Characterization of Acetyl-CoA Carboxylases in the Basal Dinoflagellate Amphidinium carterae
Abstract
:1. Introduction
2. Results
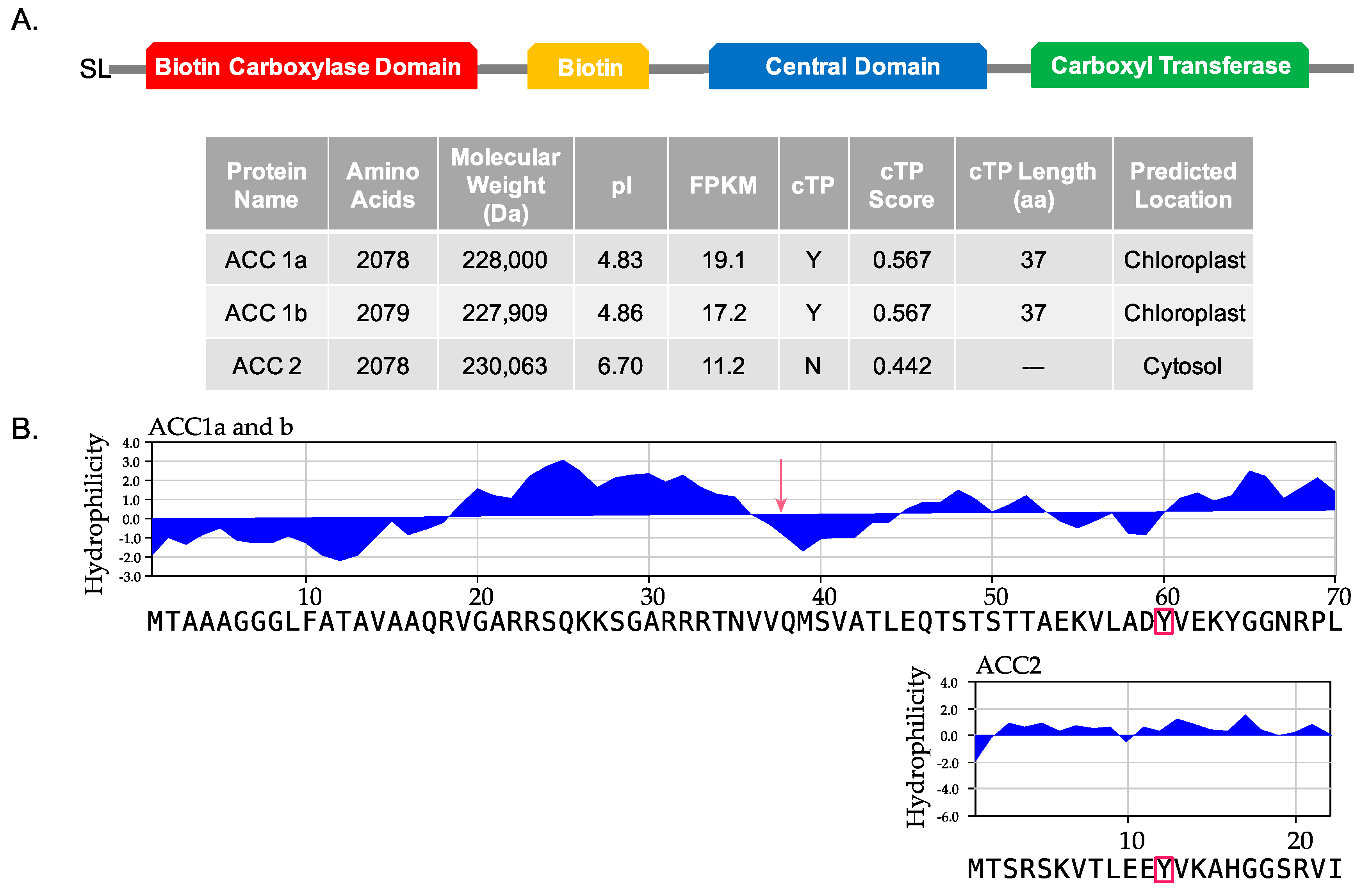
2.1. Transcriptome Analysis of Amphidinium carterae (CCMP 1314) ACCs
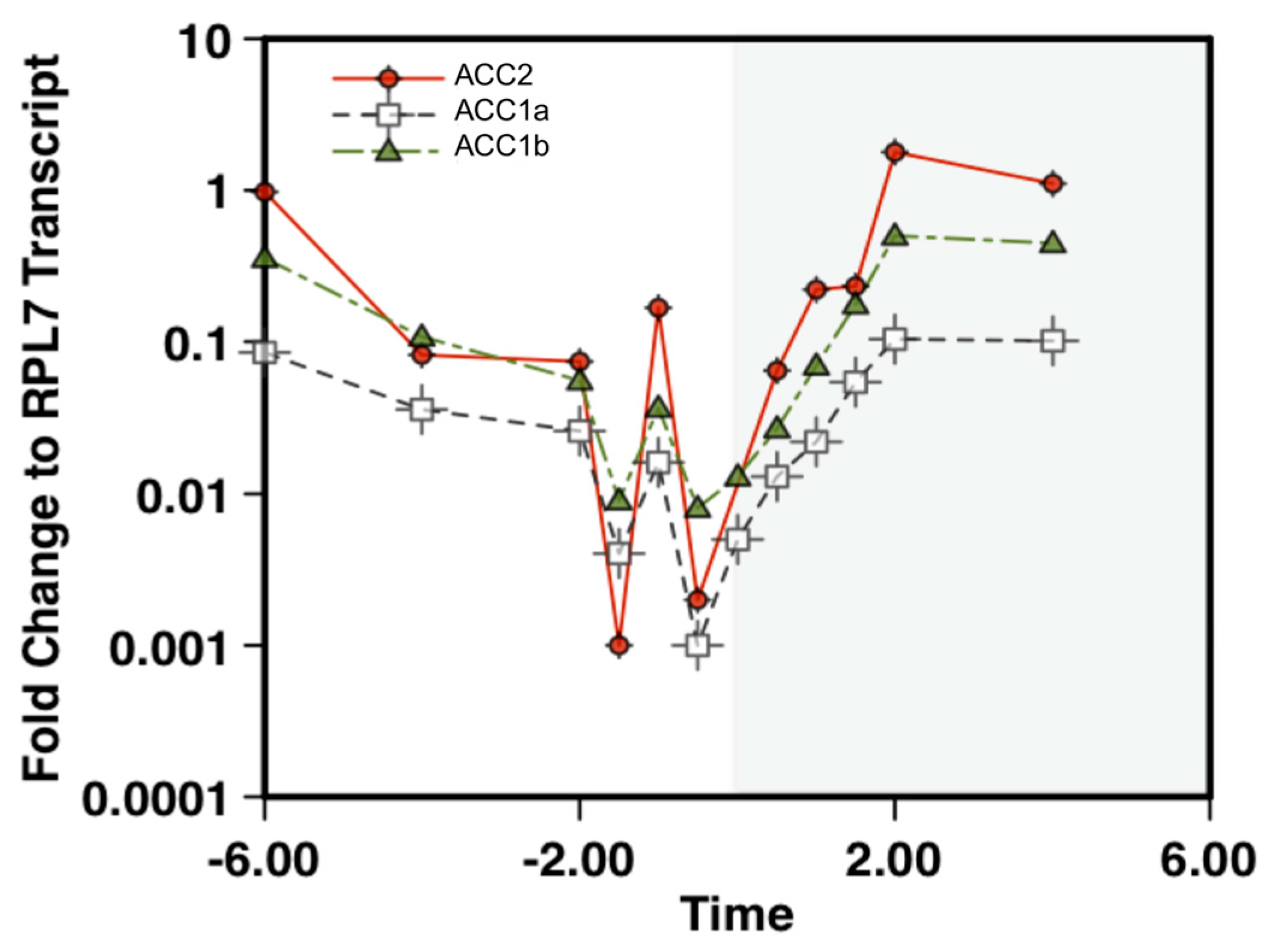
2.2. Quantification of ACC Transcript Levels in Amphidinium carterae
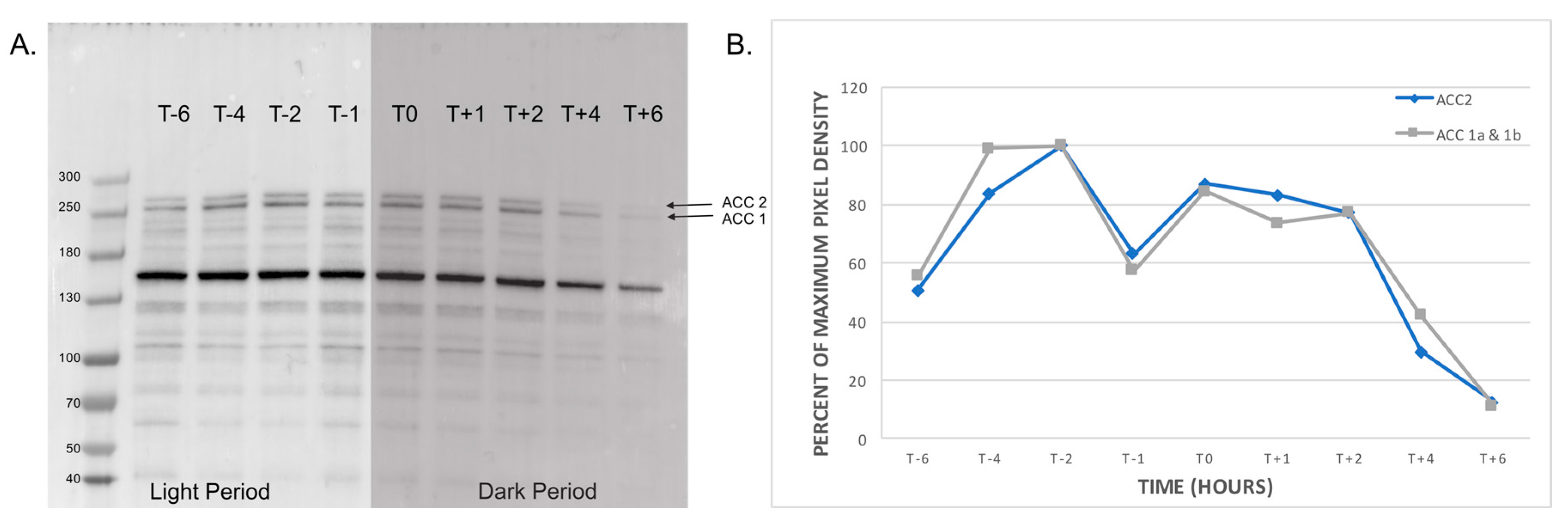
2.3. Profile of Natively Biotinylated Proteins in Amphidinium carterae
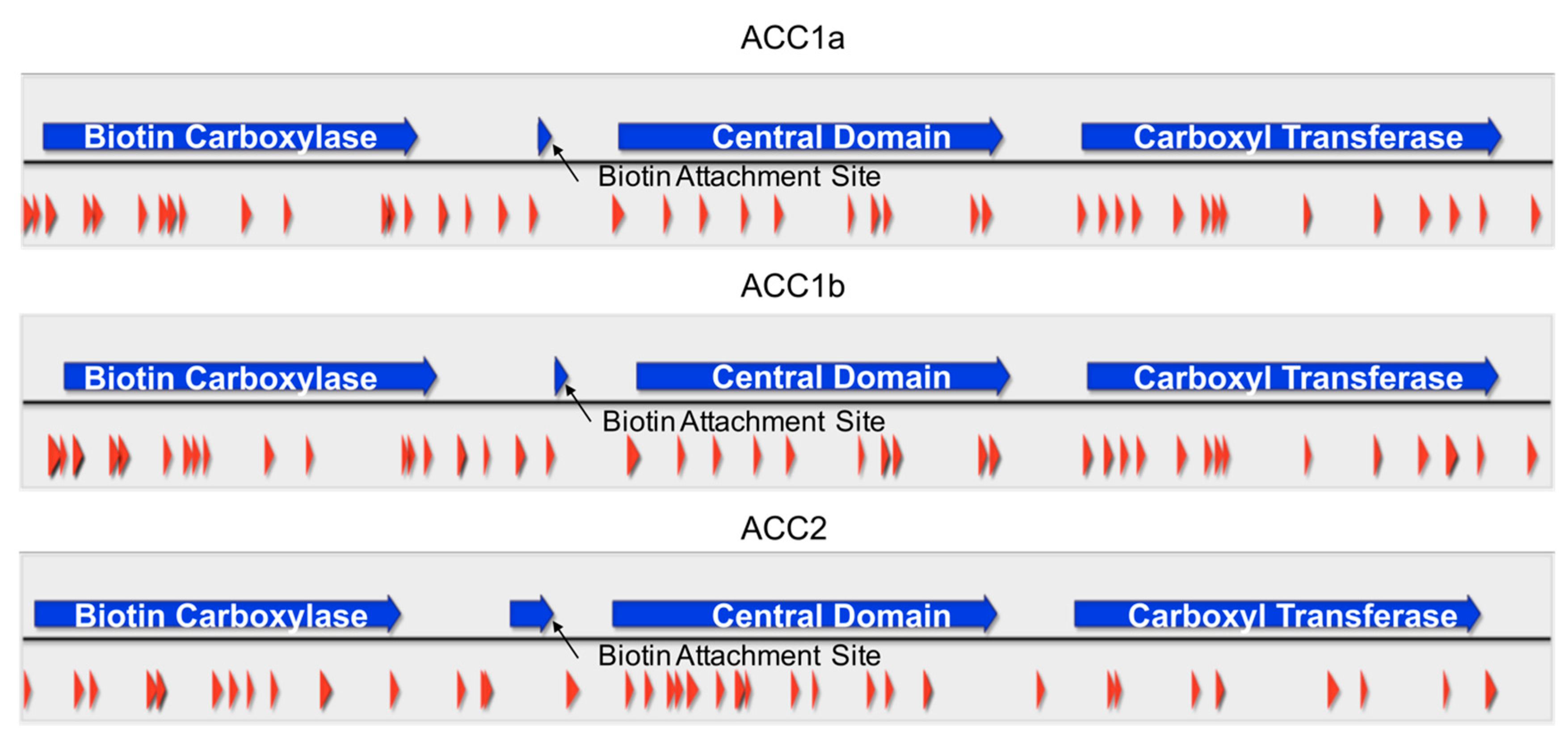
2.4. Peptide Coverage of ACCs in Amphidinium carterae
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sequence Acquisition and Searching
5.2. ChloroP Analysis of Sequences
5.3. Kyte-Doolittle Hydrophilicity Analysis
5.4. Cell Culture and Harvest for Diel Quantification
5.5. RNA Extraction and qPCR Analysis
5.6. Cell Culture and Harvest for SDS-PAGE and Mass Spectrometry
5.7. Western Blotting
5.8. Preparation of Proteins for Mass Spectrometry
5.9. Mass Spectrometry Analysis and Data Processing
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Place, A.R.; Bowers, H.A.; Bachvaroff, T.R.; Adolf, J.E.; Deeds, J.R.; Sheng, J. Karlodinium veneficum—The little dinoflagellate with a big bite. Harmful Algae 2012, 14, 179–195. [Google Scholar] [CrossRef]
- Cembella, A.D. Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia 2003, 42, 420–447. [Google Scholar] [CrossRef]
- Smayda, T.J. Bloom dynamics: Physiology, behavior, trophic effects. Limonol. Oceanogr. 1997, 42, 1132–1136. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second Generation Biofuels: High-Efficiency Microalgae for Biodiesel Production. BioEnergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Tong, L. Structure and function of biotin-dependent carboxylases. Cell. Mol. Life Sci. 2013, 70, 863–891. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, D.A.; Sherman, D.H. Molecular Genetics of Polyketides and its Comparision to Fatty Acid Biosynthesis. Annu. Rev. Genet. 1990, 24, 37–66. [Google Scholar] [CrossRef] [PubMed]
- Huerlimann, R.; Heimann, K. Comprehensive guide to acetyl-carboxylases in algae. Crit. Rev. Biotechnol. 2013, 33, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Hunkeler, M.; Stuttfeld, E.; Hagmann, A.; Imseng, S.; Maier, T. The dynamic organization of fungal acetyl-CoA carboxylase. Nat. Commun. 2016, 7, 11196. [Google Scholar] [CrossRef] [PubMed]
- Brownsey, R.W.; Zhande, R.; Boone, A.N. Isoforms of acetyl-CoA carboxylase: Structures, regulatory properties and metabolic functions. Biochem. Soc. Trans. 1997, 25, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Tong, L. Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cell. Mol. Life Sci. 2005, 62, 1784–1803. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elheiga, L.; Brinkley, W.R.; Zhong, L.; Chirala, S.S.; Woldegiorgis, G.; Wakil, S.J. The subcellular localization of acetyl-CoA carboxylase 2. Proc. Natl. Acad. Sci. USA 2000, 97, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Nagano, Y. Plant Acetyl-CoA Carboxylase: Structure, biosynthesis, regulation, and gene manipulation for plant breeding. Biosci. Biotechnol. Biochem. 2004, 68, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Houdai, T.; Matsuoka, S.; Matsumori, N.; Murata, M. Membrane-permeabilizing activities of amphidinol 3, polyene-polyhydroxy antifungal from a marine dinoflagellate. Biochim. Biophys. Acta-Biomembr. 2004, 1667, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Morsy, N.; Houdai, T.; Konoki, K.; Matsumori, N.; Oishi, T.; Murata, M. Effects of lipid constituents on membrane-permeabilizing activity of amphidinols. Bioorg. Med. Chem. 2008, 16, 3084–3090. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.; Jørgensen, M.F.; Daugbjerg, N.; Rhodes, L. Amphidinium revisited. II. Resolving species boundaries in the Amphidinium operculatum species complex (Dinophyceae), including the descriptions of Amphidinium trulla sp nov. and Amphidinium gibbosum. comb. nov. J. Phycol. 2004, 40, 366–382. [Google Scholar] [CrossRef]
- Murray, S.A.; Garby, T.; Hoppenrath, M.; Neilan, B.A. Genetic diversity, morphological uniformity and polyketide production in dinoflagellates (Amphidinium, Dinoflagellata). PLoS ONE 2012, 7, e38253. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, O.; Brunak, S.; von Heijne, G.; Nielsen, H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2007, 2, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, O.; Nielsen, H.; von Heijne, G. ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci. 1999, 8, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, O.; Nielsen, H.; Brunak, S.; von Heijne, G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 2000, 300, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Bachvaroff, T.R.; Place, A.R. Phylogenetic Analysis of Acetyl CoA Carboxylases in Dinoflagellates. In Proceedings of the 17th International Conference on Harmful Algae, Florianopolois, Brazil, 9–14 October 2016; ISSHA: Seattle, WA, USA in press. [Google Scholar]
- Williams, E.P.; Jagus, R.; Place, A.R. Discovery of non-coding RNAs in Amphidinium carterae differentially expressed over a diel cycle. In Proceedings of the 16 International Conference on Harmful Algae, Wellington, New Zealand, 27–31 October 2014; ISSHA: Seattle, WA, USA, 2015; pp. 233–237. [Google Scholar]
- Jones, G.D.; Williams, E.P.; Place, A.R.; Jagus, R.; Bachvaroff, T.R. The alveolate translation initiation factor 4E family reveals a custom toolkit for translational control in core dinoflagellates. BMC Evol. Biol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Wakil, S.J.; Stoops, J.K.; Joshi, V.C. Fatty acid synthesis and its regulation. Annu. Rev. Biochem. 1983, 52, 537–579. [Google Scholar] [CrossRef] [PubMed]
- Bachvaroff, T.R.; Handy, S.M.; Place, A.R.; Delwiche, C.F. Alveolate Phylogeny Inferred using Concatenated Ribosomal Proteins. J. Eukaryot. Microbiol. 2011, 58, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Patron, N.J.; Waller, R.F.; Archibald, J.M.; Keeling, P.J. Complex protein targeting to dinoflagellate plastids. J. Mol. Biol. 2005, 348, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Zuther, E.; Johnson, J.J.; Haselkorn, R.; McLeod, R.; Gornicki, P. Growth of Toxoplasma gondii is inhibited by aryloxyphenoxypropionate herbicides targeting acetyl-CoA carboxylase. Proc. Natl. Acad. Sci. USA 1999, 96, 13387–13392. [Google Scholar] [CrossRef] [PubMed]
- Yanai, Y.; Kawasaki, T.; Shimada, H.; Wurtele, E.S.; Nikolau, B.J.; Ichikawa, N. Genomic organization of 251 kDa acetyl-CoA carboxylase genes in Arabidopsis: Tandem gene duplication has made two differentially expressed isozymes. Plant Cell Physiol. 1995, 36, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, R.M.; Satake, M.; Wright, J.L.C. Polyketide biosynthesis in dinoflagellates: What makes it different? Nat. Prod. Rep. 2014, 31, 1101–1137. [Google Scholar] [CrossRef] [PubMed]
- Huerlimann, R.; Zenger, K.R.; Jerry, D.R.; Heimann, K. Phylogenetic analysis of nucleus-encoded acetyl-CoA carboxylases targeted at the cytosol and plastid of algae. PLoS ONE 2015, 10, e0131099. [Google Scholar] [CrossRef] [PubMed]
- Bachvaroff, T.R.; Gornik, S.G.; Concepcion, G.T.; Waller, R.F.; Mendez, G.S.; Lippmeier, J.C.; Delwiche, C.F. Dinoflagellate phylogeny revisited: Using ribosomal proteins to resolve deep branching dinoflagellate clades. Mol. Phylogenet. Evol. 2014, 70, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Berges, J.A.; Franklin, D.J.; Harrison, P.J. Evolution of an artificial seawater medium: Improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 2001, 37, 1138–1145. [Google Scholar] [CrossRef]
| Primer | Sequence | Product Size (bp) | Tm (°C) |
|---|---|---|---|
| ACC1a_F | CTTGGCGTTCTTGATGGAGT | 701 | 55.2 |
| ACC1a_R | TCATGATCTTTGCGAACTGG | 701 | 53.0 |
| ACC1b_F | TCCAAATAGGATGCCACCTC | 680 | 54.4 |
| ACC1b_R | TTGCCTGTCATGATCTTTGC | 680 | 53.5 |
| ACC2_F | ACAAACTTGTGGGGCAGTTC | 701 | 56.0 |
| ACC2_R | TCCCTGCAATACTTCCCATC | 701 | 54.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, S.; Bachvaroff, T.R.; Place, A.R. Characterization of Acetyl-CoA Carboxylases in the Basal Dinoflagellate Amphidinium carterae. Mar. Drugs 2017, 15, 149. https://doi.org/10.3390/md15060149
Haq S, Bachvaroff TR, Place AR. Characterization of Acetyl-CoA Carboxylases in the Basal Dinoflagellate Amphidinium carterae. Marine Drugs. 2017; 15(6):149. https://doi.org/10.3390/md15060149
Chicago/Turabian StyleHaq, Saddef, Tsvetan R. Bachvaroff, and Allen R. Place. 2017. "Characterization of Acetyl-CoA Carboxylases in the Basal Dinoflagellate Amphidinium carterae" Marine Drugs 15, no. 6: 149. https://doi.org/10.3390/md15060149
APA StyleHaq, S., Bachvaroff, T. R., & Place, A. R. (2017). Characterization of Acetyl-CoA Carboxylases in the Basal Dinoflagellate Amphidinium carterae. Marine Drugs, 15(6), 149. https://doi.org/10.3390/md15060149






