Effects of Tetrodotoxin in Mouse Models of Visceral Pain
Abstract
:1. Introduction
2. Results
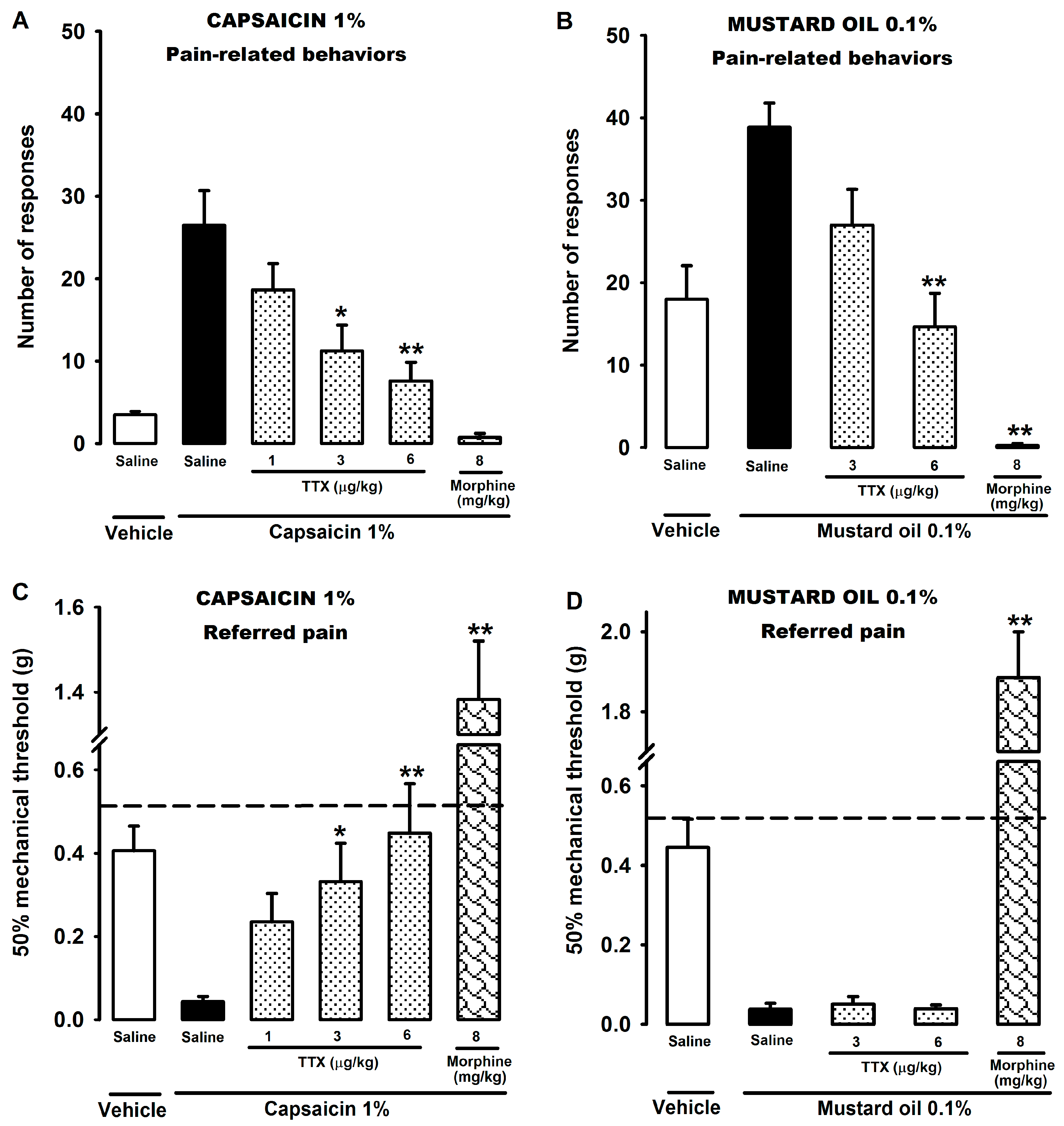
2.1. Effect of Tetrodotoxin on Visceral Pain Induced by Chemical Stimulation of the Colon
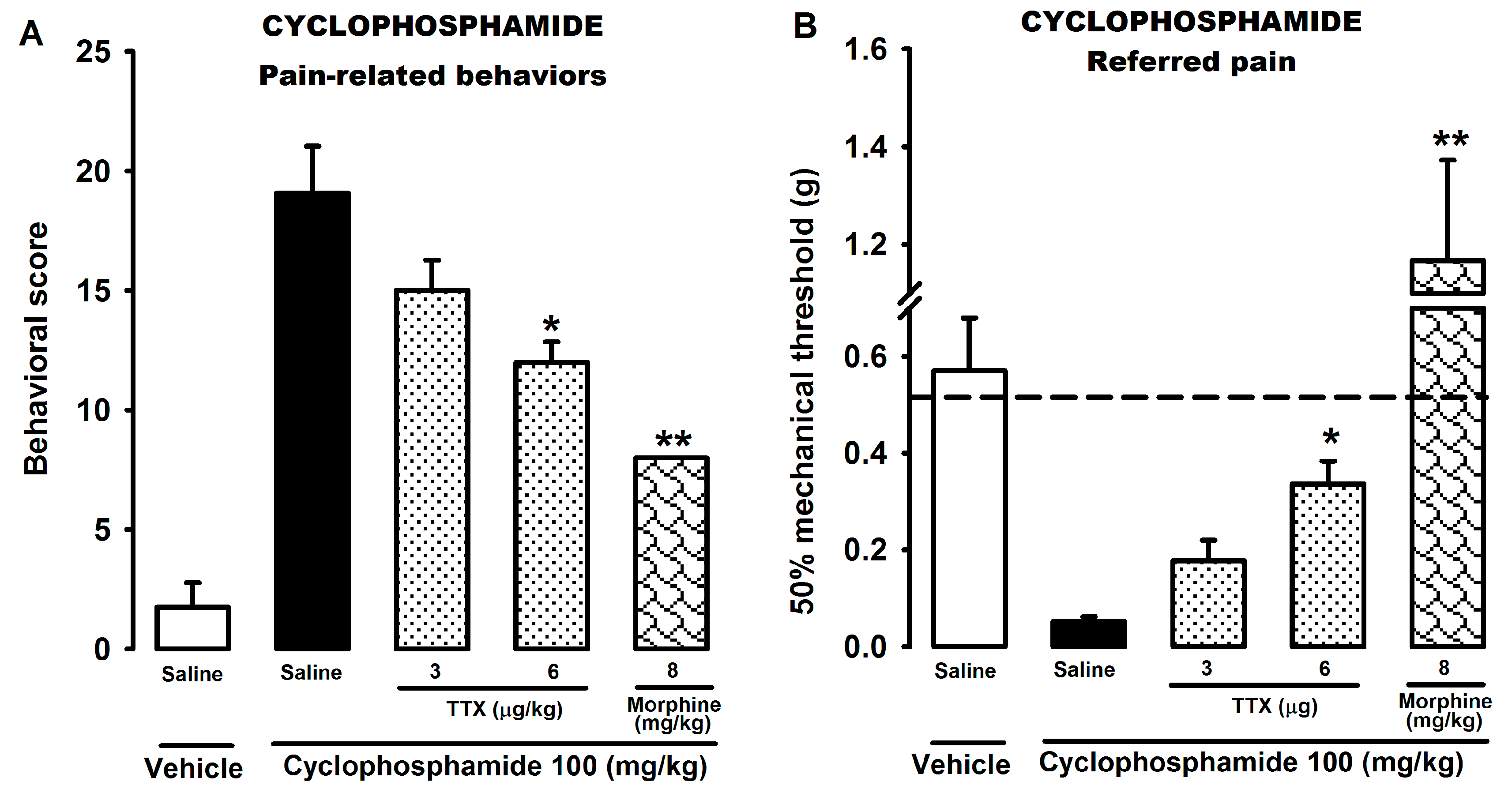
2.2. Effect of Tetrodotoxin on Cyclophosphamide-Induced Visceral Pain
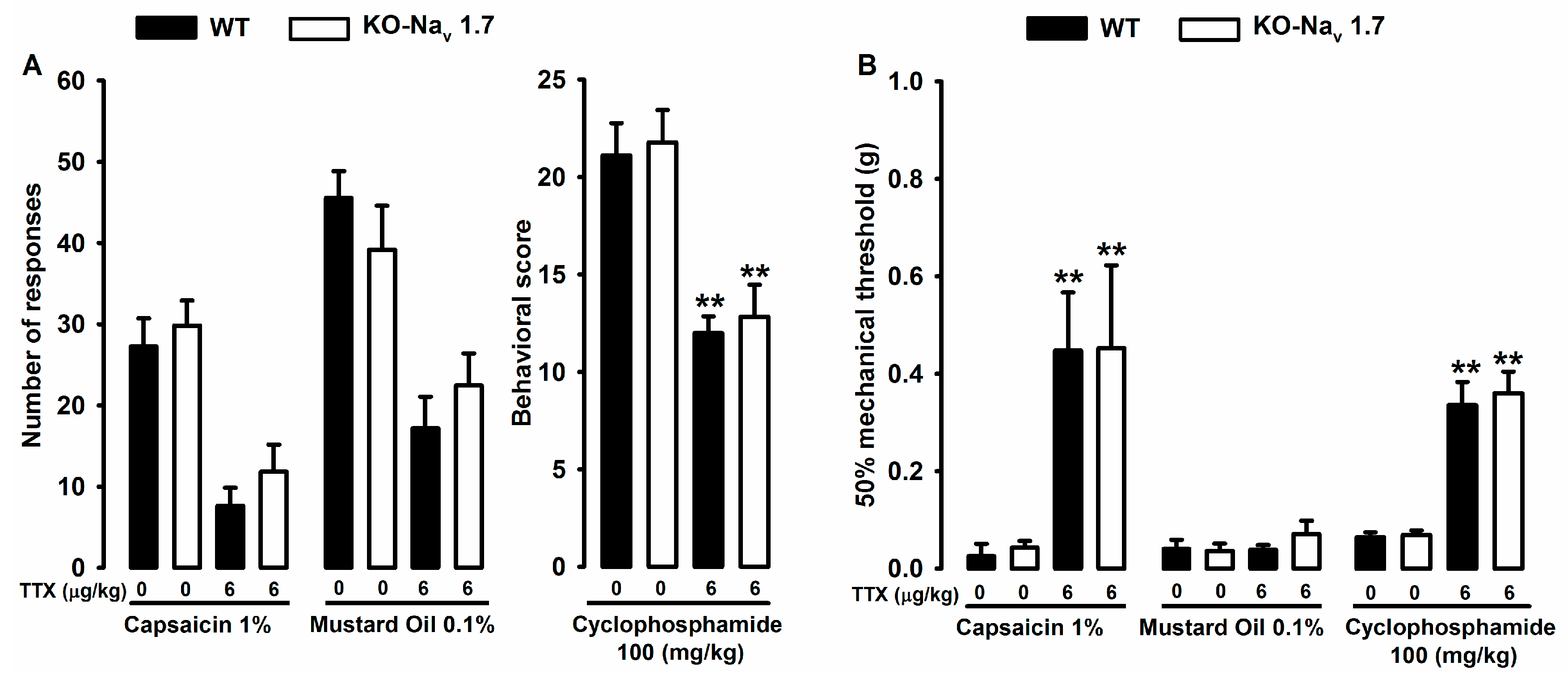
2.3. Effect of Tetrodotoxin in Nav1.7 Knockout Mice
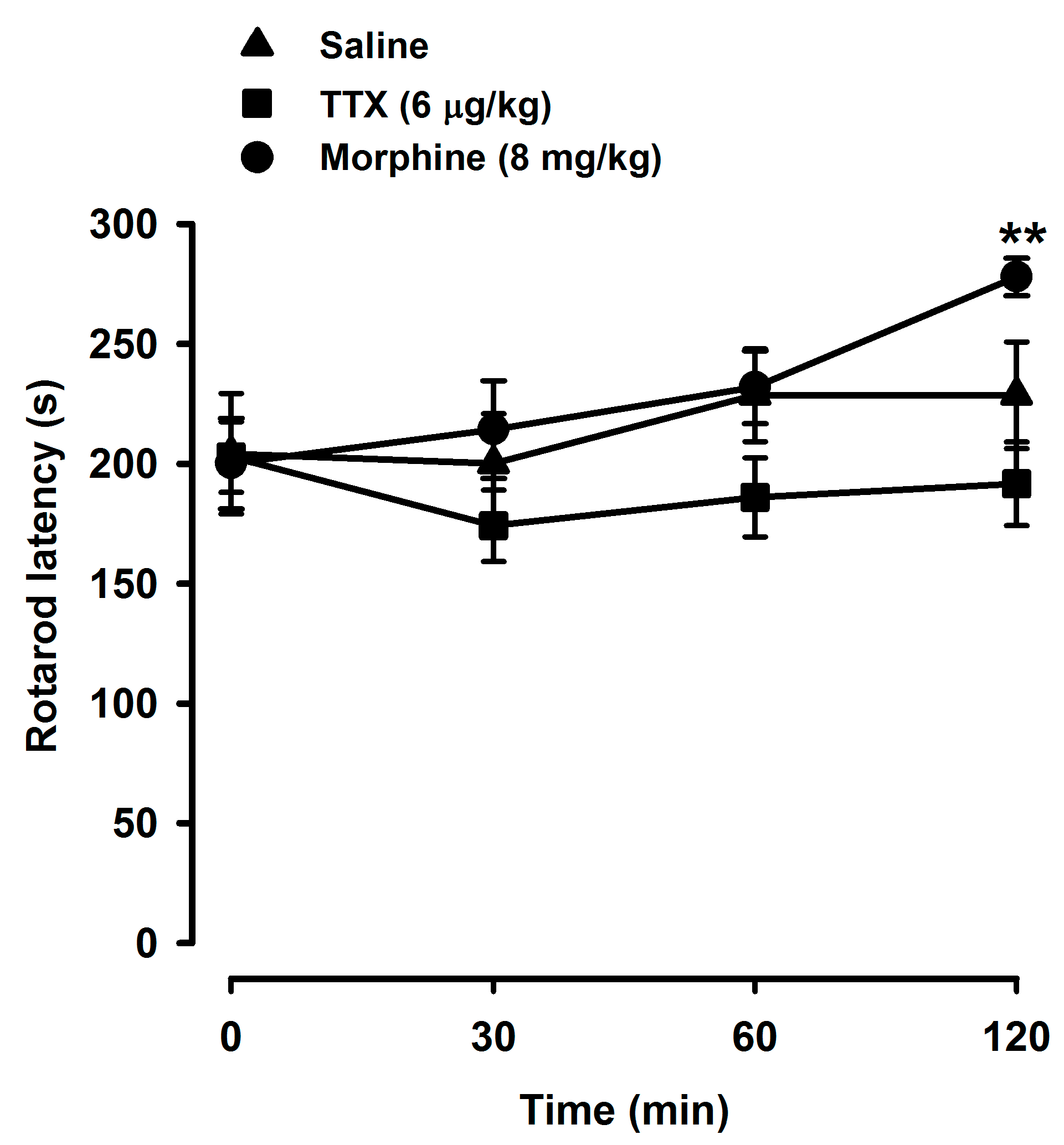
2.4. Tetrodotoxin Does Not Alter Locomotor Coordination
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs and Drug Administration
4.3. General Procedures for Evaluating Intracolonic Visceral Pain
4.4. Procedure for Evaluating Cyclophosphamide-Induced Cystitis
4.5. Rotarod Test
4.6. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cervero, F.; Laird, J.M. Visceral pain. Lancet 1999, 353, 2145–2148. [Google Scholar] [CrossRef]
- Wesselmann, U.; Baranowski, A.P.; Börjesson, M.; Curran, N.C.; Czakanski, P.P.; Giamberardino, M.A.; Ness, T.J.; Robbins, M.T.; Traub, R.J. Emerging therapies and novel approaches to visceral pain. Drug Discov. Today 2009, 6, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.S.; Gebhart, G.F. Visceral Pain. In Behavioral Neurobiology of Chronic Pain; Taylor, B.K., Finn, D.P., Eds.; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2014; pp. 171–197. [Google Scholar]
- Davis, M.P. Drug Management of Visceral Pain: Concepts from Basic Research. Pain Res. Treat. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Cervero, F. Visceral versus Somatic Pain: Similarities and Differences. Dig. Dis. 2010, 27 (Suppl. 1), 3–10. [Google Scholar] [CrossRef] [PubMed]
- Strigo, I.A.; Bushnell, M.C.; Boivin, M.; Duncan, G.H. Psychophysical analysis of visceral and cutaneous pain in human subjects. Pain 2002, 97, 235–246. [Google Scholar] [CrossRef]
- Robinson, D.R.; Gebhart, G.F. Inside information—The unique features of visceral sensation. Mol. Interv. 2008, 8, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Westlund, K. Animal Models of Visceral Pain. In Animal Models of Pain; Ma, C., Zhang, J.-M., Eds.; Neuromethods; Humana Press: New York, NY, USA, 2011; pp. 41–68. [Google Scholar]
- Lago, J.; Rodríguez, L.P.; Blanco, L.; Vieites, J.M.; Cabado, A.G. Tetrodotoxin, an Extremely Potent Marine Neurotoxin: Distribution, Toxicity, Origin and Therapeutical Uses. Mar. Drugs 2015, 13, 6384–6406. [Google Scholar] [CrossRef] [PubMed]
- Moczydlowski, E.G. The molecular mystique of tetrodotoxin. Toxicon 2013, 63, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Li, X.; Jin, L.; Zhang, F.; Inoue, M.; Yu, B.; Cao, Z. Development of a Rapid Throughput Assay for Identification of hNav1.7 Antagonist Using Unique Efficacious Sodium Channel Agonist, Antillatoxin. Mar. Drugs 2016, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N.; Ohishi, Y. Molecular Diversity of Structure and Function of the Voltage-Gated Na+ Channels. Jpn. J. Pharmacol. 2002, 88, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Marcil, J.; Walczak, J.-S.; Guindon, J.; Ngoc, A.H.; Lu, S.; Beaulieu, P. Antinociceptive effects of tetrodotoxin (TTX) in rodents. Br. J. Anaesth. 2006, 96, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Beloeil, H.; Ababneh, Z.; Chung, R.; Zurakowski, D.; Mulkern, R.V.; Berde, C.B. Effects of Bupivacaine and Tetrodotoxin on Carrageenan-induced Hind Paw Inflammation in Rats (Part 1)Hyperalgesia, Edema, and Systemic Cytokines. J. Am. Soc. Anesthesiol. 2006, 105, 128–138. [Google Scholar] [CrossRef]
- Alguacil, L.F.; Pérez-García, C.; Salas, E.; González-Martín, C.; Castillo, C.; Polanco, M.J.; Herradón, G.; Morales, L. Subcutaneous tetrodotoxin and inflammatory pain. Br. J. Anaesth. 2008, 100, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, P.; Levine, J.D. Antihyperalgesic Effect of Tetrodotoxin in Rat Models of Persistent Muscle Pain. Neuroscience 2015, 311, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.R.; Entrena, J.M.; Cendán, C.M.; Pozo, E.D.; Vela, J.M.; Baeyens, J.M. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain 2008, 137, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Kayser, V.; Viguier, F.; Ioannidi, M.; Bernard, J.-F.; Latrémolière, A.; Michot, B.; Vela, J.-M.; Buschmann, H.; Hamon, M.; Bourgoin, S. Differential anti-neuropathic pain effects of tetrodotoxin in sciatic nerve-versus infraorbital nerve-ligated rats—Behavioral, pharmacological and immunohistochemical investigations. Neuropharmacology 2010, 58, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.S.; Park, S.K.; Chung, K.; Chung, J.M. Low dose of tetrodotoxin reduces neuropathic pain behaviors in an animal model. Brain Res. 2000, 871, 98–103. [Google Scholar] [CrossRef]
- Hagen, N.A.; du Souich, P.; Lapointe, B.; Ong-Lam, M.; Dubuc, B.; Walde, D.; Love, R.; Ngoc, A.H. Tetrodotoxin for Moderate to Severe Cancer Pain: A Randomized, Double Blind, Parallel Design Multicenter Study. J. Pain Symptom Manag. 2008, 35, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.A.; Lapointe, B.; Ong–Lam, M.; Dubuc, B.; Walde, D.; Gagnon, B.; Love, R.; Goel, R.; Hawley, P.; Ngoc, A.H.; et al. A multicentre open-label safety and efficacy study of tetrodotoxin for cancer pain. Curr. Oncol. 2011, 18, e109–e116. [Google Scholar] [CrossRef] [PubMed]
- Wex Pharmaceuticals Inc. The Purpose of This Study Is to Determine If Tetrodotoxin (TTX) Is Effective in the Treatment of Pain Resulting From Chemotherapy Treatment (TTX-CINP-201). Available online: https://clinicaltrials.gov/ct2/show/NCT01655823NMLIdentifier:NCT01655823 (accessed on 4 April 2017).
- Nieto, F.R.; Cobos, E.J.; Tejada, M.Á.; Sánchez-Fernández, C.; González-Cano, R.; Cendán, C.M. Tetrodotoxin (TTX) as a Therapeutic Agent for Pain. Mar. Drugs 2012, 10, 281–305. [Google Scholar] [CrossRef] [PubMed]
- González-Cano, R.; Merlos, M.; Baeyens, J.M.; Cendán, C.M. σ1Receptors Are Involved in the Visceral Pain Induced by Intracolonic Administration of Capsaicin in Mice. J. Am. Soc. Anesthesiol. 2013, 118, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, M.H.; Nieto, F.R.; Cervero, F. Stimulation of Cutaneous Low Threshold Mechanoreceptors in Mice After Intracolonic Capsaicin Increases Spinal c-Fos Labeling in an NKCC1-Dependent Fashion. J. Pain 2013, 14, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.M.; Martinez-Caro, L.; Garcia-Nicas, E.; Cervero, F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain 2001, 92, 335–342. [Google Scholar] [CrossRef]
- Hockley, J.R.F.; González-Cano, R.; McMurray, S.; Tejada-Giraldez, M.A.; McGuire, C.; Torres, A.; Wilbrey, A.L.; Cibert-Goton, V.; Nieto, F.R.; Pitcher, T.; et al. Visceral and somatic pain modalities reveal NaV1.7-independent visceral nociceptive pathways. J. Physiol. 2017, 595, 2661–2679. [Google Scholar] [CrossRef] [PubMed]
- Wantuch, C.; Piesla, M.; Leventhal, L. Pharmacological validation of a model of cystitis pain in the mouse. Neurosci. Lett. 2007, 421, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.H. Tetrodotoxin, saxitoxin, and related substances: Their applications in neurobiology. Int. Rev. Neurobiol. 1972, 15, 83–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Huang, K.; Gao, L.; Zhang, H.; Rong, K. Toxicity of tetrodotoxin towards mice and rabbits. Wei Sheng Yan Jiu 2003, 32, 371–374. [Google Scholar] [PubMed]
- LeBars, D.; Gozariu, M.; Cadden, S.W. Animal Models of Nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar]
- Arendt-Nielsen, L.; Schipper, K.-P.; Dimcevski, G.; Sumikura, H.; Krarup, A.L.; Giamberardino, M.A.; Drewes, A.M. Viscero-somatic reflexes in referred pain areas evoked by capsaicin stimulation of the human gut. Eur. J. Pain 2008, 12, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Drewes, A.M.; Schipper, K.P.; Dimcevski, G.; Petersen, P.; Gregersen, H.; Funch-Jensen, P.; Arendt-Nielsen, L. Gut pain and hyperalgesia induced by capsaicin: A human experimental model. Pain 2003, 104, 333–341. [Google Scholar] [CrossRef]
- Schmidt, B.; Hammer, J.; Holzer, P.; Hammer, H.F. Chemical nociception in the jejunum induced by capsaicin. Gut 2004, 53, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Olivar, T.; Laird, J.M.A. Cyclophosphamide cystitis in mice: Behavioural characterisation and correlation with bladder inflammation. Eur. J. Pain 1999, 3, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Cervero, F.; Laird, J.M.A. Spinal Mechanisms of Visceral Pain and Hyperalgesia. In Synaptic Plasticity in Pain; Malcangio, M., Ed.; Springer: New York, NY, USA, 2009; pp. 289–306. [Google Scholar]
- Cendán, C.M.; Pujalte, J.M.; Portillo-Salido, E.; Baeyens, J.M. Antinociceptive effects of haloperidol and its metabolites in the formalin test in mice. Psychopharmacology 2005, 182, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Entrena, J.M.; Cobos, E.J.; Nieto, F.R.; Cendán, C.M.; Gris, G.; Del Pozo, E.; Zamanillo, D.; Baeyens, J.M. Sigma-1 receptors are essential for capsaicin-induced mechanical hypersensitivity: Studies with selective sigma-1 ligands and sigma-1 knockout mice. Pain 2009, 143, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Julius, D. TRP Channels and Pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef] [PubMed]
- Everaerts, W.; Gees, M.; Alpizar, Y.A.; Farre, R.; Leten, C.; Apetrei, A.; Dewachter, I.; van Leuven, F.; Vennekens, R.; Ridder, D.D.; et al. The Capsaicin Receptor TRPV1 Is a Crucial Mediator of the Noxious Effects of Mustard Oil. Curr. Biol. 2011, 21, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.M.A.; Olivar, T.; Roza, C.; De Felipe, C.; Hunt, S.P.; Cervero, F. Deficits in visceral pain and hyperalgesia of mice with a disruption of the tachykinin NK1 receptor gene. Neuroscience 2000, 98, 345–352. [Google Scholar] [CrossRef]
- Shin, J.-W.; Hwang, K.-S.; Kim, Y.-K.; Leem, J.-G.; Lee, C. Nonsteroidal antiinflammatory drugs suppress pain-related behaviors, but not referred hyperalgesia of visceral pain in mice. Anesth. Analg. 2006, 102, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.M.; McIntyre, M.K.; Petz, L.N.; Korz, W.; Wong, D.; Clifford, J.L. Tetrodotoxin suppresses thermal hyperalgesia and mechanical allodynia in a rat full thickness thermal injury pain model. Neurosci. Lett. 2015, 607, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.M.; Wood, J.N.; Cox, J.J. Sodium Channels and Pain. In Pain Control; Schaible, H.-G., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 39–56. [Google Scholar]
- Eijkelkamp, N.; Linley, J.E.; Baker, M.D.; Minett, M.S.; Cregg, R.; Werdehausen, R.; Rugiero, F.; Wood, J.N. Neurological perspectives on voltage-gated sodium channels. Brain 2012, 135, 2585–2612. [Google Scholar] [CrossRef] [PubMed]
- Deuis, J.R.; Zimmermann, K.; Romanovsky, A.A.; Possani, L.D.; Cabot, P.J.; Lewis, R.J.; Vetter, I. An animal model of oxaliplatin-induced cold allodynia reveals a crucial role for Nav1.6 in peripheral pain pathways. Pain 2013, 154, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Strong, J.A.; Zhang, J.-M. Local knockdown of the NaV1.6 sodium channel reduces pain behaviors, sensory neuron excitability, and sympathetic sprouting in rat models of neuropathic pain. Neuroscience 2015, 291, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Zhu, Y.; La, J.-H.; Wills, Z.P.; Gebhart, G.F. Experimental and computational evidence for an essential role of NaV1.6 in spike initiation at stretch-sensitive colorectal afferent endings. J. Neurophysiol. 2015, 113, 2618–2634. [Google Scholar] [CrossRef] [PubMed]
- Osteen, J.D.; Herzig, V.; Gilchrist, J.; Emrick, J.J.; Zhang, C.; Wang, X.; Castro, J.; Garcia-Caraballo, S.; Grundy, L.; Rychkov, G.Y.; et al. Selective spider toxins reveal a role for Nav1.1 channel in mechanical pain. Nature 2016, 534, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.A.; Stirling, L.C.; Forlani, G.; Baker, M.D.; Matthews, E.A.; Dickenson, A.H.; Wood, J.N. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc. Natl. Acad. Sci. USA 2004, 101, 12706–12711. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Nieto, F.R.; Cobos, E.J.; Entrena, J.M.; Parra, A.; García-Granados, A.; Baeyens, J.M. Antiallodynic and Analgesic Effects of Maslinic Acid, a Pentacyclic Triterpenoid from Olea europaea. J. Nat. Prod. 2013, 76, 737–740. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cano, R.; Tejada, M.Á.; Artacho-Cordón, A.; Nieto, F.R.; Entrena, J.M.; Wood, J.N.; Cendán, C.M. Effects of Tetrodotoxin in Mouse Models of Visceral Pain. Mar. Drugs 2017, 15, 188. https://doi.org/10.3390/md15060188
González-Cano R, Tejada MÁ, Artacho-Cordón A, Nieto FR, Entrena JM, Wood JN, Cendán CM. Effects of Tetrodotoxin in Mouse Models of Visceral Pain. Marine Drugs. 2017; 15(6):188. https://doi.org/10.3390/md15060188
Chicago/Turabian StyleGonzález-Cano, Rafael, Miguel Ángel Tejada, Antonia Artacho-Cordón, Francisco Rafael Nieto, José Manuel Entrena, John N. Wood, and Cruz Miguel Cendán. 2017. "Effects of Tetrodotoxin in Mouse Models of Visceral Pain" Marine Drugs 15, no. 6: 188. https://doi.org/10.3390/md15060188
APA StyleGonzález-Cano, R., Tejada, M. Á., Artacho-Cordón, A., Nieto, F. R., Entrena, J. M., Wood, J. N., & Cendán, C. M. (2017). Effects of Tetrodotoxin in Mouse Models of Visceral Pain. Marine Drugs, 15(6), 188. https://doi.org/10.3390/md15060188




