Marine Peptides as Potential Agents for the Management of Type 2 Diabetes Mellitus—A Prospect
Abstract
:1. Introduction
2. Regulation of Bioactive Peptides on the Insulin-Regulated Glucose Metabolism
2.1. Protecting Pancreatic β-Cells of Bioactive Peptides
2.2. Enhancement of Glucose-Stimulated Insulin Secretion
2.3. Regulation of Glucose Uptake and Lipid Accumulation
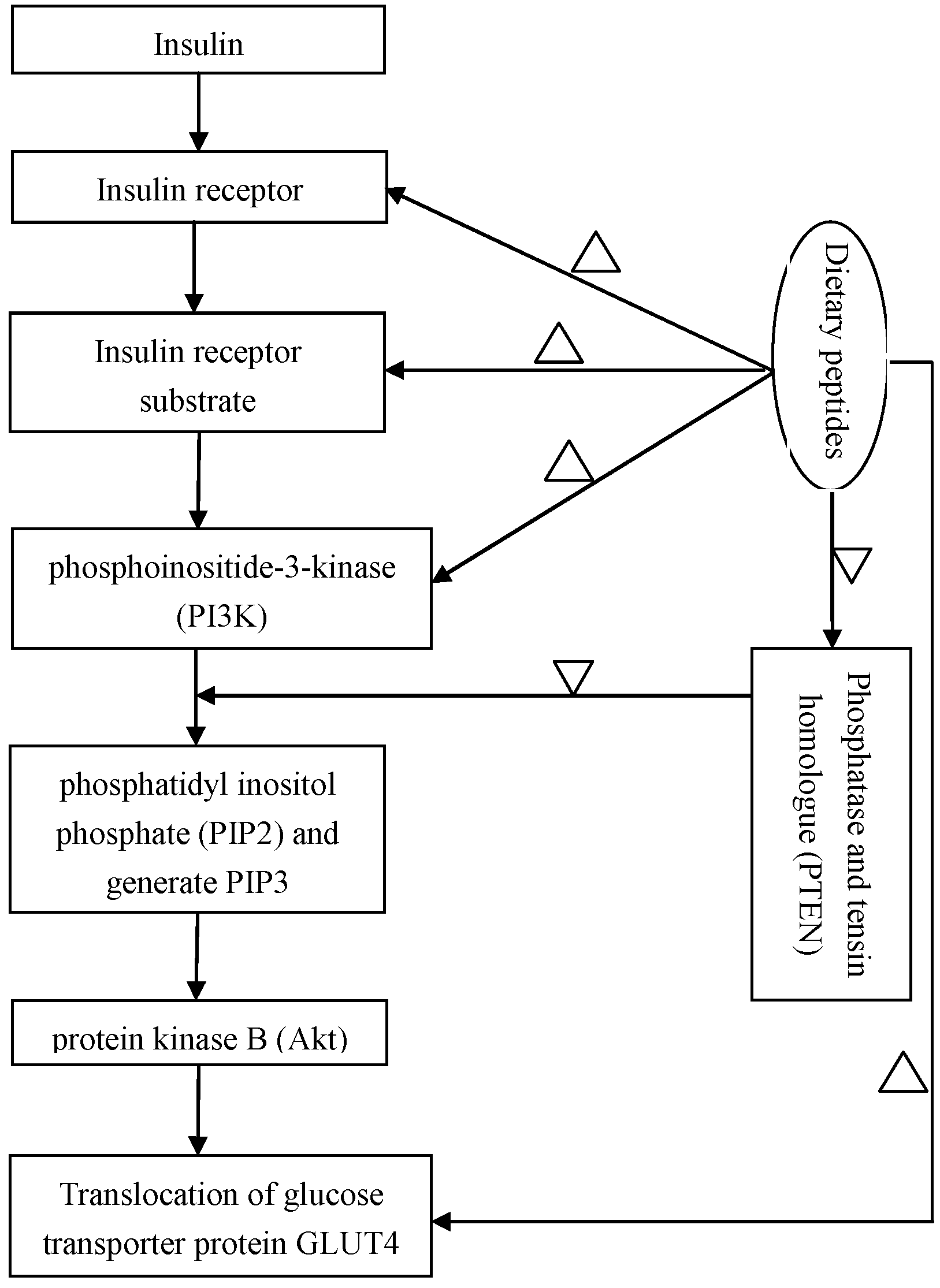
2.4. Regulation of the Insulin-Signaling Pathways
2.5. Clinical Trials
3. Inhibition of Bioactive Peptides to α-Amylase and α-Glucosidase Activities
4. The Structure Characteristics of Antidiabetes Peptides
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, J.E.; Min, S.H.; Lee, D.H.; Oh, T.J.; Kim, K.M.; Moon, J.H.; Choi, S.H.; Park, K.S.; Jang, H.C.; Lim, S. Comprehensive assessment of lipoprotein subfraction profiles according to glucose metabolism status, and association with insulin resistance in subjects with early-stage impaired glucose metabolism. Int. J. Cardiol. 2016, 225, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Leavens, K.F.; Birnbaum, M.J. Insulin signaling to hepatic lipid metabolism in health and disease. Crit. Rev. Biochem. Mol. Biol. 2011, 46, 200–215. [Google Scholar] [CrossRef] [PubMed]
- So, W.Y.; Leung, P.S. Irisin ameliorates hepatic glucose/lipid metabolism and enhances cell survival in insulin-resistant human HepG2 cells through adenosine monophosphate-activated protein kinase signaling. Int. J. Biochem. Cell Biol. 2016, 78, 237–247. [Google Scholar] [PubMed]
- Maulucci, G.; Daniel, B.; Cohen, O.; Avrahami, Y.; Sasson, S. Hormetic and regulatory effects of lipid peroxidation mediators in pancreatic β cells. Mol. Aspects Med. 2016, 49, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.F.; Peng, H.B.; Liu, G.Q.; Zhang, F.; Li, Y. Beneficial effects of oligopeptides from marine salmon skin in a rat model of type 2 diabetes. Nutrition 2010, 26, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Sree, A.; Dash, S.S.; Sethi, D.P.; Chowdhury, L. Diversity of marine bacteria producing β-glucosidase inhibitors. Microb. Cell Fact. 2013, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Hayakawa, K.; Egashira, Y.; Sanada, H. Hypocholesterolemic mechanism of Chlorella: Chlorella and its indigestible fraction enhance hepatic cholesterol catabolism through upregulation of cholesterol 7α-hydroxylase in rats. Biosci. Biotechnol. Biochem. 2007, 71, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Mello-Sampayo, C.; Luisa-Corvo, M.; Mendes, R.; Duarte, D.; Lucas, J.; Pinto, R. Insights on the safety of carotenogenic Chlorella vulgaris in rodents. Algal Res. 2013, 2, 409–915. [Google Scholar]
- Belgardt, B.F.; Ahmed, K.; Spranger, M.; Latreille, M.; Denzler, R.; Kondratiuk, N.; von, Meyenn, F.; Villena, F.N.; Herrmanns, K.; Bosco, D.; et al. The microRNA-200 family regulates pancreatic β cell survival in type 2 diabetes. Nat. Med. 2015, 21, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, N.; Leibowitz, G. Failure of β-cell adaptation in type 2 diabetes Lessons from animal models. Front. Biosci. (Landmark Ed). 2009, 14, 1099–1115. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Is oxidative stress; a link between nephrolithiasis and obesity; hypertension; diabetes; chronic kidney disease; metabolic syndrome? Urol. Res. 2012, 40, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [PubMed]
- Fernandez-Millan, E.; Cordero-Herrera, I.; Ramos, S.; Escriva, F.; Alvarez, C.; Goya, L.; Martin, M.A. Cocoa-rich diet attenuates β cell mass loss and function in young Zucker diabetic fatty rats by preventing oxidative stress and β cell apoptosis. Mol. Nutr. Food Res. 2015, 59, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Bayod, S.; Del, Valle, J.; Lalanza, J.F.; Sanchez-Roige, S.; de Luxan-Delgado, B.; Coto-Montes, A.; Canudas, A.M.; Camins, A.; Escorihuela, R.M.; Pallas, M. Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Exp. Gerontol. 2012, 47, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Carnagarin, R.; Dharmarajan, A.M.; Dass, C.R. PEDF-induced alteration of metabolism leading to insulin resistance. Mol. Cell Endocrinol. 2015, 40, 98–104. [Google Scholar]
- Cnop, M.; Igoillo-Esteve, M.; Cunha, D.A.; Ladriere, L.; Eizirik, D.L. An update on lipotoxic endoplasmic reticulum stress in pancreatic β-cells. Biochem. Soc. Trans. 2008, 36, 909–915. [Google Scholar]
- Lenzen, S. Oxidative stress the vulnerable β-cell. Biochem. Soc. Trans. 2008, 36, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Haber, E.P.; Hirabara, S.M.; Rebelato, E.L.; Procopio, J.; Morgan, D.; Oliveira-Emilio, H.C.; Carpinelli, A.R.; Curi, R. Diabetes associated cell stress and dysfunction: Role of mitochondrial and non-mitochondrial ROS production and activity. J. Physiol. 2007, 583, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Poitout, V.; Amyot, J.; Semache, M.; Zarrouki, B.; Hagman, D.; Fontes, G. Glucolipotoxicity of the pancreatic β cell. Biochim. Biophys. Acta 2010, 1801, 289–298. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Fu, A.; Robson-Doucette, C.; Allister, E.M.; Wheeler, M.B.; Screaton, R.; Harper, M.E. Glutathionylation state of uncoupling protein-2 and the control of glucose-stimulated insulin secretion. J. Biol. Chem. 2012, 287, 39673–39685. [Google Scholar] [PubMed]
- Lenzen, S.; Drinkgern, J.; Tiedge, M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996, 20, 463–466. [Google Scholar] [PubMed]
- Tiedge, M.; Lortz, S.; Drinkgern, J.; Lenzen, S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997, 46, 1733–1742. [Google Scholar] [PubMed]
- Corbett, J.A.; Wang, J.L.; Hughes, J.H.; Wolf, B.A.; Sweetland, M.A.; Lancaster, J.R.; McDaniel, M.L. Nitric oxide and cyclic GMP formation induced by interleukin 1 β in islets of Langerhans. Evidence for an effector role of nitric oxide in islet dysfunction. Biochem. J. 1992, 287, 229–235. [Google Scholar] [PubMed]
- Morgan, D.; Oliveira-Emilio, H.R.; Keane, D.; Hirata, A.E.; Santos, da, Rocha, M.; Bordin, S.; Curi, R.; Newsholme, P.; Carpinelli, A.R. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal β cell line. Diabetologia 2007, 50, 359–369. [Google Scholar] [PubMed]
- Lei, X.G.; Vatamaniuk, M.Z. Two tales of antioxidant enzymes on β cells and diabetes. Antioxid. Redox Signal. 2011, 14, 489–503. [Google Scholar] [PubMed]
- Tiedge, M.; Lortz, S.; Munday, R.; Lenzen, S. Protection against the co-operative toxicity of nitric oxide and oxygen free radicals by overexpression of antioxidant enzymes in bioengineered insulin-producing RINm5F cells. Diabetologia 1999, 42, 849–855. [Google Scholar]
- Wolf, G.; Aumann, N.; Michalska, M.; Bast, A.; Sonnemann, J.; Beck, J.F.; Lendeckel, U.; Newsholme, P.; Walther, R. Peroxiredoxin III protects pancreatic ss cells from apoptosis. J. Endocrinol. 2010, 207, 163–175. [Google Scholar] [PubMed]
- Li, X.; Chen, H.; Epstein, P.N. Metallothionein and catalase sensitize to diabetes in nonobese diabetic mice reactive oxygen species may have a protective role in pancreatic β-cells. Diabetes 2006, 55, 1592–1604. [Google Scholar] [PubMed]
- Ibrahim, M.A.; Koorbanally, N.A.; Islam, M.S. Antioxidative activity and inhibition of key enzymes linked to type-2 diabetes (α-glucosidase and α-amylase) by Khaya senegalensis. Acta Pharm. 2014, 64, 311–324. [Google Scholar] [CrossRef]
- Choi, H.K.; Willett, W.C.; Stampfer, M.J.; Rimm, E.; Hu, F.B. Dairy consumption and risk of type 2 diabetes mellitus in men a prospective study. Arch. Intern. Med. 2005, 165, 997–1003. [Google Scholar] [PubMed]
- Tremblay, A.; Gilbert, J.A. Milk products; insulin resistance syndrome and type 2 diabetes. J. Am. Coll. Nutr. 2009, 28 (Suppl. S1), 91S–102S. [Google Scholar] [PubMed]
- Nasri, R.; Abdelhedi, O.; Jemil, I.; Daoued, I.; Hamden, K.; Kallel, C.; Elfeki, A.; Lamri-Senhadji, M.; Boualga, A.; Nasri, M.; et al. Ameliorating effects of goby fish protein hydrolysates on high-fat-high-fructose diet-induced hyperglycemia; oxidative stress and deterioration of kidney function in rats. Chem-Biol. Interact. 2015, 24, 271–280. [Google Scholar]
- Ben, Khaled, H.; Ghlissi, Z.; Chtourou, Y.; Hakim, A.; Ktari, N.; Fatma, M.A.; Barkia, A.; Sahnoun, Z.; Nasri, M. Effect of protein hydrolysates from sardinelle (Sardinella. aurita) on the oxidative status and blood lipid profile of cholesterol-fed rats. Food Res. Int. 2012, 45, 60–68. [Google Scholar]
- Ktari, N.; Nasri, R.; Mnafgui, K.; Hamden, K.; Belguith, O.; Boudaouara, T.; El, Feki, A.; Nasri, M. Antioxidative and ACE inhibitory activities of protein hydrolysates from zebra blenny (Salaria. basilisca) in alloxan-induced diabetic rats. Process Biochem. 2014, 49, 890–897. [Google Scholar]
- Oseguera, Toledo, M.E.; Gonzalez, de, Mejia, E.; Sivaguru, M.; Amaya-Llano, S.L. Common bean (Phaseolus. vulgaris L.) protein-derived peptides increased insulin secretion; inhibited lipid accumulation; increased glucose uptake and reduced the phosphatase and tensin homologue activation in vitro. J. Func. Foods 2016, 27, 160–177. [Google Scholar]
- Fernández-Tomé, S.; Ramos, S.; Cordero-Herrera, I.; Recio, I.; Goya, L.; Hernández-Ledesma, B. In vitro chemo-protective effect of bioactive peptide lunasin against oxidative stress in human HepG2 cells. Food Res. Int. 2014, 62, 793–800. [Google Scholar]
- Lee, K.W.; Kim, S.J. Uptake of modified LDLs in HepG2 cells and cholesterol accumulation by modified LDLs in THP-1 macrophages. Toxicol. Lett. 2010, 196, s243. [Google Scholar] [CrossRef]
- Han, D.N.; Zhang, D.H.; Wang, L.P.; Zhang, Y.S. Protective effect of β-casomorphin-7 on cardiomyopathy of streptozotocin-induced diabetic rats via inhibition of hyperglycemia and oxidative stress. Peptides 2013, 44, 120–126. [Google Scholar] [CrossRef]
- Donath, M.Y.; Storling, J.; Maedler, K.; Mandrup-Poulsen, T. Inflammatory mediators and islet β-cell failure a link between type 1 and type 2 diabetes. J. Mol. Med. (Berl.). 2003, 81, 455–470. [Google Scholar] [CrossRef]
- Cherng, J.Y.; Liu, C.C.; Shen, C.R.; Lin, H.H.; Shih, M.F. Beneficial effects of Chlorella-11 peptide on blocking LPS-induced macrophage activation and alleviating thermal injury-induced inflammation in rats. Int. J. Immuno. Pathol. Pharmacol. 2010, 23, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Adipokines in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1062–1079. [Google Scholar] [PubMed]
- Moran, T.H.; Dailey, M.J. Minireview Gut peptides targets for antiobesity drug development? Endocrinology 2009, 150, 2526–2530. [Google Scholar] [PubMed]
- Perry, B.; Wang, Y. Appetite regulation and weight control the role of gut hormones. Nutr. Diabetes 2012, 2, e26. [Google Scholar] [CrossRef] [PubMed]
- Troke, R.C.; Tan, T.M.; Bloom, S.R. The future role of gut hormones in the treatment of obesity. Ther. Adv. Chronic Dis. 2014, 5, 4–14. [Google Scholar]
- Nongonierma, A.B.; FitzGerald, R.J. Inhibition of dipeptidyl peptidase IV (DPP-IV) by proline containing casein-derived peptides. J. Funct. Foods 2013, 5, 1909–1917. [Google Scholar]
- Omar, B.; Ahlkvist, L.; Yamada, Y.; Seino, Y.; Ahren, B. Incretin hormone receptors are required for normal β cell development and function in female mice. Peptides 2016, 79, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A.; Carter, J.D.; McDonald, A.R. Natural regulation of rat intestinal cholecystokinin gene expression. J. Clin. Invest. 1988, 81, 2015–2019. [Google Scholar]
- Caron, J.; Domenger, D.; Belguesmia, Y.; Kouach, M.; Lesage, J.; Goossens, J.F.; Dhulster, P.; Ravallec, R.; Cudennec, B. Protein digestion and energy homeostasis How generated peptides may impact intestinal hormones? Food Res. Int. 2016, 88, 310–318. [Google Scholar]
- Harnedy, P.A.; O'Keeffe, M.B.; FitzGerald, R.J. Purification and identification of dipeptidyl peptidase (DPP) IV inhibitory peptides from the macroalga Palmaria. palmata. Food Chem. 2015, 172, 400–406. [Google Scholar] [PubMed]
- Wang, T.Y.; Hsieh, C.H.; Hung, C.C.; Jao, C.L.; Chen, M.C.; Hsu, K.C. Fish skin gelatin hydrolysates as dipeptidyl peptidase IV inhibitors and glucagon-like peptide-1 stimulators improve glycaemic control in diabetic rats: A comparison between warm- and cold-water fish. J. Func. Foods 2015, 18, 330–340. [Google Scholar]
- Huang, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Dipeptidyl-peptidase IV inhibitory activity of peptides derived from tuna cooking juice hydrolysates. Peptides 2012, 35, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yan, J.; Yu, Y.; Qi, Y. Screening and identification of DPP-IV inhibitory peptides from deer skin hydrolysates by an integrated approach of LC–MS/MS and in silico analysis. J. Funct. Foods 2015, 18, 344–357. [Google Scholar] [CrossRef]
- Lacroix, I.M.; Li-Chan, E.C. Overview of food products and natural constituents with antidiabetic properties and their putative mechanisms of action a natural approach to complement pharmacotherapy in the management of diabetes. Mol. Nutr. Food Res. 2014, 58, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Ohshiba, Y.; Mogami, O. Novel dipeptidyl peptidase-4-inhibiting peptide derived from β-lactoglobulin. J. Pharmacol. Sci. 2011, 117, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Silveira, S.T.; Martinez-Maqueda, D.; Recio, I.; Hernandez-Ledesma, B. Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem. 2013, 141, 1072–1077. [Google Scholar] [PubMed]
- Uenishi, K. Diabetes mellitus and osteoporosis. Natural therapy of diabetes related osteoporosis. Clin. Calcium. 2012, 22, 1398–1402. [Google Scholar] [PubMed]
- Lacroix, I.M.; Li-Chan, E.C. Inhibition of dipeptidyl peptidase (DPP)-IV and α-glucosidase activities by pepsin-treated whey proteins. J. Agric. Food Chem. 2013, 61, 7500–7506. [Google Scholar] [CrossRef]
- Lacroix, I.M.; Li-Chan, E.C. Isolation and characterization of peptides with dipeptidyl peptidase-IV inhibitory activity from pepsin-treated bovine whey proteins. Peptides 2014, 54, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, I.M.; Li-Chan, E.C. Comparison of the susceptibility of porcine and human dipeptidyl-peptidase IV to inhibition by protein-derived peptides. Peptides 2015, 69, 19–25. [Google Scholar] [PubMed]
- Lacroix, I.M.E.; Meng, G.; Cheung, I.W.Y.; Li-Chan, E.C.Y. Do whey protein-derived peptides have dual dipeptidyl-peptidase IV and angiotensin I-converting enzyme inhibitory activities? J. Funct. Foods 2016, 21, 87–96. [Google Scholar] [CrossRef]
- Tulipano, G.; Sibilia, V.; Caroli, A.M.; Cocchi, D. Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides 2011, 32, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Le Maux, S.; Nongonierma, A.B.; FitzGerald, R.J. Improved short peptide identification using HILIC-MS/MS retention time prediction model based on the impact of amino acid position in the peptide sequence. Food Chem. 2015, 17, 3847–3854. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides 2013, 39, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; Mooney, C.; Shields, D.C.; FitzGerald, R.J. In silico approaches to predict the potential of milk protein-derived peptides as dipeptidyl peptidase IV (DPP-IV) inhibitors. Peptides 2014, 57, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Strategies for the discovery; identification and validation of milk protein-derived bioactive peptides. Trends. Food Sci. Tec. 2016, 50, 26–43. [Google Scholar]
- De Souza Rocha, T.; Hernandez, L.M.R.; Chang, Y.K.; de Mejía, E.G. Impact of germination and enzymatic hydrolysis of cowpea bean (Vigna. unguiculata) on the generation of peptides capable of inhibiting dipeptidyl peptidase IV. Food Res. Int. 2014, 64, 799–809. [Google Scholar]
- Nongonierma, A.B.; Le, Maux, S.; Dubrulle, C.; Barre, C.; FitzGerald, R.J. Quinoa (Chenopodium. quinoa Willd.) protein hydrolysates with In Vitro dipeptidyl peptidase IV (DPP-IV) inhibitory and antioxidant properties. J. Cereal Sci. 2015, 65, 112–118. [Google Scholar]
- Hatanaka, T.; Inoue, Y.; Arima, J.; Kumagai, Y.; Usuki, H.; Kawakami, K.; Kimura, M.; Mukaihara, T. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem. 2012, 134, 797–802. [Google Scholar] [PubMed]
- Velarde-Salcedo, A.J.; Barrera-Pacheco, A.; Lara-Gonzalez, S.; Montero-Morán, G.M.; Díaz-Gois, A.; de Mejia, E.G.; Barba, de la Rosa, A.P. In Vitro inhibition of dipeptidyl peptidase IV by peptides derived from the hydrolysis of amaranth (Amaranthus hypochondriacus l.) proteins. Food Chem. 2013, 136, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Shuai, T.; Yi, L.J.; Wang, Y.; Song, G.M. Effect of case management on patients with type 2 diabetes mellitus: A meta-analysis. Chinese Nursing Research 2016, 3, 71–76. [Google Scholar]
- Jong-Yuh, C.; Mei-Fen, S. Potential hypoglycemic effects of Chlorella in streptozotocin-induced diabetic mice. Life Sci. 2005, 77, 980–990. [Google Scholar] [CrossRef]
- Jeong, H.; Kwon, H.J.; Kim, M.K. Hypoglycemic effect of Chlorella vulgaris intake in type 2 diabetic Goto-Kakizaki and normal Wistar rats. Nutr. Res. Prac. 2009, 3, 23–30. [Google Scholar]
- Lu, J.; Zeng, Y.; Hou, W.; Zhang, S.; Li, L.; Luo, X.; Xi, W.; Chen, Z.; Xiang, M. The soybean peptide aglycin regulates glucose homeostasis in type 2 diabetic mice via IR/IRS1 pathway. J. Nutr. Biochem. 2012, 23, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Veloso, R.V.; Latorraca, M.Q.; Arantes, V.C.; Reis, M.A.; Ferreira, F.; Boschero, A.C.; Carneiro, E.M. Soybean diet improves insulin secretion through activation of cAMP/PKA pathway in rats. J. Nutr. Biochem. 2008, 19, 778–784. [Google Scholar] [PubMed]
- Roblet, C.; Akhtar, M.J.; Mikhaylin, S.; Pilon, G.; Gill, T.; Marette, A.; Bazinet, L. Enhancement of glucose uptake in muscular cell by peptide fractions separated by electrodialysis with filtration membrane from salmon frame protein hydrolysate. J. Funct. Foods 2016, 22, 337–346. [Google Scholar]
- Kwon, D.Y.; Daily, J.W.; Kim, H.J.; Park, S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010, 30, 1–13. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Bringe, N.A.; Berhow, M.A.; Gonzalez, de Mejia, E. Β-conglycinin embeds active peptides that inhibit lipid accumulation in 3T3-L1 adipocytes In Vitro. J. Agric. Food Chem. 2008, 56, 10533–10543. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Dia, V.P.; Berhow, M.; Bringe, N.A.; Gonzalez de Mejia, E. Protein hydrolysates from β-conglycinin enriched soybean genotypes inhibit lipid accumulation and inflammation In Vitro. Mol. Nutr. Food Res. 2009, 53, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.D.; Kim, E.; Lee, J.H.; Hyun, C.K. Gly-Ala-Gly-Val-Gly-Tyr: A novel synthetic peptide; improves glucose transport and exerts beneficial lipid metabolic effects in 3T3-L1 adipoctyes. Eur. J. Pharmacol. 2011, 650, 479–485. [Google Scholar] [CrossRef]
- Hammé, V.; Sannier, F.; Piot, J.M.; Bordenave-Juchereau, S. Effects of lactokinins from fermented acid goat whey on lipid content and adipogenesis of immortalised human adipocytes. Int. Dairy J. 2010, 20, 642–645. [Google Scholar] [CrossRef]
- Yim, M.J.; Hosokawa, M.; Mizushina, Y.; Yoshida, H.; Saito, Y.; Miyashita, K. Suppressive effects of Amarouciaxanthin A on 3T3-L1 adipocyte differentiation through downregulation of PPARγ and C/EBPα mRNA expression. J. Agric. Food Chem. 2011, 59, 1646–1652. [Google Scholar] [PubMed]
- Ben, Henda, Y.; Laamari, M.; Lanneluc, I.; Travers, M.A.; Agogué, H.; Arnaudin, I.; Bridiau, N.; Maugard, T.; Piot, J.M.; Sannier, F.; et al. Di and tripeptides from marine sources can target adipogenic process and contribute to decrease adipocyte number and functions. J. Funct. Foods 2015, 17, 1–10. [Google Scholar]
- Wang, L.L.; Hao, S.; Zhang, S.; Guo, L.J.; Hu, C.Y.; Zhang, G.; Gao, B.; Zhao, J.J.; Jiang, Y.; Tian, W.G.; et al. PTEN/PI3K/AKT protein expression is related to clinicopathologic features and prognosis in breast cancer with axillary lymph node metastases. Hum. Pathol. 2017, 61, 49–57. [Google Scholar] [PubMed]
- Govers, R. Molecular mechanisms of GLUT4 regulation in adipocytes. Diabetes Metab. 2014, 40, 400–410. [Google Scholar] [CrossRef]
- Morino, K.; Neschen, S.; Bilz, S.; Sono, S.; Tsirigotis, D.; Reznick, R.M.; Moore, I.; Nagai, Y.; Samuel, V.; Sebastian, D.; et al. Muscle-specific IRS-1 Ser->Ala transgenic mice are protected from fat-induced insulin resistance in skeletal muscle. Diabetes 2008, 57, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Bozulic, L.; Hemmings, B.A. PIKKing on PKB regulation of PKB activity by phosphorylation. Curr. Opin. Cell Biol. 2009, 21, 256–261. [Google Scholar] [PubMed]
- Davis, J.; Higginbotham, A.; O'Connor, T.; Moustaid-Moussa, N.; Tebbe, A.; Kim, Y.C.; Cho, K.W.; Shay, N.; Adler, S.; Peterson, R.; et al. Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann. Nutr. Metab. 2007, 51, 42–52. [Google Scholar] [PubMed]
- Nordentoft, I.; Jeppesen, P.B.; Hong, J.; Abudula, R.; Hermansen, K. Increased insulin sensitivity and changes in the expression profile of key insulin regulatory genes and β cell transcription factors in diabetic KKAy-mice after feeding with a soy bean protein rich diet high in isoflavone content. J. Agric. Food Chem. 2008, 56, 4377–4385. [Google Scholar] [CrossRef] [PubMed]
- Han, B.K.; Lee, H.J.; Lee, H.S.; Suh, H.J.; Park, Y. Hypoglycaemic effects of functional tri-peptides from silk in differentiated adipocytes and streptozotocin-induced diabetic mice. J. Sci. Food Agric. 2016, 96, 116–121. [Google Scholar]
- Huang, K.C.; Huang, H.J.; Chen, C.C.; Chang, C.T.; Wang, T.Y.; Chen, R.H.; Chen, Y.C.; Tsai, F.J. Susceptible gene of stasis-stagnation constitution from genome-wide association study related to cardiovascular disturbance and possible regulated traditional Chinese medicine. BMC Complement. Altern. Med. 2015, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, C.E.; Klemm, D.J. Adipose tissue insulin sensitivity and macrophage recruitment Does PI3K pick the pathway? Adipocyte 2013, 21, 135–142. [Google Scholar]
- Zhang, Y.; Hai, J.; Cao, M.; Zhang, Y.; Pei, S.; Wang, J.; Zhang, Q. Silibinin ameliorates steatosis and insulin resistance during non-alcoholic fatty liver disease development partly through targeting IRS-1/PI3K/Akt pathway. Int. Immunopharmacol. 2013, 17, 714–720. [Google Scholar] [PubMed]
- Ishihara, K.; Oyaizu, S.; Fukuchi, Y.; Mizunoya, W.; Segawa, K.; Takahashi, M.; Mita, Y.; Fukuya, Y.; Fushiki, T.; Yasumoto, K. A soybean peptide isolate diet promotes postprandial carbohydrate oxidation and energy expenditure in type II diabetic mice. J. Nutr. 2003, 133, 752–757. [Google Scholar] [PubMed]
- Zhu, K.N.; Jiang, C.H.; Tian, Y.S.; Xiao, N.; Wu, Z.F.; Ma, Y.L.; Lin, Z.; Fang, S.Z.; Shang, X.L.; Liu, K.; et al. Two triterpeniods from Cyclocarya paliurus (βl) Iljinsk (Juglandaceae) promote glucose uptake in 3T3-L1 adipocytes: The relationship to AMPK activation. Phytomedicine 2015, 22, 837–846. [Google Scholar]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [PubMed]
- Mizoguchi, T.; Takehara, I.; Masuzawa, T.; Saito, T.; Naoki, Y. Nutrigenomic studies of effects of Chlorella on subjects with high-risk factors for lifestyle-related disease. J. Med. Food 2008, 11, 395–404. [Google Scholar] [PubMed]
- Panahi, Y.; Ghamarchehreh, M.E.; Beiraghdar, F.; Zare, R.; Jalalian, H.R.; Sahebkar, A. Investigation of the effects of Chlorella vulgaris supplementation in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Hepatogastroenterology 2012, 59, 2099–2103. [Google Scholar] [PubMed]
- Ebrahimi-Mameghani, M.; Sadeghi, Z.; Farhangi, M.A.; Vaghef-Mehrabany, E.; Aliashrafi, S. Glucose homeostasis, insulin resistance and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: Beneficial effects of supplementation with microalgae Chlorella vulgaris: A double-blind placebo-controlled randomized clinical trial. Clin. Nutr. 2016. [Google Scholar] [CrossRef]
- Jo, B.H.; Lee, C.S.; Song, H.R.; Lee, H.G.; Oh, H.M. Development of novel microsatellite markers for strain-specific identification of Chlorella vulgaris. J. Microbiol. Biotechnol. 2014, 24, 1189–1195. [Google Scholar]
- Kim, M.; Kim, E.; Kwak, H.S.; Jeong, Y. The ingredients in Saengshik; a formulated health food; inhibited the activity of α-amylase and α-glucosidase as anti-diabetic function. Nutr. Res. Pract. 2014, 8, 602–606. [Google Scholar] [PubMed]
- Ren, Y.; Liang, K.; Jin, Y.; Zhang, M.; Chen, Y.; Wu, H.; Lai, F. Identification and characterization of two novel α-glucosidase inhibitory oligopeptides from hemp (Cannabis sativa l.) seed protein. J. Funct. Foods 2016, 26, 439–450. [Google Scholar] [CrossRef]
- Ngoh, Y.Y.; Gan, C.Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus. vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [PubMed]
- Siow, H.L.; Gan, C.Y. Extraction; identification; and structure–activity relationship of antioxidative and α-amylase inhibitory peptides from cumin seeds (Cuminum. cyminum). J. Funct. Foods 2016, 22, 1–12. [Google Scholar] [CrossRef]
- Siow, H.L.; Lim, T.S.; Gan, C.Y. Development of a workflow for screening and identification of α-amylase inhibitory peptides from food source using an integrated Bioinformatics-phage display approach Case study-Cumin seed. Food Chem. 2017, 214, 67–76. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejia, E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus. vulgaris l.) proteins; their characterization and biological potential. Food. Funct. 2016, 7, 713–727. [Google Scholar]
- Uraipong, C.; Zhao, J. Rice bran protein hydrolysates exhibit strong in vitro α-amylase; β-glucosidase and ACE-inhibition activities. J. Sci. Food Agric. 2016, 96, 1101–1110. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, Y.; Zhao, W.; Liu, J.; Chen, F. Anti-diabetic activity peptides from albumin against α-glucosidase and α-amylase. Food Chem. 2012, 135, 2078–2085. [Google Scholar] [CrossRef]
- Mojica, L.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Characterization of peptides from common bean protein isolates and their potential to inhibit markers of type-2 diabetes; hypertension and oxidative stress. J. Sci. Food Agric. 2016. [Google Scholar] [CrossRef]
- Engel, M.; Hoffmann, T.; Wagner, L.; Wermann, M.; Heiser, U.; Kiefersauer, R.; Huber, R.; Bode, W.; Demuth, H.U.; Brandstetter, H. The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism. Proc. Natl. Acad. Sci. USA 2003, 100, 5063–5068. [Google Scholar] [CrossRef]
- Juillerat-Jeanneret, L. Dipeptidyl peptidase IV and its inhibitors therapeutics for type 2 diabetes and what else? J. Med. Chem. 2014, 57, 2197–2212. [Google Scholar]
- Nongonierma, A.B.; FitzGerald, R.J. Susceptibility of milk protein-derived peptides to dipeptidyl peptidase IV (DPP-IV) hydrolysis. Food Chem. 2014, 145, 845–852. [Google Scholar]
- Lu, I.L.; Tsai, K.C.; Chiang, Y.K.; Jiaang, W.T.; Wu, S.H.; Mahindroo, N.; Chien, C.H.; Lee, S.J.; Chen, X.; Chao, Y.S.; et al. A three-dimensional pharmacophore model for dipeptidyl peptidase IV inhibitors. Eur. J. Med. Chem. 2008, 43, 1603–1611. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Gaudel, C.; Murray, B.A.; Flynn, S.; Kelly, P.M.; Newsholme, P.; FitzGerald, R.J. Insulinotropic properties of whey protein hydrolysates and impact of peptide fractionation on insulinotropic response. Int. Dairy J. 2013, 32, 163–168. [Google Scholar]
- Dixon, G.; Nolan, J.; McClenaghan, N.; Flatt, P.R.; Newsholme, P. A comparative study of amino acid consumption by rat islet cells and the clonal β-cell line BRIN-BD11-the functional significance of l-alanine. J. Endocrinol. 2003, 179, 447–454. [Google Scholar]
- Bender, K.; Newsholme, P.; Brennan, L.; Maechler, P. The importance of redox shuttles to pancreatic β-cell energy metabolism and function. Biochem. Soc. Trans. 2006, 34, 811–814. [Google Scholar] [CrossRef]
- Power, O.; Hallihan, A.; Jakeman, P. Human insulinotropic response to oral ingestion of native and hydrolysed whey protein. Amino Acids 2009, 37, 333–339. [Google Scholar] [CrossRef]
- Horner, K.; Drummond, E.; Brennan, L. Bioavailability of milk protein-derived bioactive peptides a glycaemic management perspective. Nutr. Res. Rev. 2016, 29, 91–101. [Google Scholar] [CrossRef]
- Manders, R.J.; Koopman, R.; Sluijsmans, W.E.; van den Berg, R.; Verbeek, K.; Saris, W.H.; Wagenmakers, A.J.; van Loon, L.J. Co-ingestion of a protein hydrolysate with or without additional leucine effectively reduces postprandial blood glucose excursions in Type 2 diabetic men. J. Nutr. 2006, 136, 1294–1299. [Google Scholar]
- Manders, R.J.; Praet, S.F.; Meex, R.C.; Koopman, R.; De Roos, A.L.; Wagenmakers, A.J.; Saris, W.H.; Van, Loon, L.J. Protein hydrolysate/leucine co-ingestion reduces the prevalence of hyperglycemia in type 2 diabetic patients. Diabetes Care 2006, 29, 2721–2722. [Google Scholar]
- Ngoh, Y.Y.; Lim, T.S.; Gan, C.Y. Screening and identification of five peptides from pinto bean with inhibitory activities against α-amylase using phage display technique. Enzyme. Microb. Technol. 2016, 89, 76–84. [Google Scholar]
- Ochiai, T.; Sugita, T.; Kato, R.; Okochi, M.; Honda, H. Screening of an α-amylase inhibitor peptide by photolinker-peptide array. Biosci. Biotechnol. Biochem. 2012, 76, 819–824. [Google Scholar] [CrossRef]
| Food | Precursor Protein | Peptide Sequence | IC 50 (μM) | Reference |
|---|---|---|---|---|
| Plant Protein | Macroalga Palmaria palmate protein | ILAP | 43.40 | [50] |
| LLAP | 53.67 | |||
| MAGVDHI | 159.37 | |||
| Collagen | Halibut skin gelatin | SPGSSGPQGFTG | 101.6 | [51] |
| GPVGPAGNPGANGLN | 81.3 | |||
| PPGPTGPRGQPGNIGF | 146.7 | |||
| Tilapia skin gelatin | IPGDPGPPGPPGP | 65.4 | ||
| LPGERGRPGAPGP | 76.8 | |||
| GPKGDRGLPGPPGRDG | 89.6 | |||
| Tuna cooking juice hydrolysates | PACGGFWISGRPG | 96.4 | [52] | |
| CAYQWQRPVDRIR | 78 | |||
| PGVGGPLGPIGPCYE | 116.1 | |||
| Collagen | Deer skin protein | GPVGXAGPPGK | 83.3 | [53] |
| GPVGPSGPXGK | 93.7 | |||
| Milk protein | α-Lactalbumin | LKPTPEGDL | 45 | [54] |
| LAHKALCSEKL | 165 | |||
| LCSEKLDQ | 186 | |||
| TKCEVFRE | 166 | |||
| β-Lactalbumin | VAGTWY | 174 | [55] | |
| IPAVF | 44.7 | [56] | ||
| Atlantic salmon collagen/gelatin | GPAE | 49.6 | [52] | |
| GPGA | 41.9 | |||
| Gouda-type cheese | VPITPTL | 110 | [57] | |
| VPITPT | 130 | |||
| LPQNIPPL | 46 | |||
| VAGTWY | 174 | |||
| LPQ | 82 | |||
| Whey protein | LAHKALCSEKL | 165 | [58,59,60,61,62,63] | |
| WLAHKALCSEKLDQ | 141 | |||
| LKPTPEGDL | 45 9 | |||
| LKPTPEGDLEIL | 57 | |||
| WLAHKALCSEKLDQ | 141 | |||
| WR | 31.4 | |||
| IPIQY | 28.2 | |||
| WCKDDQNPHS | 75.0 | |||
| TKCEVFRE | 166 | |||
| IPA | 49 | |||
| VA3, VL, WL, WI | <170 | |||
| LKPTPEGDLE | 42 | |||
| LKALPMH | 193 | |||
| Milk protein | WA | 92.6 | [46,64,65,66] | |
| WR | 37.8 | |||
| WK | 40.6 | |||
| LPYPY | 108.3 | |||
| WQ | 120.3 | |||
| WI | 138.7 | |||
| WN | 148.5 | |||
| YPYY | 194.4 | |||
| Milk protein | Milk protein | WN | 148.5 | [46,64,65,66] |
| IP | 149.6 | |||
| IPI | 3.5 | |||
| IPIQY | 35.2 | |||
| FLQP | 65.3 | |||
| WV | 65.7 | |||
| LPVPQ | 48.2 | |||
| IPM | 73.9 | |||
| HL | 143.2 | |||
| VA | 168.2 | |||
| WL | 43.6 | |||
| WP | 44.5 |
| Ingredient | Peptides Sequence | IC 50 | Precursors | Reference |
|---|---|---|---|---|
| α-amylase | PPHMLP | 1.97 (mg mL−1) | Pinto bean | [103] |
| PLPWGAGF | 8.96 (mg mL−1) | |||
| PPHMGGP | 14.63 (mg mL−1) | |||
| PLPLHMLP | 18.45 (mg mL−1) | |||
| LSSLEMGSLGALFVCM | 20.56 (mg mL−1) | |||
| FFRSKLLSDGAAAAKGALLPQYW | 0.02 (μM) | Cumin seed protein | [104] | |
| RCMAFLLSDGAAAAQQLLPQYW | 0.04 (μM) | |||
| DPAQPNYPWTAVLVFRH | 0.03 (μM) | |||
| RCMAFLLSDGAAAAQQLLPQYW | 0.04 (μM) | Cumin seed protein | [105] | |
| DPAQPNYPW TAVLVFRH | 0.15 (μM) | |||
| WEVM | - | Black bean protein | [106] | |
| AKSPLF | - | |||
| <3 kDa fraction | - | Rice bran protein | [107] | |
| KLPGF | 120.0 ± 4.0 (μM) | Albumin | [108] | |
| NVLQPS | 110.0 ± 6.2 (μM) | |||
| α-glucosidase | - | 36.3%–50.1% mg−1 DW | Bean protein | [109] |
| TTGGKGGK | - | Black bean protein | [107] | |
| KLPGF | 59.5 ± 5.7 (μM) | Albumin | [108] | |
| NVLQPS | 100.0 ± 5.7 (μM) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, E.-Q.; Zhu, S.-S.; He, M.-J.; Luo, F.; Fu, C.-Z.; Zou, T.-B. Marine Peptides as Potential Agents for the Management of Type 2 Diabetes Mellitus—A Prospect. Mar. Drugs 2017, 15, 88. https://doi.org/10.3390/md15040088
Xia E-Q, Zhu S-S, He M-J, Luo F, Fu C-Z, Zou T-B. Marine Peptides as Potential Agents for the Management of Type 2 Diabetes Mellitus—A Prospect. Marine Drugs. 2017; 15(4):88. https://doi.org/10.3390/md15040088
Chicago/Turabian StyleXia, En-Qin, Shan-Shan Zhu, Min-Jing He, Fei Luo, Cheng-Zhan Fu, and Tang-Bin Zou. 2017. "Marine Peptides as Potential Agents for the Management of Type 2 Diabetes Mellitus—A Prospect" Marine Drugs 15, no. 4: 88. https://doi.org/10.3390/md15040088
APA StyleXia, E.-Q., Zhu, S.-S., He, M.-J., Luo, F., Fu, C.-Z., & Zou, T.-B. (2017). Marine Peptides as Potential Agents for the Management of Type 2 Diabetes Mellitus—A Prospect. Marine Drugs, 15(4), 88. https://doi.org/10.3390/md15040088





