Urinary Excretion of Tetrodotoxin Modeled in a Porcine Renal Proximal Tubule Epithelial Cell Line, LLC-PK1
Abstract
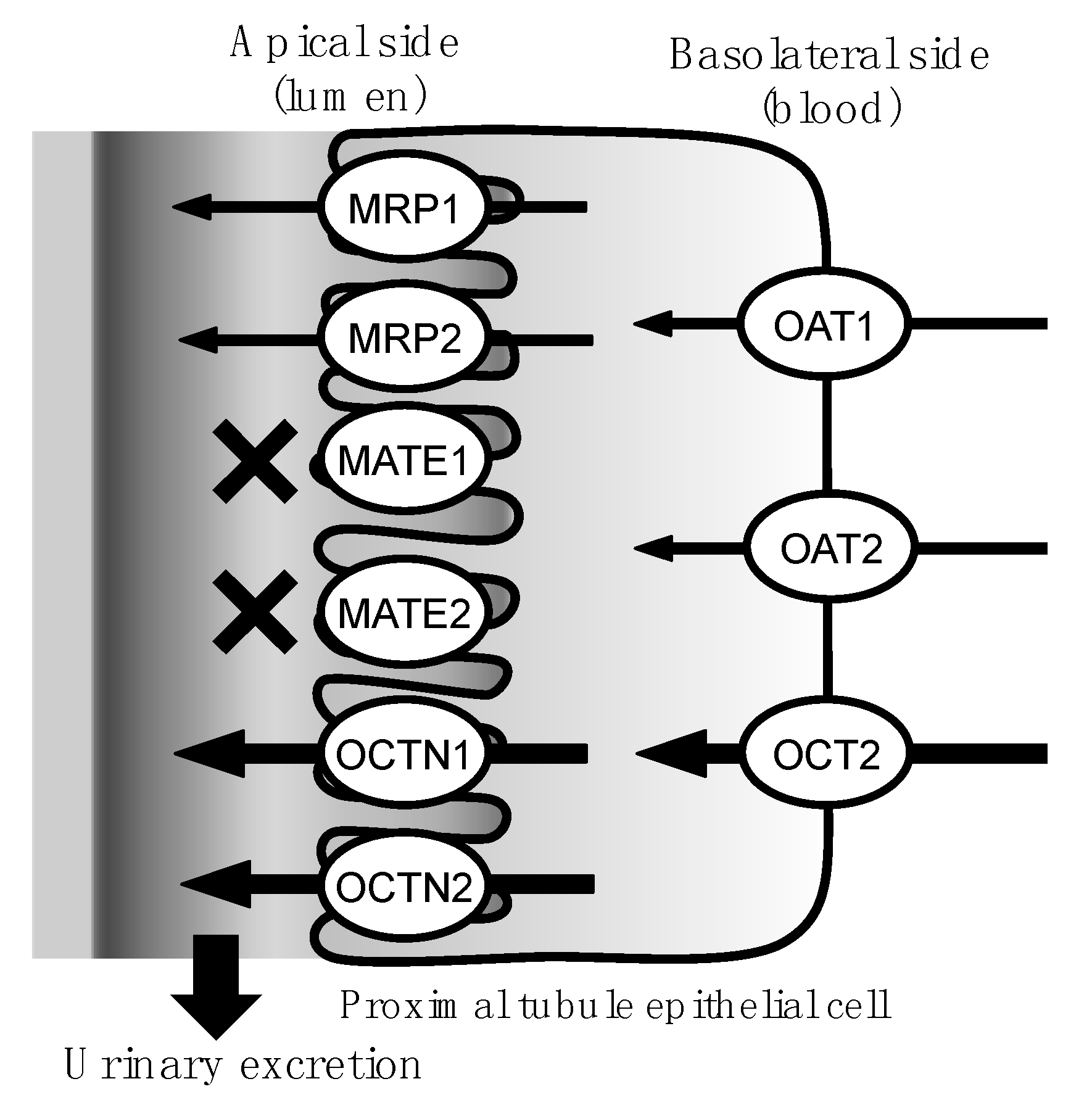
1. Introduction
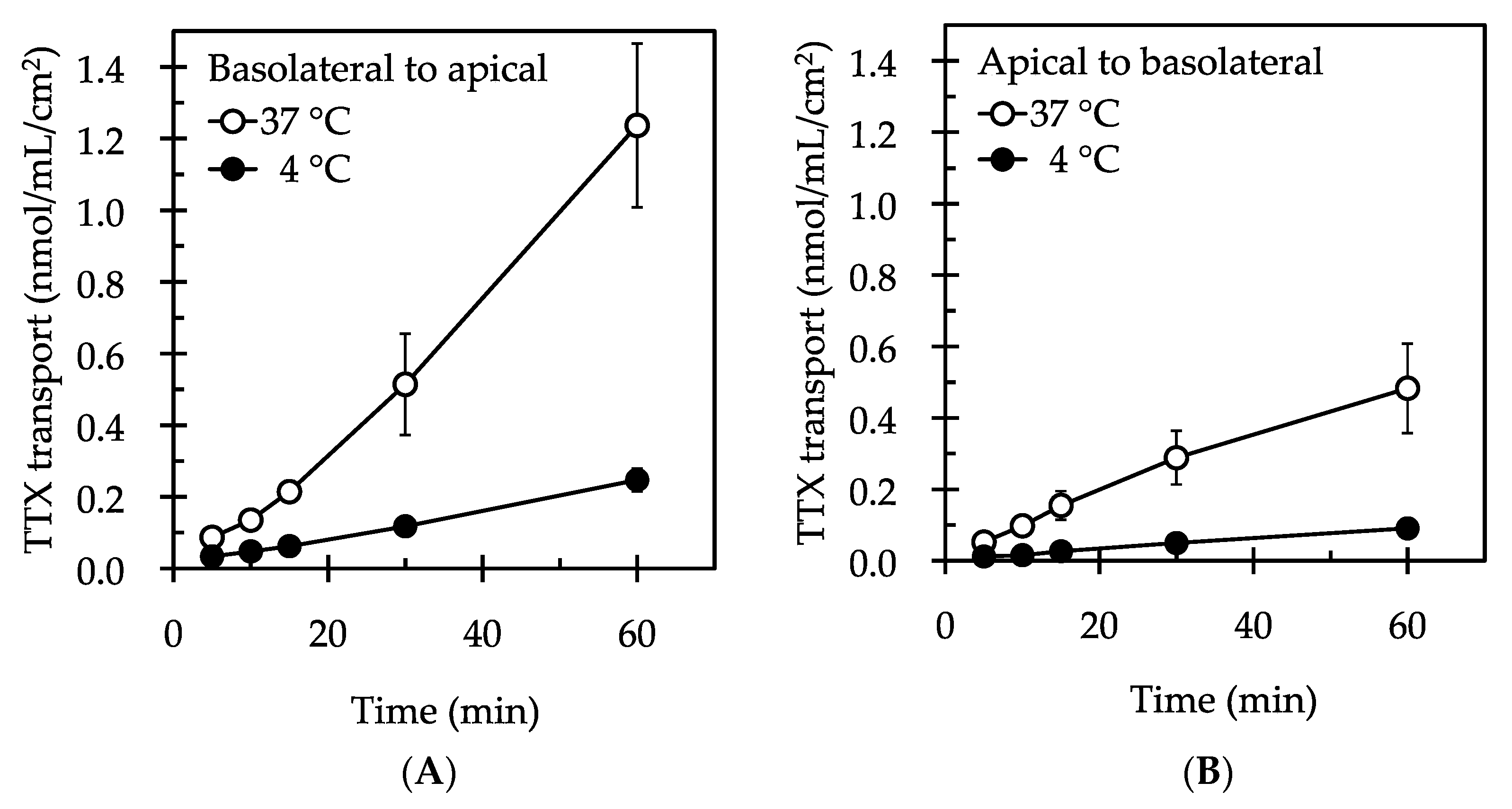
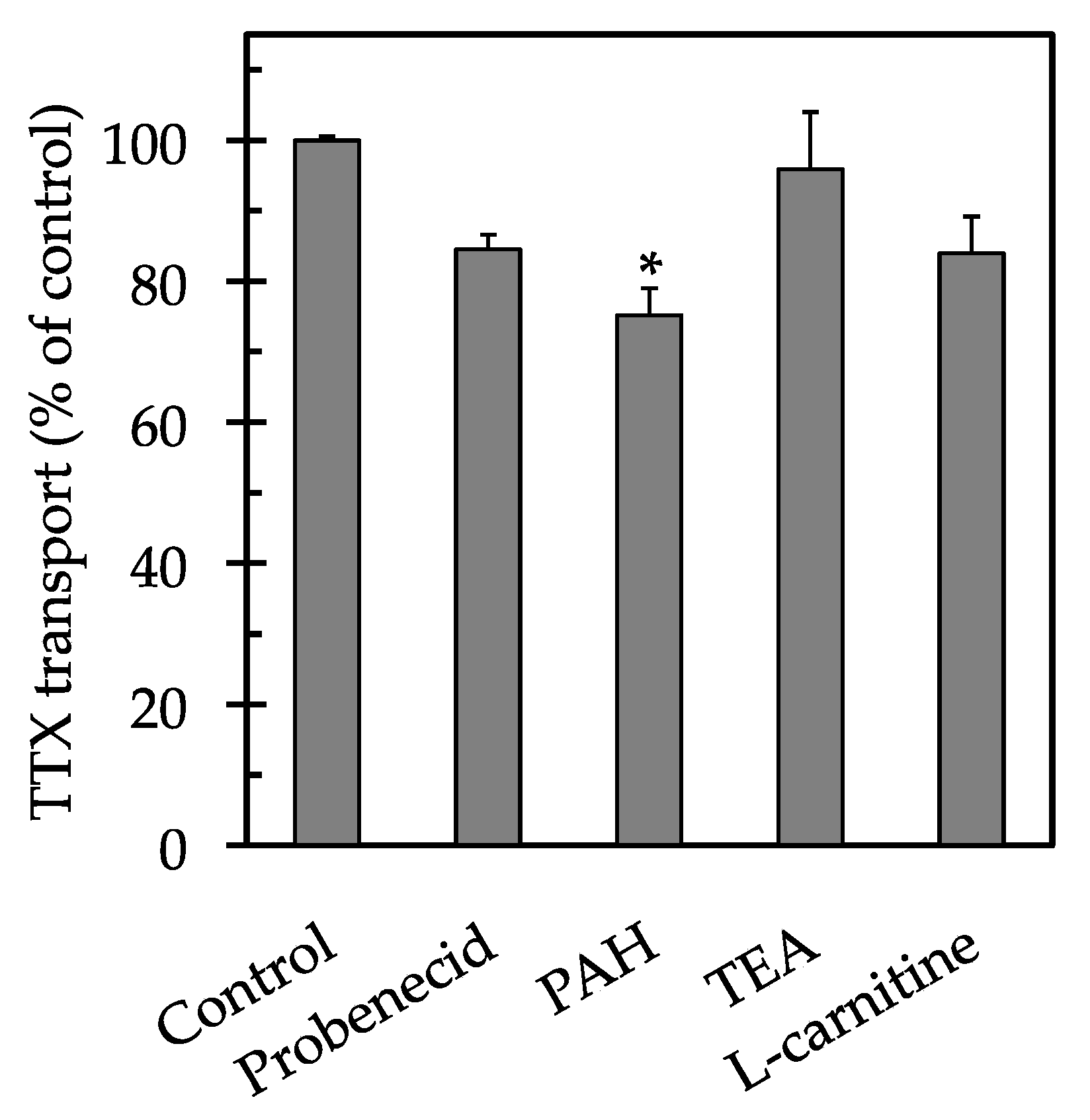
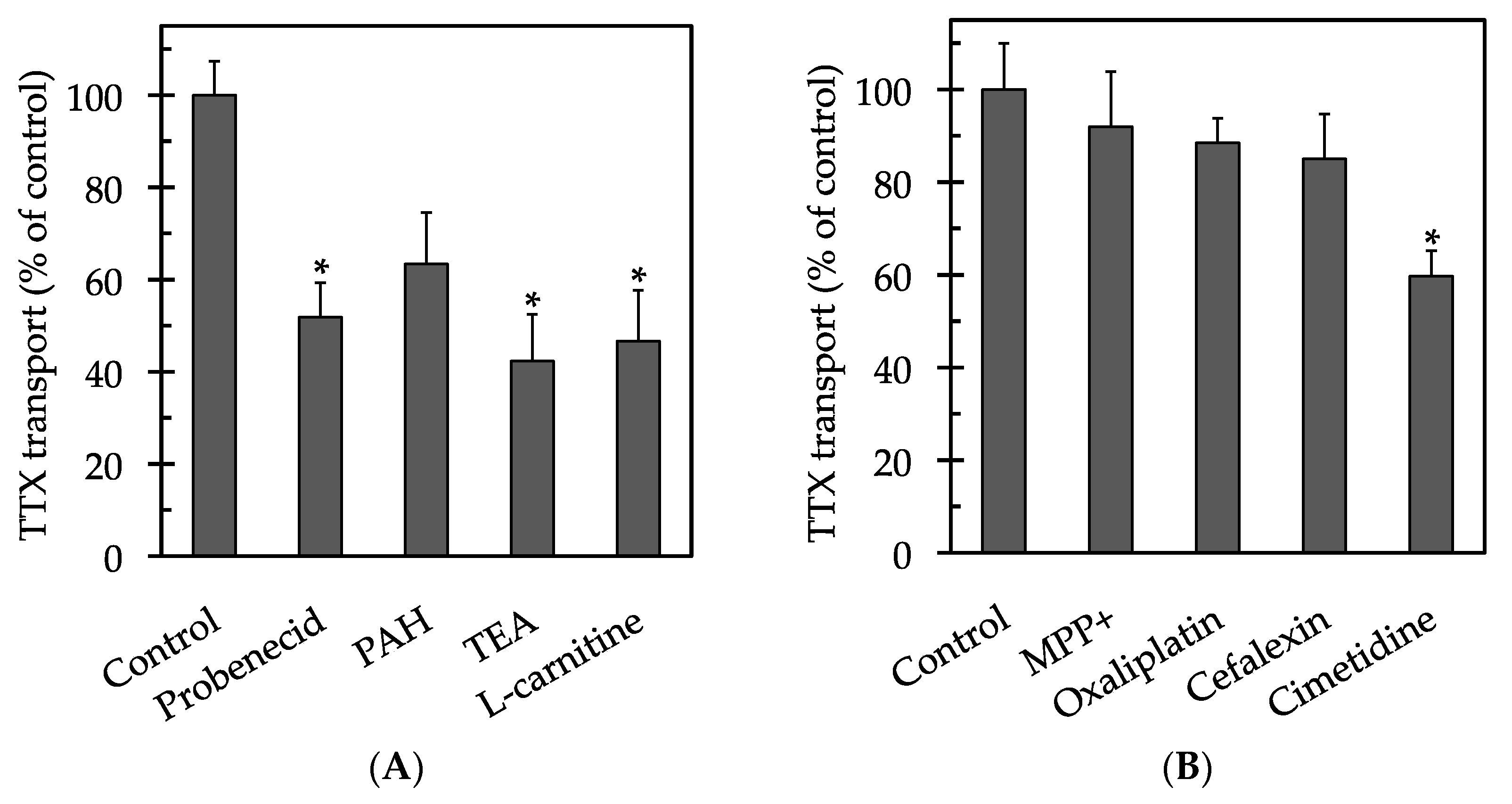
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Transepithelial Electrical Resistance (TEER) Measurements
4.4. Transport Studies
4.5. Determination of TTX Levels by LC-MS/MS
4.6. Pharmacokinetics and Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Narahashi, T.; Moore, J.W.; Scott, W.R. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J. Gen. Physiol. 1964, 47, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Takata, M.; Moore, J.W.; Kao, C.Y.; Fuhrman, F.A. Blockage of sodium conductance increase in lobster giant axon by tarichatoxin (tetrodotoxin). J. Gen. Physiol. 1966, 49, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Noguchi, T. Distribution and origin of tetrodotoxin. J. Toxicol. Toxin Rev. 2001, 20, 11–33. [Google Scholar] [CrossRef]
- Habu, J.; Kim, M.; Katayama, M.; Komiya, H. Jomon subsistence-settlement systems at the Sannai Maruyama site. Bull. Indo-Pac. Prehist. Assoc. 2001, 21, 9–21. [Google Scholar]
- Ishida, Y.; Yamada, A.; Adachi, H.; Yagisawa, I.; Tadokoro, K.; Geiger, H.J. Salmon distribution in the northern Japan during the Jomon Period, 2000–8000 years ago, and its implications for future global warming. NPAFC Bull. 2009, 5, 287–292. [Google Scholar]
- O’Connor, S.; Ono, R.; Clarkson, C. Pelagic fishing at 42,000 years before the present and the maritime skills of modern humans. Science 2011, 334, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; Son, J.; Wang, F.; Maclean, C.J.; Lin, C.S.; Ujma, J.; Balit, C.R.; Smith, B.; Milder, D.G.; Kiernan, M.C. Puffer fish poisoning: A potentially life-threatening condition. Med. J. Aust. 2002, 177, 650–653. [Google Scholar] [PubMed]
- Kanchanapongkul, J. Tetrodotoxin poisoning following ingestion of the toxic eggs of the horseshoe crab Carcinoscorpius rotundicauda, a case series from 1994 through 2006. Southeast Asian J. Trop. Med. Public Health 2008, 39, 303–306. [Google Scholar] [PubMed]
- Fernández-Ortega, J.F.; Morales-de los Santos, J.M.; Herrera-Gutiérrez, M.E.; Fernández-Sánchez, V.; Loureo, P.R.; Rancaño, A.A.; Téllez-Andrade, A. Seafood intoxication by tetrodotoxin: First case in Europe. J. Emerg. Med. 2010, 39, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Islam, Q.T.; Razzak, M.A.; Islam, M.A.; Bari, M.I.; Basher, A.; Chowdhury, F.R.; Sayeduzzaman, A.B.; Ahasan, H.A.; Faiz, M.A.; Arakawa, O.; et al. Puffer fish poisoning in Bangladesh: Clinical and toxicological results from large outbreaks in 2008. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.S.; Quek, L.S.; Lim, E.K.; Ngo, A. A case report of puffer fish poisoning in Singapore. Case Rep. Med. 2013, 2013, 206971. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.M.D.S.; Mendes, T.M.; Adão, A.; Junior, V.H. Poisoning after ingestion of pufferfish in Brazil: Report of 11 cases. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Lin, C.L.; Chen, C.H.; Hsieh, C.H.; Jen, H.C.; Jian, S.J.; Hwang, D.F. Toxin and species identification of toxic octopus implicated into food poisoning in Taiwan. Toxicon 2014, 91, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Heegaard, W.G.; Deeds, J.R.; McGrath, S.C.; Handy, S.M. Tetrodotoxin poisoning outbreak from imported dried puffer fish-Minneapolis, Minnesota, 2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 63, 1222–1225. [Google Scholar] [PubMed]
- You, J.; Yue, Y.; Xing, F.; Xia, W.; Lai, S.; Zhang, F. Tetrodotoxin poisoning caused by Goby fish consumption in southeast China: A retrospective case series analysis. Clinics 2015, 70, 24–29. [Google Scholar] [CrossRef]
- Noguchi, T.; Ebesu, J.S.M. Puffer poisoning: Epidemiology and treatment. J. Toxicol. Toxin Rev. 2001, 20, 1–10. [Google Scholar] [CrossRef]
- Isbister, G.K.; Kiernan, M.C. Neurotoxic marine poisoning. Lancet Neurol. 2005, 4, 219–228. [Google Scholar] [CrossRef]
- Hwang, D.F.; Noguchi, T. Tetrodotoxin poisoning. Adv. Food Nutr. Res. 2007, 52, 141–236. [Google Scholar] [PubMed]
- Oda, K.; Araki, K.; Totoki, T.; Shibasaki, H. Nerve conduction study of human tetrodotoxication. Neurology 1989, 39, 743–745. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.A.; Schneider, J.J.; Isbister, G.K. Use of high performance liquid chromatography to measure tetrodotoxin in serum and urine of poisoned patients. Toxicon 2004, 44, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Tsai, Y.H.; Deng, J.F.; Cheng, C.A.; Ho, P.H.; Hwang, D.F. Identification of tetrodotoxin in a marine gastropod (Nassarius glans) responsible for human morbidity and mortality in Taiwan. J. Food Prot. 2005, 68, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Yu, C.F.; Tam, S.; Yu, P.H. Rapid screening of tetrodotoxin in urine and plasma of patients with puffer fish poisoning by HPLC with creatinine correction. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sasaya, M.; Oda, M.; Endo, T.; Saitoh, H.; Takada, M. The transport of ciprofloxacin in cultured kidney epithelial cells LLC-PK1. Biol. Pharm. Bull. 1997, 20, 887–891. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soares-Da-Silva, P.; Serrão, M.P. Molecular modulation of inward and outward apical transporters of l-dopa in LLC-PK1 cells. Am. J. Physiol. Ren. Physiol. 2000, 279, F736–F746. [Google Scholar]
- Fouda, A.K.; Fauth, C.; Roch-Ramel, F. Transport of organic cations by kidney epithelial cell line LLC-PK1. J. Pharmacol. Exp. Ther. 1990, 252, 286–292. [Google Scholar] [PubMed]
- Saito, H.; Yamamoto, M.; Inui, K.; Hori, R. Transcellular transport of organic cation across monolayers of kidney epithelial cell line LLC-PK1. Am. J. Physiol. 1992, 262, C59–C66. [Google Scholar] [PubMed]
- Saladik, D.T.; Soler, A.P.; Lewis, S.A.; Mullin, J.M. Cell division does not increase transepithelial permeability of LLC-PK1 cell sheets. Exp. Cell Res. 1995, 220, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A. Impact of transporter-mediated drug absorption, distribution, elimination and drug interactions in antimicrobial chemotherapy. J. Infect. Chemother. 2006, 12, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, G.; Wolff, N.A. Structure of renal organic anion and cation transporters. Am. J. Physiol. Ren. Physiol. 2000, 278, F853–F866. [Google Scholar]
- Motohashi, H.; Nakao, Y.; Masuda, S.; Katsura, T.; Kamba, T.; Ogawa, O.; Inui, K. Precise comparison of protein localization among OCT, OAT, and MATE in human kidney. J. Pharm. Sci. 2013, 102, 3302–3308. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, J. Renal drug transporters and their significance in drug-drug interactions. Acta Pharm. Sin. B 2016, 6, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Inui, K.; Masuda, S.; Saito, H. Cellular and molecular aspects of drug transport in the kidney. Kidney Int. 2000, 58, 944–958. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Otsuki, Y.; Hashimoto, Y.; Inui, K. Kinetic analysis of tetraethylammonium transport in the kidney epithelial cell line, LLC-PK1. Pharm. Res. 1997, 14, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Gründemann, D.; Babin-Ebell, J.; Martel, F.; Ording, N.; Schmidt, A.; Schömig, E. Primary structure and functional expression of the apical organic cation transporter from kidney epithelial LLC-PK1 cells. J. Biol. Chem. 1997, 272, 10408–10413. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kishi, Y.; Takahashi, S.; Hirata, Y. Tetrodotoxin. Tetrahedron 1965, 21, 2059–2088. [Google Scholar] [CrossRef]
- Kungsuwan, A.; Nagashima, Y.; Noguchi, T.; Shida, Y.; Suvapeepan, S.; Suwansakornkul, P.; Hashimoto, K. Tetrodotoxin in the horseshoe crab Carcinoscorpius rotundicauda inhabiting Thailand. Nippon Suisan Gakkaishi 1987, 53, 261–266. [Google Scholar] [CrossRef]
- Feller, N.; Broxterman, H.J.; Währer, D.C.; Pinedo, H.M. ATP-dependent efflux of calcein by the multidrug resistance protein (MRP): No inhibition by intracellular glutathione depletion. FEBS Lett. 1995, 368, 385–388. [Google Scholar] [CrossRef]
- Evers, R.; Zaman, G.J.; van Deemter, L.; Jansen, H.; Calafat, J.; Oomen, L.C.; Oude-Elferink, R.P.; Borst, P.; Schinkel, A.H. Basolateral localization and export activity of the human multidrug resistance-associated protein in polarized pig kidney cells. J. Clin. Investig. 1996, 97, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Goh, L.B.; Spears, K.J.; Yao, D.; Ayrton, A.; Morgan, P.; Roland, W.C.; Friedberg, T. Endogenous drug transporters in vitro and in vivo models for the prediction of drug disposition in man. Biochem. Pharmacol. 2002, 64, 1569–1578. [Google Scholar] [CrossRef]
- Tamai, I.; Ohashi, R.; Nezu, J.; Yabuuchi, H.; Oku, A.; Shimane, M.; Sai, Y.; Tsuji, A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J. Biol. Chem. 1998, 273, 20378–20382. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, H.; Tamai, I.; Nezu, J.; Sakamoto, K.; Oku, A.; Shimane, M.; Sai, Y.; Tsuji, A. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. J. Pharmacol. Exp. Ther. 1999, 289, 768–773. [Google Scholar] [PubMed]
- Tanihara, Y.; Masuda, S.; Sato, T.; Katsura, T.; Ogawa, O.; Inui, K. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H+-organic cation antiporters. Biochem. Pharmacol. 2007, 74, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Matsumoto, T.; Morimoto, R.; Arioka, S.; Omote, H.; Moriyama, Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl. Acad. Sci. USA 2005, 103, 17923–17928. [Google Scholar] [CrossRef] [PubMed]
- Tahara, H.; Kusuhara, H.; Endou, H.; Koepsell, H.; Imaoka, T.; Fuse, E.; Sugiyama, Y. A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J. Pharmacol. Exp. Ther. 2005, 315, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Tanuma, D.; Tsutsumi, K.; Jeon, J.K.; Ishizaki, S.; Nagashima, Y. Plasma protein binding of tetrodotoxin in the marine puffer fish Takifugu rubripes. Toxicon 2010, 55, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.Y.; Lai, S.L.; Chen, S.S.; Hwang, D.F. Tetrodotoxin intoxication in a uraemic patient. J. Neurol. Neurosurg. Psychiatry 1999, 67, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Sunwoo, M.K.; Sunwoo, I.N. Serial electrophysiological changes in uraemic patients with tetrodotoxin intoxication. Clin. Neurophysiol. 2011, 122, 2310–2311. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, R.; Nakata, Y.; Kameoka, M.; Hayashi, N.; Watanabe, K.; Yagi, K. A case of tetrodotoxin intoxication in a uremic patient. Chudoku Kenkyu 2007, 20, 141–145. (In Japanese) [Google Scholar] [PubMed]
- Fong, B.M.; Tam, S.; Tsui, S.H.; Leung, K.S. Development and validation of a high-throughput double solid phase extraction-liquid chromatography-tandem mass spectrometry method for the determination of tetrodotoxin in human urine and plasma. Talanta 2011, 83, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Inui, K.; Saito, H.; Hori, R. H+-gradient-dependent active transport of tetraethylammonium cation in apical-membrane vesicles isolated from kidney epithelial cell line LLC-PK1. Biochem. J. 1985, 227, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kimura, O.; Sasaya, M.; Takada, M.; Sakata, M. Na+- and energy-dependent transport of cadmium into LLC-PK1 cells. Biol. Pharm. Bull. 1995, 18, 1689–1693. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Masago, M.; Takaai, M.; Sakata, J.; Horie, A.; Ito, T.; Ishida, K.; Taguchi, M.; Hashimoto, Y. Membrane transport mechanisms of quinidine and procainamide in renal LLC-PK1 and intestinal LS180 cells. Biol. Pharm. Bull. 2010, 33, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Takaai, M.; Suzuki, H.; Ishida, K.; Tahara, K.; Hashimoto, Y. Pharmacokinetic analysis of transcellular transport of levofloxacin across LLC-PK1 and Caco-2 cell monolayers. Biol. Pharm. Bull. 2007, 30, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kiriake, A.; Ishizaki, S.; Watabe, S.; Nagashima, Y. Biliary excretion of tetrodotoxin in the cultured pufferfish Takifugu rubripes juvenile after intramuscular administration. Toxicon 2015, 93, 98–102. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, T.; Ishizaki, Y.; Mochizuki, K.; Aoyagi, M.; Mitoma, Y.; Ishizaki, S.; Nagashima, Y. Urinary Excretion of Tetrodotoxin Modeled in a Porcine Renal Proximal Tubule Epithelial Cell Line, LLC-PK1. Mar. Drugs 2017, 15, 225. https://doi.org/10.3390/md15070225
Matsumoto T, Ishizaki Y, Mochizuki K, Aoyagi M, Mitoma Y, Ishizaki S, Nagashima Y. Urinary Excretion of Tetrodotoxin Modeled in a Porcine Renal Proximal Tubule Epithelial Cell Line, LLC-PK1. Marine Drugs. 2017; 15(7):225. https://doi.org/10.3390/md15070225
Chicago/Turabian StyleMatsumoto, Takuya, Yui Ishizaki, Keika Mochizuki, Mitsuru Aoyagi, Yoshiharu Mitoma, Shoichiro Ishizaki, and Yuji Nagashima. 2017. "Urinary Excretion of Tetrodotoxin Modeled in a Porcine Renal Proximal Tubule Epithelial Cell Line, LLC-PK1" Marine Drugs 15, no. 7: 225. https://doi.org/10.3390/md15070225
APA StyleMatsumoto, T., Ishizaki, Y., Mochizuki, K., Aoyagi, M., Mitoma, Y., Ishizaki, S., & Nagashima, Y. (2017). Urinary Excretion of Tetrodotoxin Modeled in a Porcine Renal Proximal Tubule Epithelial Cell Line, LLC-PK1. Marine Drugs, 15(7), 225. https://doi.org/10.3390/md15070225




