New Cytotoxic Secondary Metabolites from Marine Bryozoan Cryptosula pallasiana
Abstract
:1. Introduction
2. Results
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
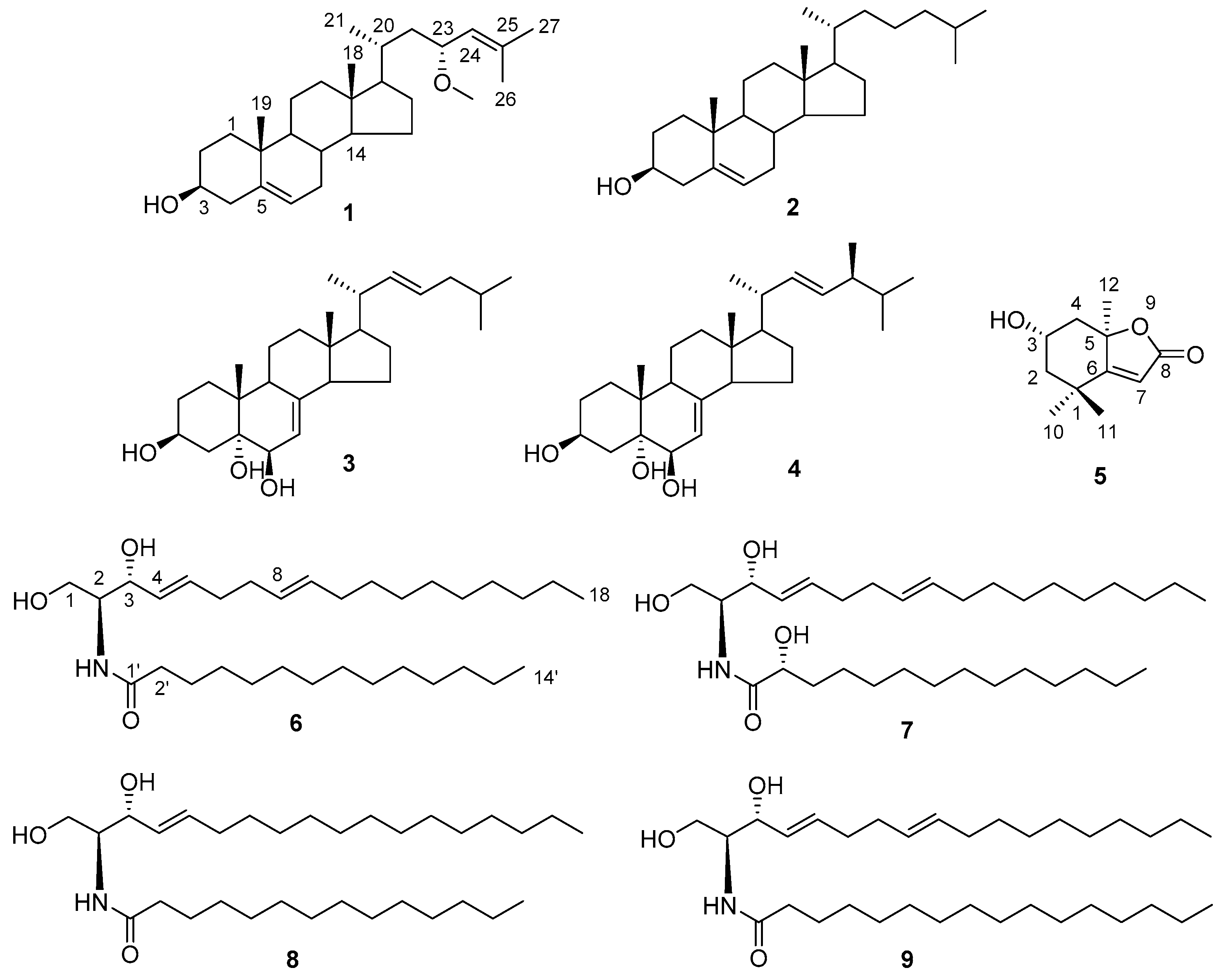
3.3.1. (23R)-Methoxycholest-5,24-dien-3β-ol (1)
3.3.2. (2S,3R,4E,8E)-2-(Tetradecanoylamino)-4,8-octadecadien-l,3-diol (6)
3.3.3. (2S,3R,2′R,4E,8E)-2-(Tetradecanoylamino)-4,8-octadecadien-l,3,2′-triol (7)
3.4. Methanolysis of Compounds 6 and 7
3.5. MTT Cytotoxicity Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CC | Column Chromatography |
| HPLC | High-Performance Liquid Chromatography |
| MTT | Microculture Tetrazolium |
| ATCC | American Type Culture Collection |
| RPMI | Roswell Park Memorial Institute |
| LCB | Long Chain Base |
| FAB | Fatty Acid Base |
| TD-DFT | Time-Dependent Density Functional Theory |
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine nature products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed]
- Blackman, A.J.; Walls, J.T. Bryozoan secondary metabolites and their chemical ecology. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 1995; pp. 73–112. [Google Scholar]
- Pejin, B.; Mojovic, M.; Savic, A.G. Novel antitumour natural products from the phylum Bryozoa. Biol. Serbica 2013, 35, 3–14. [Google Scholar]
- Tian, X.R.; Tang, H.F.; Li, Y.S.; Lin, H.W.; Tong, X.Y.; Ma, N. Alkaloids from marine bryozoan Cryptpsula pallasiana. Biochem. Syst. Ecol. 2010, 38, 1250–1252. [Google Scholar] [CrossRef]
- Tian, X.R.; Tang, H.F.; Li, Y.S.; Lin, H.W.; Chen, X.L.; Ma, N.; Yao, M.N.; Zhang, P.H. New cytotoxic oxygenated sterols from the marine bryozoan Cryptosula pallasiana. Mar. Drugs 2011, 9, 162–183. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.R.; Tang, H.F.; Li, Y.S.; Lin, H.W.; Zhang, X.Y.; Feng, J.T.; Zhang, X. Studies on the chemical constituents from marine bryozoan Cryptosula pallasiana. Rec. Nat. Prod. 2015, 9, 628–632. [Google Scholar]
- Xiao, D.; Deng, S.; Zeng, L. Studies on the chemical constituents of the marine sponge Clathria fasciculate from the South China Sea. Chin. J. Mar. Drugs 2002, 2, 1–4. [Google Scholar]
- Madaio, A.; Piccialli, V.; Sica, D.; Corriero, G. New polyhydroxysterols from the dictyoceratid sponges Hipposoingia communis, Spongia officinalis, Ircinia variabilis, and Spongionella gracilis. J. Nat. Prod. 1989, 52, 952–961. [Google Scholar] [CrossRef]
- Kimura, J.; Maki, N. New loliolide derivatives from the brown alga Undaria pinnatifida. J. Nat. Prod. 2002, 65, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y. Six kinds of N-acylsphingosines from the Chinese gorgonian Junceella squamata. Chin. J. Mar. Drugs 1995, 2, 1–4. [Google Scholar]
- Babu, U.V.; Bhandari, S.P.S.; Garg, H.S. Temno-sides A and B, two new glycosphingolipids from the Sea, urchin Temnopleurus toreumaticus of the Indian coast. J. Nat. Prod. 1997, 60, 732–734. [Google Scholar] [CrossRef]
- Calderon, G.J.; Castellanos, L.; Duque, C.; Echigo, S.; Hara, N.; Fujimoto, Y. Ophirasterol, a new C31 sterol from the marine sponge Topsentia ophiraphidites. Steroids 2004, 69, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.K.U.; Dobson, J.F.; Petersilka, M. Density functional theory of time-dependent phenomena. In Density Functional Theory II; Nalewajski, R.F., Ed.; Springer: Berlin, Germany, 1996; Volume 181, p. 81. [Google Scholar]
- Casida, M.E. Recent Advances in Density Functional Methods, Part I; Chong, D.P., Ed.; World Scientific: Singapore, 1995; pp. 155–192. [Google Scholar]
- Gross, E.K.U.; Kohn, W. Time-dependent density functional theory. In Density Functional Theory of Many-Fermion Systems; Advances in Quantum Chemistry; Elsevier: Amsterdam, The Netherlands, 1990; Volume 21, pp. 255–291. [Google Scholar]
- Runge, E.; Gross, E.K.U. Density-functional theory for time-dependent systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Kong, F.; Wang, Y.; Liu, P.; Dong, T.; Zhu, W. Thiodiketopiperazines from the marine-derived fungus Phoma sp. OUCMDZ-1847. J. Nat. Prod. 2014, 77, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, Y.; Guo, L.; Wang, Y.; Liu, P.; Zhu, W. Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar. Drugs 2014, 12, 3970–3981. [Google Scholar] [CrossRef] [PubMed]
- Chebaane, K.; Guyot, M. Occurrence of erythro-docosasphinga-4,8-dienine, as an ester, in Anemonia sulcata. Tetrahedron Lett. 1986, 27, 1495–1496. [Google Scholar] [CrossRef]
- Colsch, B.; Afonso, C.; Popa, I.; Portoukalian, J.; Fournier, F.; Tabet, J.-C.; Baumann, N. Characterization of the ceramide moieties of sphingoglycolipids from mouse brain by ESI-MS/MS: Identification of ceramides containing sphingadienine. J. Lipid Res. 2004, 45, 281–286. [Google Scholar] [CrossRef] [PubMed]
- De Hann, J.W.; Van de Ven, L.J.M. Configurations and conformations in acyclic unsaturated hydrocarbons. A 13C-NMR study. Org. Magn. Reson. 1973, 5, 147–153. [Google Scholar] [CrossRef]
- Shi, J.; Seo, Y. Isolation of new ceramides from the gorgonian Acabaria undulate. J. Nat. Prod. 1995, 58, 948–953. [Google Scholar]
- Nakagawa, M.; Tsuruoka, A.; Yoshida, J.; Hino, T. Total synthesis of (+)-erythro-N-Lauroyldocosasphinga-4,8-dienine from Anemonia sulcata and determination of the absolute configuration. J. Chem. Soc. Chem. Commun. 1990, 603–605. [Google Scholar] [CrossRef]
- Szulc, Z.M.; Bai, A.; Bielawski, J.; Mayroo, N.; Miller, D.E.; Gracz, H.; Hannun, Y.A.; Bielawska, A. Synthesis, NMR characterization and divergent biological actions of 2′-hydroxy-ceramide/dihydroceramide stereoisomers in MCF7 cells. Bioorg. Med. Chem. 2010, 18, 7565–7579. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.R.; Tang, H.F.; Feng, J.T.; Li, Y.S.; Lin, H.W.; Fan, X.P.; Zhang, X. Neritinaceramides A–E, new ceramides from the marine bryozoan Bugula neritina inhabiting South China Sea and their cytotoxicity. Mar. Drugs 2014, 12, 1987–2003. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.R.; Tang, H.F.; Li, Y.S.; Lin, H.W.; Ma, N.; Zhang, W.; Yao, M.N. Ceramides and cerebrosides from the marine bryozoan Bugula neritina inhabiting South China Sea. J. Asian Nat. Prod. 2009, 11, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
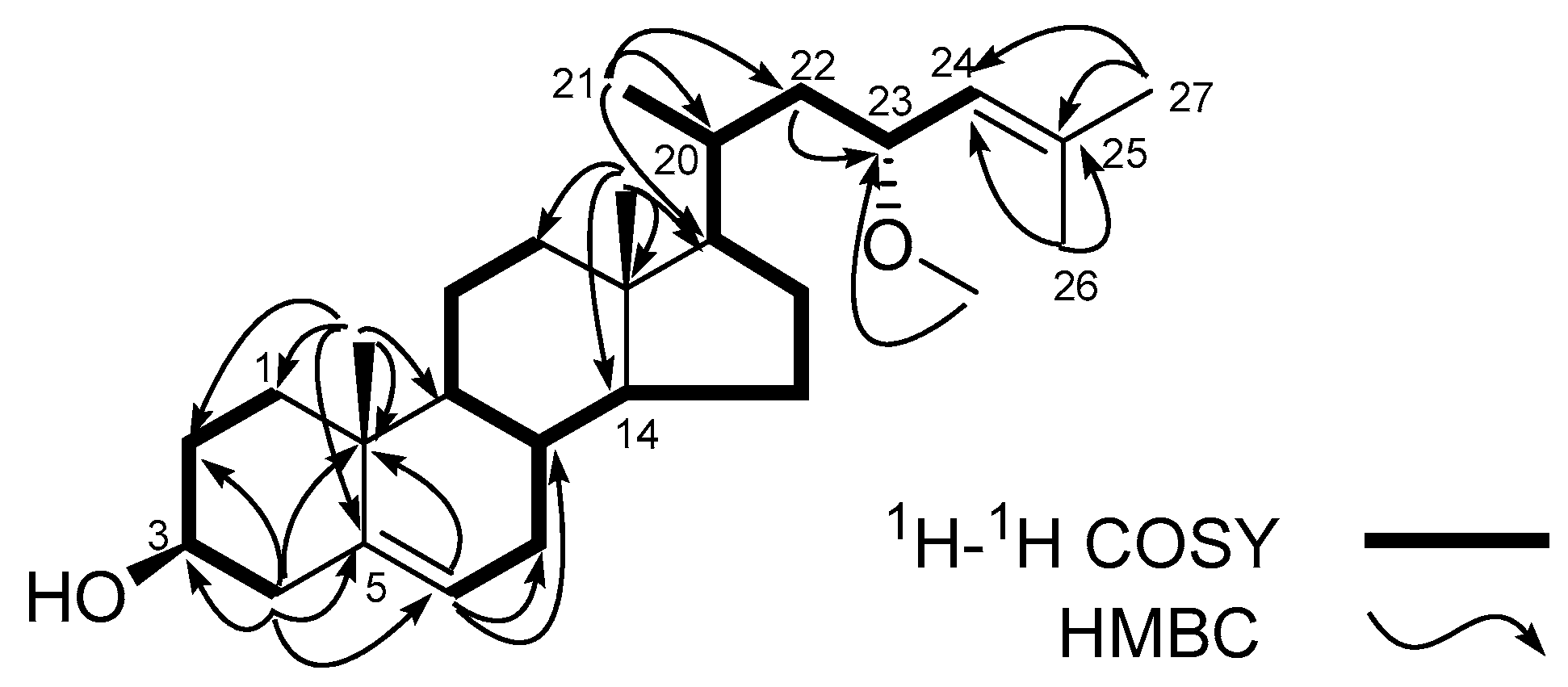
 ) for 1.
) for 1.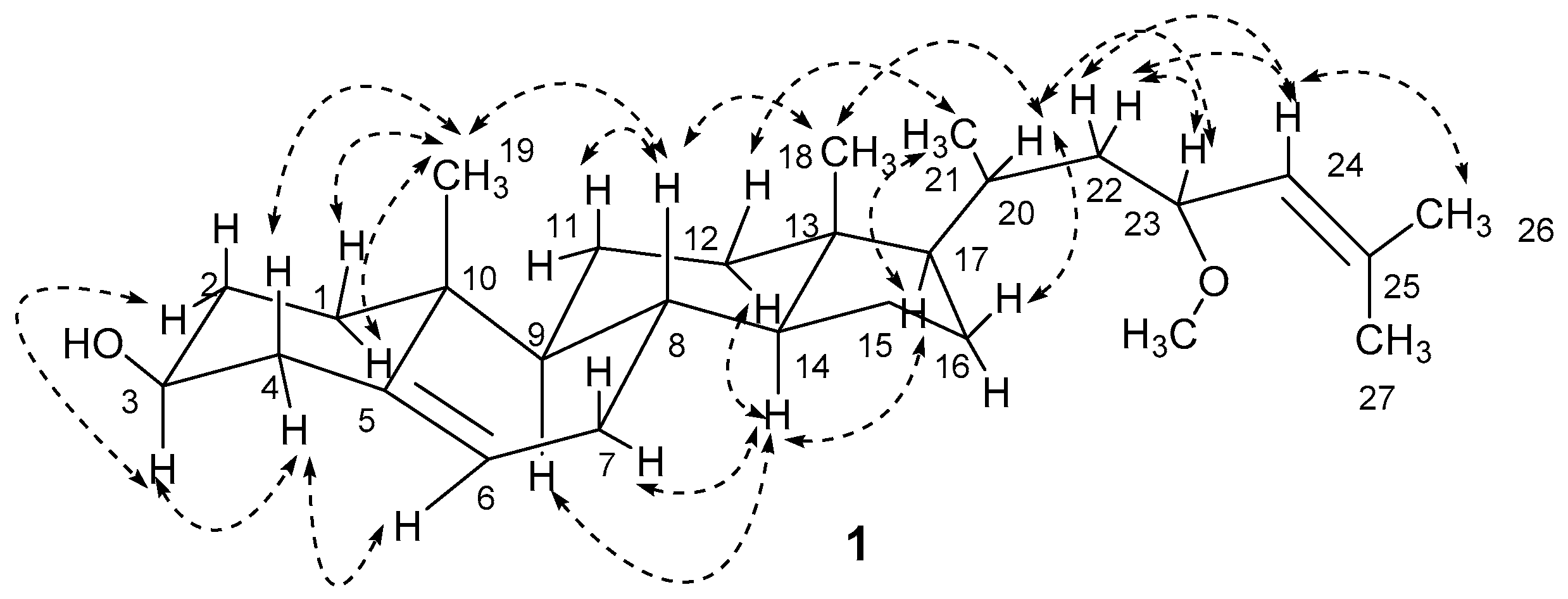
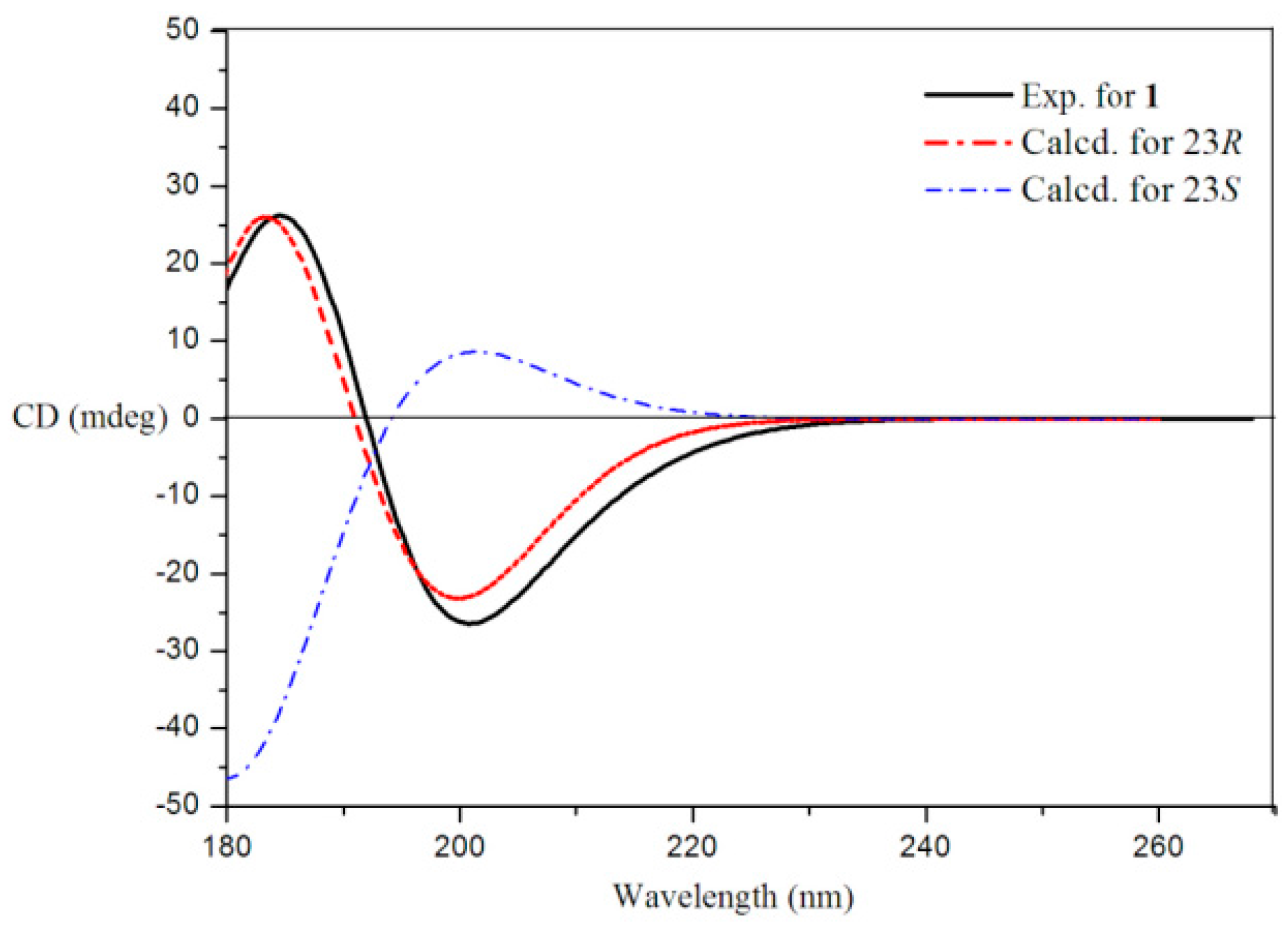

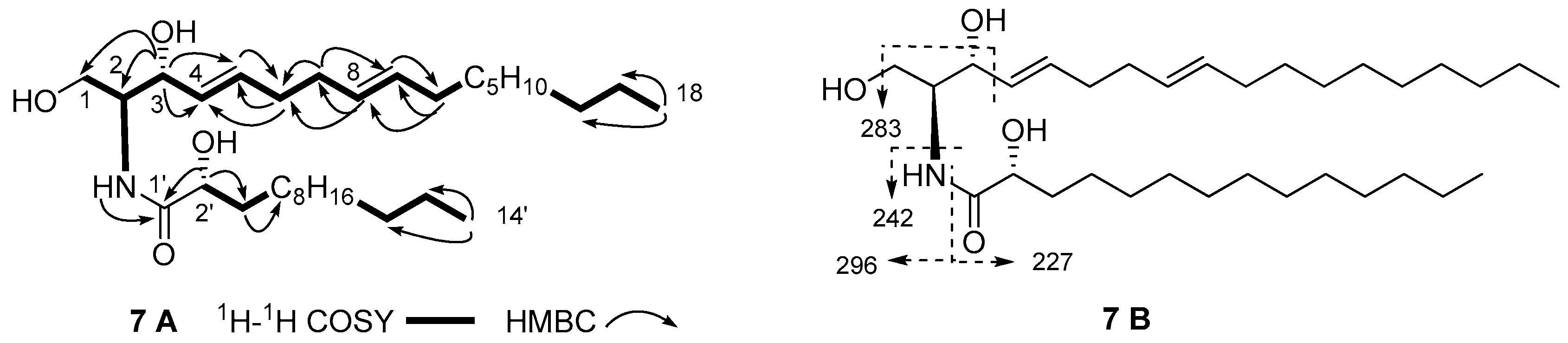
| No. | δC | δH mult., J in Hz | No. | δC | δH mult., J in Hz |
|---|---|---|---|---|---|
| 1 | 37.3 | α 1.86, m | 13 | 42.5 | – |
| β 1.08, m | 14 | 56.9 | 1.04, m | ||
| 2 | 31.7 | α 1.84, m | 15 | 24.3 | α 1.57, m |
| β 1.50, m | β 1.07, m | ||||
| 3 | 71.8 | 3.52, m | 16 | 28.2 | α 1.83, m |
| 4 | 42.3 | α 2.28, m | β 1.30, m | ||
| β 2.23, m | 17 | 56.8 | 1.01, m | ||
| 5 | 140.7 | – | 18 | 12.0 | 0.73, s |
| 6 | 121.7 | 5.35, t (2.5) | 19 | 19.4 | 1.00, s |
| 7 | 31.9 | α 1.50, m | 20 | 32.5 | 1.66, m |
| β 1.97, m | 21 | 18.7 | 0.95, d (5.9) | ||
| 8 | 31.9 | 1.50, m | 22 | 42.5 | α 0.92, m |
| β 1.68, m | |||||
| 9 | 50.1 | 0.93, m | 23 | 74.8 | 3.94, dt (9.3, 3.1) |
| 10 | 36.5 | – | 24 | 127.0 | 5.03, td (9.0, 2.6, 1.3) |
| 11 | 21.1 | α 1.43, m | 25 | 134.5 | – |
| β 1.49, m | 26 | 18.2 | 1.67, d (1.0) | ||
| 12 | 39.8 | α 1.16, m | 27 | 25.9 | 1.74, d (1.1) |
| β 2.03, m | 23-OCH3 | 55.7 | 3.21, s |
| No. | 6 | 7 | ||
|---|---|---|---|---|
| δC | δH mult., J in Hz | δC | δH mult., J in Hz | |
| 1 | 62.5 | 3.70, dd (11.3, 3.3) | 62.2 | 3.72, dd (11.0, 3.0) |
| 3.90, m | 3.89, m | |||
| 2 | 54.5 | 3.95, dd (11.3, 3.5) | 54.4 | 3.92, br d (11.0) |
| 3 | 74.7 | 4.32, m | 74.7 | 4.30, m |
| 4 | 129.2 | 5.54, dd (15.4, 6.4) | 129.2 | 5.52, dd (15.5, 6.0) |
| 5 | 133.6 | 5.78, dt (15.4, 6.6) | 133.5 | 5.75, dt (15.5, 6.1) |
| 6 | 32.3 | 1.97, q (6.7) | 32.4 | 1.96, q (6.5) |
| 7 | 32.1 | 2.12, m | 32.2 | 2.11, m |
| 8 | 131.4 | 5.42, dt (15.2, 6.3) | 131.3 | 5.41, m |
| 9 | 129.0 | 5.37, dt (15.2, 6.4) | 129.0 | 5.38, m |
| 10 | 32.6 | 2.08, m | 32.2 | 2.07, m |
| 11~15 | 29.2–29.7 | 1.26, br s | 29.2–29.7 | 1.26, br s |
| 16 | 31.9 | 1.26, br s | 32.0 | 1.26, br s |
| 17 | 22.7 | 1.26, br s | 22.7 | 1.26, br s |
| 18 | 14.1 | 0.88, t (6.8) | 14.1 | 0.87, t (7.0) |
| 1′ | 174.0 | – | 175.0 | – |
| 2′ | 36.9 | 2.23, t (7.5) | 72.4 | 4.12, dd (7.8, 3.4) |
| 3′ | 25.8 | 1.64, m | 34.8 | 2.16, t (3.3) |
| 4′ | 29.2–29.7 | 1.26, br s | 31.8 | 1.26, br s |
| 5′ | 29.2–29.7 | 1.26, br s | 25.1 | 1.63, m |
| 6′~11′ | 29.2–29.7 | 1.26, br s | 29.2–29.7 | 1.26, br s |
| 12′ | 31.9 | 1.26, br s | 31.9 | 1.26, br s |
| 13′ | 22.7 | 1.26, br s | 22.7 | 1.26, br s |
| 14′ | 14.1 | 0.88, t (6.8) | 14.1 | 0.87, t (7.0) |
| NH | – | 6.25, d (7.5) | – | 6.20, d (7.8) |
| Compound | HL-60 | HepG-2 | SGC-7901 |
|---|---|---|---|
| 1 | 17.64 ± 0.32 | 12.34 ± 0.12 | 18.37 ± 0.17 |
| 2 | >100 | >100 | >100 |
| 3 | >100 | >100 | >100 |
| 4 | >100 | >100 | >100 |
| 5 | 6.29 ± 0.11 | 4.12 ± 0.15 | 7.32 ± 0.26 |
| 6 | 32.26 ± 0.23 | 26.69 ± 0.21 | 27.14 ± 0.30 |
| 7 | 25.32 ± 0.17 | 21.13 ± 0.13 | 22.74 ± 0.16 |
| 8 | 35.72 ± 0.36 | 28.53 ± 0.24 | 30.31 ± 0.14 |
| 9 | 58.15 ± 0.28 | 46.21 ± 0.17 | 45.79 ± 0.12 |
| Adriamycin b | 2.51 ± 0.14 | 2.73 ± 0.23 | 2.65 ± 0.17 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.-R.; Gao, Y.-Q.; Tian, X.-L.; Li, J.; Tang, H.-F.; Li, Y.-S.; Lin, H.-W.; Ma, Z.-Q. New Cytotoxic Secondary Metabolites from Marine Bryozoan Cryptosula pallasiana. Mar. Drugs 2017, 15, 120. https://doi.org/10.3390/md15040120
Tian X-R, Gao Y-Q, Tian X-L, Li J, Tang H-F, Li Y-S, Lin H-W, Ma Z-Q. New Cytotoxic Secondary Metabolites from Marine Bryozoan Cryptosula pallasiana. Marine Drugs. 2017; 15(4):120. https://doi.org/10.3390/md15040120
Chicago/Turabian StyleTian, Xiang-Rong, Yan-Qing Gao, Xiao-Lin Tian, Jiao Li, Hai-Feng Tang, Yu-Shan Li, Hou-Wen Lin, and Zhi-Qing Ma. 2017. "New Cytotoxic Secondary Metabolites from Marine Bryozoan Cryptosula pallasiana" Marine Drugs 15, no. 4: 120. https://doi.org/10.3390/md15040120
APA StyleTian, X.-R., Gao, Y.-Q., Tian, X.-L., Li, J., Tang, H.-F., Li, Y.-S., Lin, H.-W., & Ma, Z.-Q. (2017). New Cytotoxic Secondary Metabolites from Marine Bryozoan Cryptosula pallasiana. Marine Drugs, 15(4), 120. https://doi.org/10.3390/md15040120