.; Hsu, C.H.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Bioactive cembranoids from the soft coral Sinularia crassa. Mar. Drugs 2011, 9, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, C.Y.; Lin, Y.F.; Wen, Z.H.; Su, J.H.; Kuo, Y.H.; Chiang, M.Y.; Sheu, J.H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 2008, 71, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.F.; Xu, S.H.; Yin, P.H.; Peng, C.H.; Liao, L. NMR spectral assignments of two sterols from a soft coral Sinularia brassica. Magn. Reson. Chem. 2011, 49, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Liaw, C.C.; Chen, B.W.; Chen, P.C.; Su, J.H.; Sung, P.J.; Dai, C.F.; Chiang, M.Y.; Sheu, J.H. Withanolide-based steroids from the cultured soft coral Sinularia brassica. J. Nat. Prod. 2013, 76, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Ahmed, A.F.; Su, J.H.; Sung, P.J.; Hwang, T.L.; Chiang, P.L.; Dai, C.F.; Liaw, C.C.; Sheu, J.H. Bioactive new withanolides from the cultured soft coral Sinularia brassica. Bioorg. Med. Chem. Lett. 2017, 27, 3267–3271. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; He, H.; Qiu, F. Natural withanolides: An overview. Nat. Prod. Rep. 2011, 28, 705–740. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.Y.; Chiu, C.C.; Chang, F.R.; Chen, J.Y.F.; Hwang, C.C.; Hseu, Y.C.; Yang, H.L.; Lee, A.Y.L.; Tsai, M.T.; Guo, Z.L.; et al. 4β-Hydroxywithanolide E from Physalis peruviana (golden berry) inhibits growth of human lung cancer cells through DNA damage, apoptosis and G(2)/M arrest. BMC Cancer 2010, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Ksebati, M.B.; Schmitz, F.J. Minabeolides: A group of withanolides from a soft coral, Minabea sp. J. Org. Chem. 1988, 53, 3926–3929. [Google Scholar] [CrossRef]
- Chao, C.H.; Chou, K.J.; Wen, Z.H.; Wang, G.H.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Paraminabeolides A–F, cytotoxic and anti-inflammatory marine withanolides from the soft coral Paraminabea acronocephala. J. Nat. Prod. 2011, 74, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Chang, C.W.; Tseng, Y.J.; Lee, J.; Sung, P.J.; Su, J.H.; Hwang, T.L.; Dai, C.F.; Wang, H.C.; Sheu, J.H. Bioactive steroids from the Formosan soft coral Umbellulifera petasites. Mar. Drugs 2016, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.W.; Kleiman, R. Degradation of linoleic-acid hydroperoxides by a cysteine FeCl3 catalyst as a model for similar biochemical reactions. II. Specificity in formation of fatty-acid epoxides. Biochim. Biophys. Acta 1981, 665, 113–125. [Google Scholar] [CrossRef]
- Voigt, B.; Porzel, A.; Bruhn, C.; Wagner, C.; Merzweiler, K.; Adam, G. Synthesis of 24-epicathasterone and related brassinosteroids with modified side chain. Tetrahedron 1997, 53, 17039–17054. [Google Scholar] [CrossRef]
- Cheng, T.C.; Din, Z.H.; Su, J.H.; Wu, Y.J.; Liu, C.I. Sinulariolide suppresses cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 and urokinase through the PI3K/AKT/mTOR signaling pathway in human bladder cancer cells. Mar. Drugs 2017, 15, 238. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Huang, C.Y.; Tseng, W.R.; Chiang, P.L.; Hwang, T.L.; Su, J.H.; Sung, P.J.; Dai, C.F.; Sheu, J.H. Klyflaccisteroids K–M, bioactive steroidal derivatives from a soft coral Klyxum flaccidum. Bioorg. Med. Chem. Lett. 2017, 27, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.R.; Huang, C.Y.; Tsai, Y.Y.; Lin, Y.S.; Hwang, T.L.; Su, J.H.; Sung, P.J.; Dai, C.F.; Sheu, J.H. New cytotoxic and anti-inflammatory steroids from the soft coral Klyxum flaccidum. Bioorg. Med. Chem. Lett. 2016, 26, 3253–3257. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, C.; Senese, M.; Aiello, A.; Luciano, P.; Fiorucci, S.; D’Amore, C.; Carino, A.; Menna, M. Phallusiasterol C, a new disulfated steroid from the mediterranean tunicate Phallusia fumigata. Mar. Drugs 2016, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chen, Y.C.; Chan, W.Y. Marine natural products with anti-inflammatory activity. Appl. Microbiol. Biot. 2016, 100, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parondoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F.I. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Hsieh, P.W.; Chang, Y.J.; Chung, P.J.; Kuo, L.M.; Hwang, T.L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
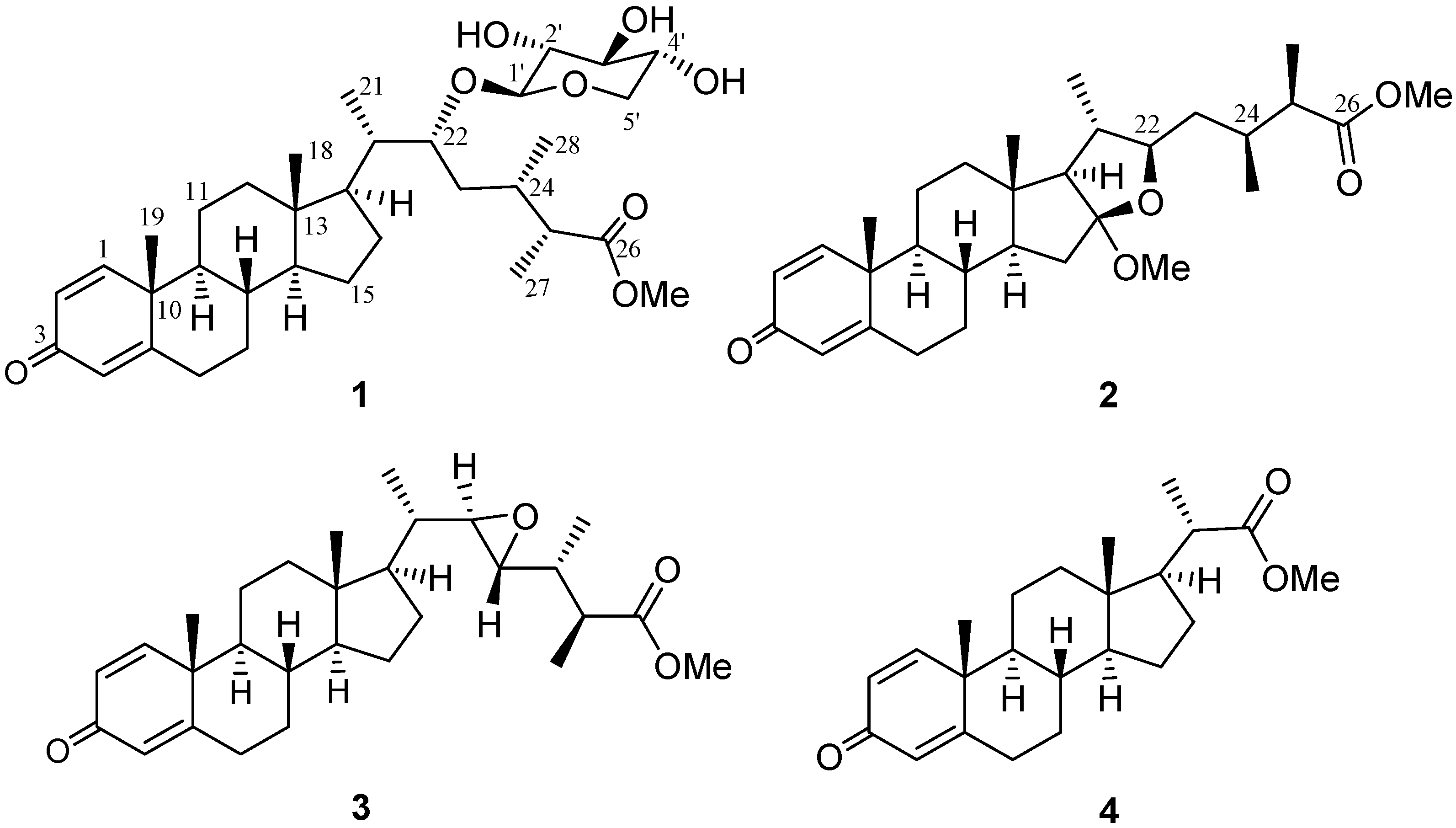

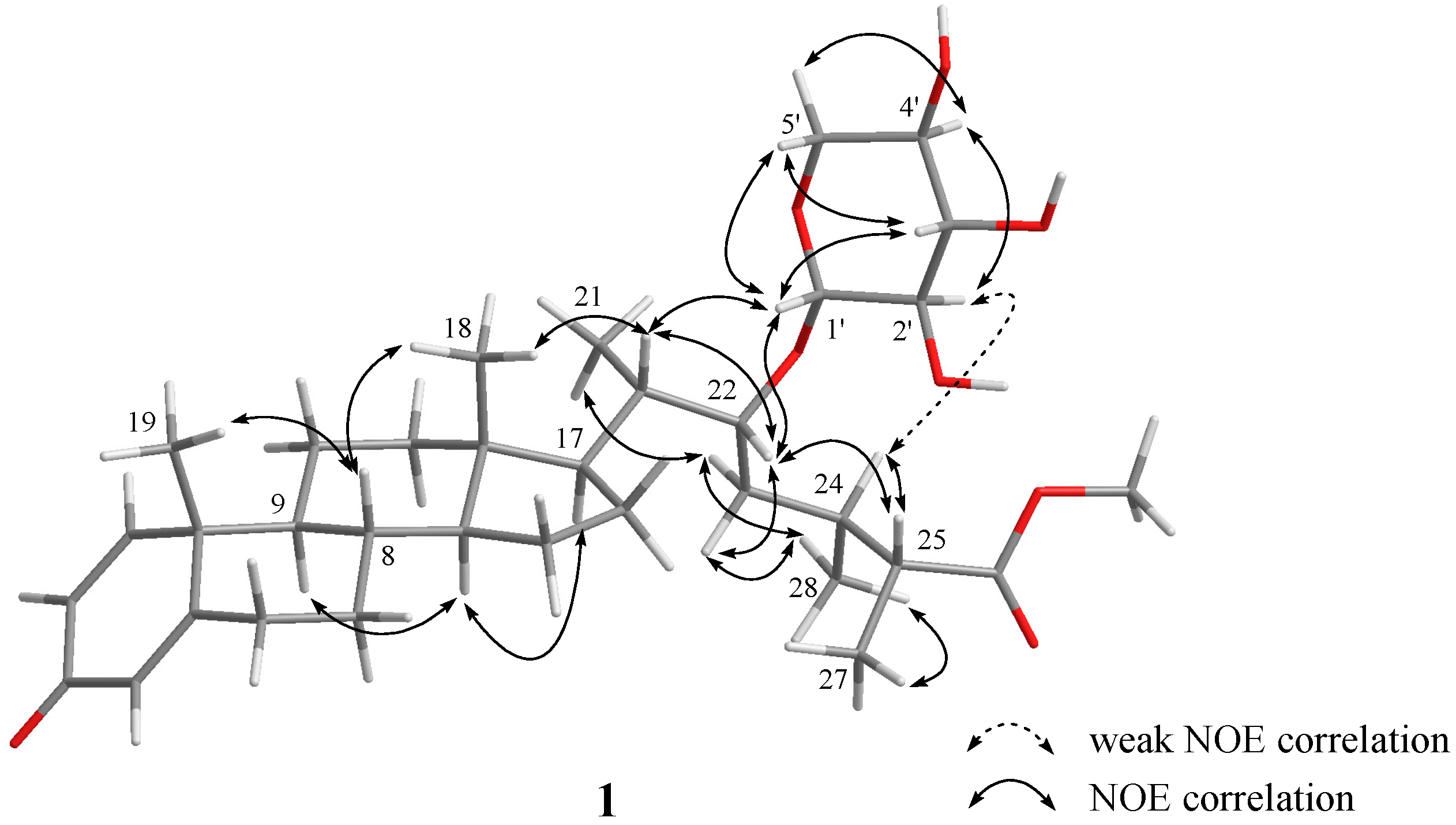
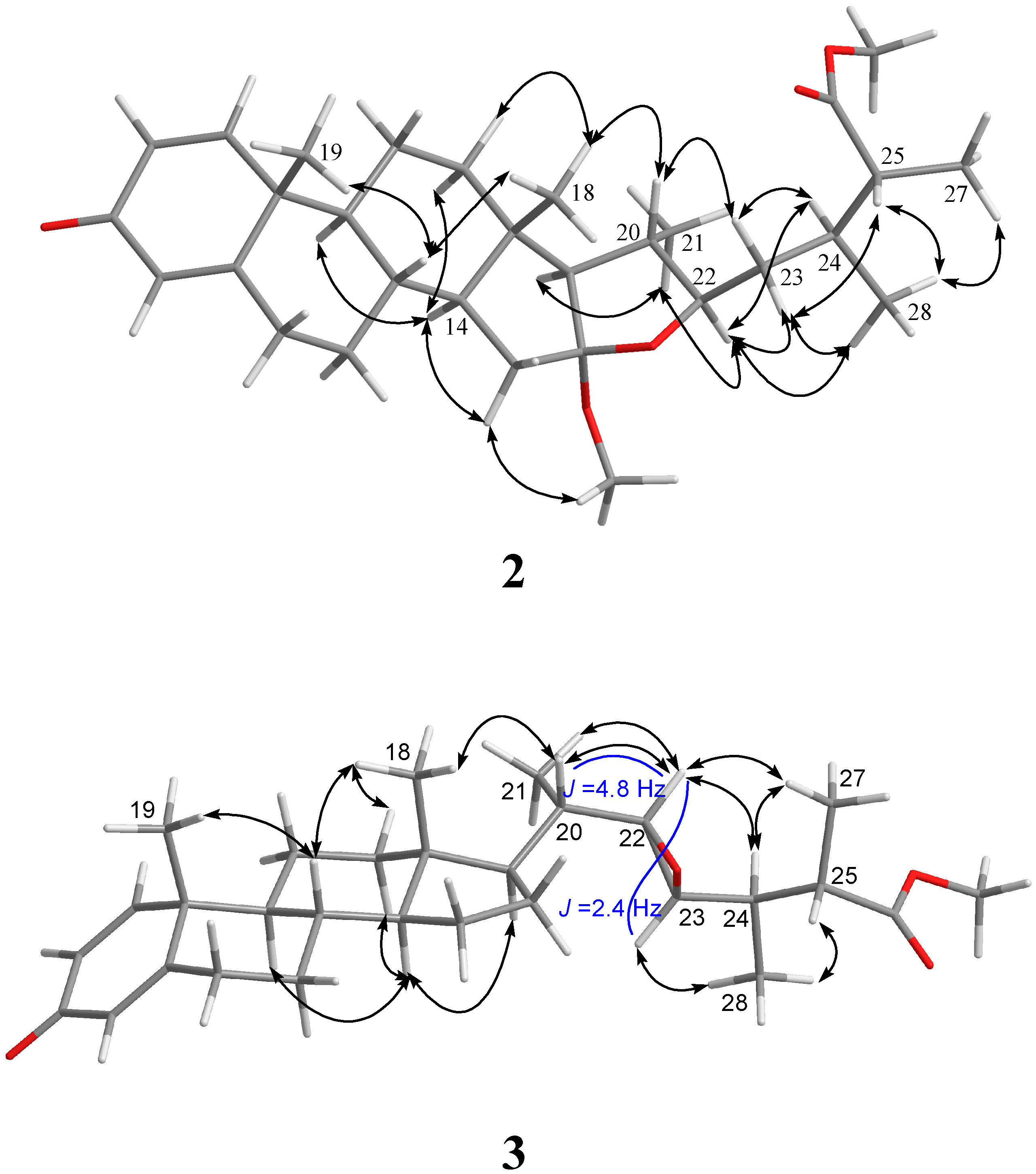
| 1 | |||||
|---|---|---|---|---|---|
| Position | δC a (Mult.) b | δH c (J in Hz) | Position | δC (Mult.) | δH (J in Hz) |
| 1 | 156.0, CH | 7.05 d (10.0) | 1′ | 104.5, CH | 4.30 d (7.0) |
| 2 | 127.5, CH | 6.23 d (10.0) | 2′ | 73.4, CH | 3.42 dd (8.5, 7.0) |
| 3 | 186.5, C | 3′ | 75.9, CH | 3.52 dd (8.5, 8.5) | |
| 4 | 123.8, CH | 6.07 s | 4′ | 69.5, CH | 3.75 ddd (9.5, 8.5, 5.0) |
| 5 | 169.3, C | 5′ | 64.9, CH2 | 4.01 dd (12.0, 5.0); | |
| 6 | 32.8, CH2 | 2.47 ddd (12.5, 12.5, 4.5) | 3.30 d (12.0, 9.5) | ||
| 2.36 br d (12.5) | 26-OMe | 52.1, CH3 | 3.71 s | ||
| 7 | 33.6, CH2 | 1.95 m; 1.04 m | |||
| 8 | 35.5, CH | 1.63 m | |||
| 9 | 52.3, CH | 1.04 m | |||
| 10 | 43.6, C | ||||
| 11 | 22.8, CH2 | 1.70 m | |||
| 12 | 39.4, CH2 | 2.04 m; 1.16 m | |||
| 13 | 43.0, C | ||||
| 14 | 55.0, CH | 0.99 m | |||
| 15 | 24.5, CH2 | 1.66 m; 1.20 m | |||
| 16 | 27.5, CH2 | 1.68 m; 1.34 m | |||
| 17 | 52.6, CH | 1.06 m | |||
| 18 | 11.9, CH3 | 0.76 s | |||
| 19 | 18.7, CH3 | 1.23 s | |||
| 20 | 39.5, CH | 2.04 m | |||
| 21 | 12.6, CH3 | 0.92 d (6.5) | |||
| 22 | 81.5, CH | 3.63 br d (11.0) | |||
| 23 | 32.3, CH2 | 1.43 ddd (14.5, 11.0, 3.5); | |||
| 1.28 m | |||||
| 24 | 31.5, CH | 2.29 m | |||
| 25 | 40.9, CH | 2.62 qd (6.0, 4.0) | |||
| 26 | 177.7, C | ||||
| 27 | 9.9, CH3 | 1.02 d (7.0) | |||
| 28 | 16.3, CH3 | 0.85 d (7.0) | |||
| 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|
| Position | δC a (Mult.) b | δH c (J in Hz) | δC d (Mult.) | δH e (J in Hz) | δC a (Mult.) | δH c (J in Hz) |
| 1 | 155.7, CH | 7.04 d (10.0) | 156.0, CH | 7.05 d (10.4) | 155.8, CH | 7.05 d (10.0) |
| 2 | 127.6, CH | 6.23 d (10.0) | 127.5, CH | 6.23 d (10.4) | 127.5, CH | 6.23 d (10.0) |
| 3 | 186.3, C | 186.5, C | 186.4, C | |||
| 4 | 123.9, CH | 6.07 s | 123.8, CH | 6.07 s | 123.9, CH | 6.07 s |
| 5 | 168.9, C | 169.4, C | 169.2, C | |||
| 6 | 32.7, CH2 | 2.46 ddd (12.0, 12.0, 4.0) | 32.9, CH2 | 2.47 m | 32.8, CH2 | 2.46 ddd (13.0, 13.0, 4.0) |
| 2.36 br d (12.0) | 2.37 m | 2.36 m | ||||
| 7 | 33.5, CH2 | 1.90 m; 1.07 m | 33.6, CH2 | 1.96 m; 1.05 m | 33.5, CH2 | 1.94 m; 1.05 m |
| 8 | 35.1, CH | 1.76 m | 35.5, CH | 1.60 m | 35.5, CH | 1.64 m |
| 9 | 52.2, CH | 1.09 m | 52.4, CH | 1.06 m | 52.2, CH | 1.08 m |
| 10 | 43.6, C | 43.6, C | 43.5, C | |||
| 11 | 22.4, CH2 | 1.69 m | 22.8, CH2 | 1.66 m | 22.8, CH2 | 1.71 m |
| 12 | 38.8, CH2 | 1.71 m | 39.3, CH2 | 1.99 m | 39.2, CH2 | 1.97 ddd (13.0, 3.0, 3.0) |
| 1.21 m | 1.21 m | 1.28 m | ||||
| 13 | 40.9, C | 43.0, C | 42.7, C | |||
| 14 | 54.6, CH | 1.36 dd (12.0, 5.5) | 55.0, CH | 1.02 m | 55.0, CH | 1.08 m |
| 15 | 33.5, CH2 | 1.96 dd (12.0, 5.5) | 24.6, CH2 | 1.63 m | 24.4, CH2 | 1.62 m |
| 1.31 dd (12.0, 12.0) | 1.17 m | 1.19 m | ||||
| 16 | 117.9, C | 26.9, CH2 | 1.93 m; 1.60 m | 27.0, CH2 | 1.70 m; 1.30 m | |
| 17 | 70.9, CH | 1.65 m | 55.8, CH | 1.30 m | 52.7, CH | 1.60 m |
| 18 | 15.3, CH3 | 0.83 s | 12.2, CH3 | 0.73 s | 12.2, CH3 | 0.76 s |
| 19 | 19.2, CH3 | 1.24 s | 18.7, CH3 | 1.23 s | 18.7, CH3 | 1.23 s |
| 20 | 38.1, CH | 1.74 m | 38.5, CH | 1.30 m | 42.4, CH | 2.43 m |
| 21 | 18.8, CH3 | 1.02 d (7.0) | 15.9, CH3 | 0.99 d (7.2) | 17.0, CH3 | 1.18 d (7.0) |
| 22 | 86.9, CH | 3.69 m | 63.9, CH | 2.59 dd (4.8, 2.4) | 177.1, C | |
| 23 | 38.5, CH2 | 1.53 m; 1.48 m | 59.1, CH | 2.52 dd (7.6, 2.4) | ||
| 24 | 33.3, CH | 2.07 dddq (6.5, 6.5, 6.5, 6.5) | 39.2, CH | 1.54 m | ||
| 25 | 43.6, CH | 2.49 dq (6.5, 6.5) | 42.9, CH | 2.48 m | ||
| 26 | 176.8, C | 175.6, C | ||||
| 27 | 11.9, CH3 | 1.08 d (6.5) | 14.5, CH3 | 1.21 d (7.2) | ||
| 28 | 16.3, CH3 | 0.93 d (6.5) | 14.8, CH3 | 1.02 d (7.2) | ||
| 16-OMe | 49.3, CH3 | 3.20 s | ||||
| 22-OMe | 51.4, CH3 | 3.65 s | ||||
| 26-OMe | 51.4, CH3 | 3.67 s | 51.5, CH3 | 3.69 s | ||
| Cell lines IC50 (μM) | ||||
|---|---|---|---|---|
| Compound | P388D1 | MOLT-4 | K-562 | HT-29 |
| 1 | 37.2 ± 4.0 | 37.8 ± 5.6 | ― b | ― b |
| 2 | 9.7 ± 1.2 | 6.0 ± 0.4 | 5.2 ± 0.8 | 7.6 ± 2.3 |
| 3 | 5.7 ± 1.8 | 5.3 ± 1.3 | 12.1 ± 2.4 | 10.4 ± 2.2 |
| 4 | 24.4 ± 4.8 | 31.2 ± 7.0 | 21.3 ± 3.7 | 36.5 ± 7.9 |
| 5-Fluorouracil a | 6.2 ± 0.7 | 6.9 ± 1.3 | 33.1 ± 8.9 | 7.7 ± 0.8 |
| Compounds | Superoxide Anion | Elastase Release | ||||
|---|---|---|---|---|---|---|
| IC50 (μM) a | Inh % b | IC50 (μM) a | Inh % b | |||
| 1 | >10 | 24.8 ± 6.5 | * | >10 | 35.6 ± 1.3 | *** |
| 2 | >10 | 19.4 ± 5.0 | * | >10 | 39.0 ± 2.3 | *** |
| 3 | >10 | 27.7 ± 1.3 | *** | 6.6 ± 1.7 | 58.8 ± 4.0 | *** |
| 4 | 8.4 ± 1.1 | 53.6 ± 1.8 | *** | 6.5 ± 1.1 | 66.3 ± 6.0 | *** |
| Idelalisib | 0.07 ± 0.01 | 102.8 ± 2.2 | *** | 0.3 ± 0.1 | 99.6 ± 4.2 | *** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Su, J.-H.; Liaw, C.-C.; Sung, P.-J.; Chiang, P.-L.; Hwang, T.-L.; Dai, C.-F.; Sheu, J.-H. Bioactive Steroids with Methyl Ester Group in the Side Chain from a Reef Soft Coral Sinularia brassica Cultured in a Tank. Mar. Drugs 2017, 15, 280. https://doi.org/10.3390/md15090280
Huang C-Y, Su J-H, Liaw C-C, Sung P-J, Chiang P-L, Hwang T-L, Dai C-F, Sheu J-H. Bioactive Steroids with Methyl Ester Group in the Side Chain from a Reef Soft Coral Sinularia brassica Cultured in a Tank. Marine Drugs. 2017; 15(9):280. https://doi.org/10.3390/md15090280
Chicago/Turabian StyleHuang, Chiung-Yao, Jui-Hsin Su, Chih-Chuang Liaw, Ping-Jyun Sung, Pei-Lun Chiang, Tsong-Long Hwang, Chang-Feng Dai, and Jyh-Horng Sheu. 2017. "Bioactive Steroids with Methyl Ester Group in the Side Chain from a Reef Soft Coral Sinularia brassica Cultured in a Tank" Marine Drugs 15, no. 9: 280. https://doi.org/10.3390/md15090280
APA StyleHuang, C.-Y., Su, J.-H., Liaw, C.-C., Sung, P.-J., Chiang, P.-L., Hwang, T.-L., Dai, C.-F., & Sheu, J.-H. (2017). Bioactive Steroids with Methyl Ester Group in the Side Chain from a Reef Soft Coral Sinularia brassica Cultured in a Tank. Marine Drugs, 15(9), 280. https://doi.org/10.3390/md15090280






