Metabolic Disorder in Chronic Obstructive Pulmonary Disease (COPD) Patients: Towards a Personalized Approach Using Marine Drug Derivatives
Abstract
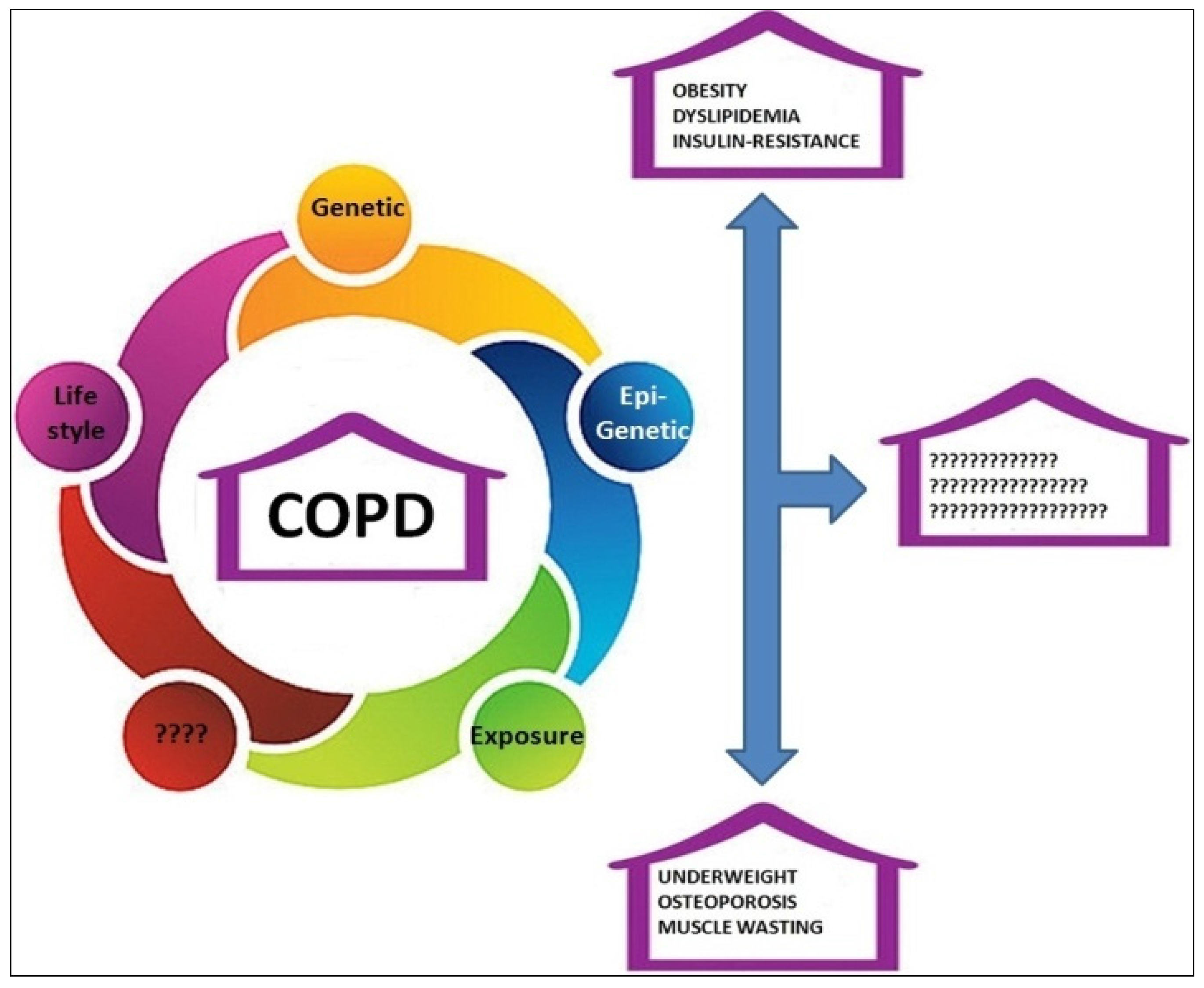
:1. Introduction
2. COPD and Obesity
3. Treatment of Obesity
Marine Drugs and Obesity and COPD
- Adipocyte fat storage and mobilization;
- Adipocyte oxidative metabolism through the stimulation of mitochondrial biogenesis and fatty acid oxidation;
- Adipocyte glucose utilization and insulin sensitivity (Akt phosphorylation);
- Secretion of adipokines; and
- Mitigation of adipose tissue inflammation through production of pro-inflammatory chemokines/cytokines, reduction of M1 macrophage infiltration/-6 derived pro-inflammatory lipid mediators production, being substrates for the formation of some specialized pro-resolving lipid mediator (SPMs), namely resolvins, protectins, and maresins.
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martinez, F.D. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2016, 375, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, P.G.; Agusti, A.; Roche, N.; Singh, D.; Martinez, F.J. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: Making progress towards personalised management. Lancet 2015, 385, 1789–1798. [Google Scholar] [CrossRef]
- Vanfleteren, L.E.; Spruit, M.A.; Wouters, E.F.; Franssen, F.M. Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir. Med. 2016, 4, 911–924. [Google Scholar] [CrossRef]
- Perret, J.L.; Walters, E.H.; Abramson, M.J.; McDonald, C.F.; Dharmage, S.C. The independent and combined effects of lifetime smoke exposures and asthma as they relate to COPD. Expert Rev. Respir. Med. 2014, 8, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Stoller, J.K.; Lacbawan, F.L.; Aboussouan, L.S. Alpha-1 Antitrypsin Deficiency. In GeneReviews® [Internet]; Pagon, R.A., Adam, M.P., Ardinger, H.H., Wallace, S.E., Amemiya, A., Bean, L.J.H., Bird, T.D., Ledbetter, N., Mefford, H.C., Smith, R.J.H., et al., Eds.; University of Washington: Seattle, WA, USA, 2006; pp. 1993–2017. [Google Scholar]
- Halldén, S.; Sjögren, M.; Hedblad, B.; Engström, G.; Hamrefors, V.; Manjer, J.; Melander, O. Gene variance in the nicotinic receptor cluster (CHRNA5-CHRNA3-CHRNB4) predicts death from cardiopulmonary disease and cancer in smokers. J. Intern. Med. 2016, 279, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Freathy, R.M.; Kazeem, G.R.; Morris, R.W.; Johnson, P.C.; Paternoster, L.; Ebrahim, S.; Hattersley, A.T.; Hill, A.; Hingorani, A.D.; Holst, C.; et al. Genetic variation at CHRNA5-CHRNA3-CHRNB4 interacts with smoking status to influence body mass index. Int. J. Epidemiol. 2011, 40, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.V.; Hallmans, G.; Hu, F.B.; Renström, F.; Franks, P.W. Smoking status, snus use, and variation at the CHRNA5-CHRNA3-CHRNB4 locus in relation to obesity: The GLACIER study. Am. J. Epidemiol. 2013, 178, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Glass, K.; Liu, Y.Y.; Silverman, E.K.; Crapo, J.D.; Tal-Singer, R.; Bowler, R.; Dy, J.; Cho, M.; Castaldi, P. COPD subtypes identified by network-based clustering of blood gene expression. Genomics 2016, 107, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.A.; Estépar, R.S.; Washko, G.R. Computed Tomographic Airway Morphology in Chronic Obstructive Pulmonary Disease. Remodeling or Innate Anatomy? Ann. Am. Thorac. Soc. 2016, 13, 4–9. [Google Scholar] [CrossRef] [PubMed]
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Baffi, C.W.; Wood, L.; Winnica, D.; Strollo, P.J., Jr.; Gladwin, M.T.; Que, L.G.; Holguin, F. Metabolic Syndrome and the Lung. Chest 2016, 149, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Marquis, K.; Maltais, F.; Duguay, V.; Bezeau, A.M.; LeBlanc, P.; Jobin, J.; Poirier, P. The metabolic syndrome in patients with chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. 2005, 25, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Cebron Lipovec, N.; Beijers, R.J.; van den Borst, B.; Doehner, W.; Lainscak, M.; Schols, A.M. The Prevalence of Metabolic Syndrome in Chronic Obstructive Pulmonary Disease: A Systematic Review. COPD 2016, 3, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kisiliaou, A.; Lococo, F.; Prinzi, G.; Fini, M.; Bonassi, S.; Russo, P. Pharmacological Management of Chronic Obstructive Lung Disease (COPD) in the Post-Genome Era. Evidence from a Real-World Perspective. Curr. Med. Chem. 2017. accepted. [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease. Available online: http://www.goldcopd.it/materiale/2015/GOLD_Pocket_2015.pdf (accessed on 24 October 2016).
- Carcache de Blanco, E. Review of Drugs of Natural Origin. In A Treatise of Pharmacognosy, 7th Revised ed.; Samuelsson, G., Bohlin, L., Eds.; Apotekarsocieteten, Swedish Pharmaceutical Society: Stockholm, Sweden, 2015; pp. 1–807. [Google Scholar]
- Li, J.W.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, G.; Busnelli, M.; Manzini, S.; Parolini, C. Nutraceuticals and Bioactive Components from Fish for Dyslipidemia and Cardiovascular Risk Reduction. Mar. Drugs. 2016, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Todorčević, M.; Hodson, L. The Effect of Marine Derived n-3 Fatty Acids on Adipose Tissue Metabolism and Function. J. Clin. Med. 2015, 5, E3. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, L.; Laiglesia, L.M.; Huerta, A.E.; Martínez, J.A.; Moreno-Aliaga, M.J. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 2015, 121, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; Montes de Oca, M.; Mendez, R.A.; Pinto Plata, V.; Cabral, H.J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rio, F.; Soriano, J.B.; Miravitlles, M.; Muñoz, L.; Duran-Tauleria, E.; Sánchez, G.; Sobradillo, V.; Ancochea, J. Impact of obesity on the clinical profile of a population-based sample with chronic obstructive pulmonary disease. PLoS ONE 2014, 9, e105220. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, D.E.; Ciavaglia, C.E.; Neder, J.A. When obesity and chronic obstructive pulmonary disease collide. Physiological and clinical consequences. Ann. Am. Thorac. Soc. 2014, 11, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Joppa, P.; Tkacova, R.; Franssen, F.M.; Hanson, C.; Rennard, S.I.; Silverman, E.K.; McDonald, M.L.; Calverley, P.M.; Tal-Singer, R.; Spruit, M.A.; et al. Sarcopenic Obesity Functional Outcomes and Systemic Inflammation in Patients With Chronic Obstructive Pulmonary Disease. J. Am. Med. Dir. Assoc. 2016, 17, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in bodymass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Lambert, A.A.; Putcha, N.; Drummond, M.B.; Boriek, A.M.; Hanania, N.A.; Kim, V.; Kinney, G.L.; McDonald, M.N.; Brigham, E.P.; Wise, R.A.; et al. Obesity is Associated with Increased Morbidity in Moderate to Severe COPD. Chest 2017, 151, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Chittal, P.; Babu, A.S.; Lavie, C.J. Obesity paradox: Does fat alter outcomes in chronic obstructive pulmonary disease? COPD 2015, 12, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Çolak, Y.; Afzal, S.; Lange, P.; Nordestgaard, B.G. High body mass index and risk of exacerbations and pneumonias in individuals with chronic obstructive pulmonary disease: Observational and genetic risk estimates from the Copenhagen General Population Study. Int. J. Epidemiol. 2016, 45, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Nigro, E.; Monaco, M.L.; Matera, M.G.; Scudiero, O.; Mazzarella, G.; Daniele, A. The burden of obesity in asthma and COPD: Role of adiponectin. Pulm. Pharmacol. Ther. 2017, 43, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Makita, H.; Östling, J.; Thomsen, L.H.; Konno, S.; Nagai, K.; Shimizu, K.; Pedersen, J.H.; Ashraf, H.; Bruijnzeel, P.L.; et al. Lower leptin/adiponectin ratio and risk of rapid lung function decline in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2014, 11, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.M.; Jeong, B.H.; Woo, S.Y.; Kim, S.Y.; Kim, H.; Lee, J.H.; Lim, S.Y.; Rhee, C.K.; Yoo, K.H.; Lee, J.H.; et al. Association of plasma adipokines with chronic obstructive pulmonary disease severity and progression. Ann. Am. Thorac. Soc. 2015, 12, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Pajvani, U.B.; Du, X.; Combs, T.P.; Berg, A.H.; Rajala, M.W.; Schulthess, T.; Engel, J.; Brownlee, M.; Scherer, P.E. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J. Biol. Chem. 2003, 278, 9073–9085. [Google Scholar] [CrossRef] [PubMed]
- Tsao, T.S.; Murrey, H.E.; Hug, C.; Lee, D.H.; Lodish, H.F. Oligomerization state-dependent activation of NF-κB signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30). J. Biol. Chem. 2002, 277, 29359–29362. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Kit, B.K.; Ogden, C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012, 307, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.M. The Fat Controller: Adipocyte Development. PLoS Biol. 2012, 10, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Berrigan, D.; Troiano, R.P.; Graubard, B.I. BMI and mortality: The limits of epidemiological evidence. Lancet 2016, 388, 734–736. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 24 October 2016).
- Cawley, J.; Meyerhoefer, C.; Biener, A.; Hammer, M.; Wintfeld, N. Savings in Medical Expenditures Associated with Reductions in Body Mass Index Among US Adults with Obesity, by Diabetes Status. Pharmacoeconomics 2015, 33, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Assfalg, M.; Bertini, I.; Colangiuli, D.; Luchinat, C.; Schäfer, H.; Schütz, B.; Spraul, M. Evidence of different metabolic phenotypes in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Ghini, V.; Saccenti, E.; Tenori, L.; Assfalg, M.; Luchinat, C. Allostasis and Resilience of the Human Individual Metabolic Phenotype. J. Proteome Res. 2015, 14, 2951–2962. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Makowski, L. Nutrition and metabolic correlates of obesity and inflammation: Clinical considerations. J. Nutr. 2015, 145, 1131S–1136S. [Google Scholar] [CrossRef] [PubMed]
- Koen, N.; Du Preez, I.; Loots, D.T. Metabolomics and Personalized Medicine. Adv. Protein Chem. Struct. Biol. 2016, 102, 53–78. [Google Scholar] [PubMed]
- Kim, G.W.; Lin, J.E.; Blomain, E.S.; Waldman, S. New advances in models and strategies for developing anti-obesity drugs. Expert Opin. Drug Discov. 2013, 8, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.H.; Shukla, A.P.; Igel, L.I.; Kumar, R.B.; Aronne, L. Pharmacotherapy for Obesity. Endocrinol. Metab. Clin. N. Am. 2016, 45, 521–538. [Google Scholar] [CrossRef] [PubMed]
- FDA. Press Announcements. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm (accessed on 24 October 2016).
- EMA. News and Event. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2015/01/news_detail_002255.jsp&mid=WC0b01ac058004d5c1 (accessed on 24 October 2016).
- Jia, W.; Gao, W.Y.; Yan, Y.Q.; Wang, J.; Xu, Z.H.; Zheng, W.J.; Xiao, P.G. The rediscovery of ancient Chinese herbal formulas. Phytother. Res. 2004, 18, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Preto, M.; Vasconcelos, V.; Urbatzka, R. Obesity: The Metabolic Disease, Advances on Drug Discovery and Natural Product Research. Curr. Top. Med. Chem. 2016, 16, 2577–2604. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Lorusso, D.; Italiano, A.; Kaye, S.B.; Aracil, M.; Tanović, A.; D’Incalci, M. Trabectedin as a chemotherapy option for patients with BRCA deficiency. Cancer Treat. Rev. 2016, 50, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Sousa, E.; Kijjoa, A.; Pinto, M.M. Marine Natural Products as Models to Circumvent Multidrug Resistance. Molecules 2016, 21, 892. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Cesario, A. New anticancer drugs from marine cyanobacteria. Curr. Drug Targets 2012, 13, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Del Bufalo, A.; Fini, M. Deep sea as a source of novel-anticancer drugs: Update on discovery and preclinical/clinical evaluation in a systems medicine perspective. EXCLI J. 2015, 14, 228–236. [Google Scholar] [PubMed]
- Russo, P.; Kisialiou, A.; Lamonaca, P.; Moroni, R.; Prinzi, G.; Fini, M. New Drugs from Marine Organisms in Alzheimer’s Disease. Mar. Drugs 2015, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Nastrucci, C.; Cesario, A.; Russo, P. New anticancer drugs from marine cyanobacteria. Anticancer drug discovery from the marine environment. Recent Pat. Anticancer Drug Discov. 2012, 7, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Label. FDA. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021654s041lbl.pdf (accessed on 24 October 2016).
- Gold, D.R.; Litonjua, A.A.; Carey, V.J.; Manson, J.E.; Buring, J.E.; Lee, I.M.; Gordon, D.; Walter, J.; Friedenberg, G.; Hankinson, J.L.; et al. Lung VITAL: Rationale, design, and baseline characteristics of an ancillary study evaluating the effects of vitamin D and/or marine omega-3 fatty acid supplements on acute exacerbations of chronic respiratory disease, asthma control, pneumonia and lung function in adults. Contemp. Clin Trials 2016, 47, 185–195. [Google Scholar] [PubMed]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Griinari, M.; Berge, K.; Vik, H.; Hubacher, R.; Rains, T.M. Krill oil supplementation increases plasma concentrations of eicosapentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutr. Res. 2009, 9, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Banni, S.; Carta, G.; Murru, E.; Cordeddu, L.; Giordano, E.; Sirigu, A.R.; Berge, K.; Vik, H.; Maki, K.C.; Di Marzo, V.; et al. Krill oil significantly decreases 2-arachidonoylglycerol plasma levels in obese subjects. Nutr. Metab. 2011, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Höper, A.C.; Salma, W.; Khalid, A.M.; Hafstad, A.D.; Sollie, S.J.; Raa, J.; Larsen, T.S.; Aasum, E. Oil from the marine zooplankton Calanus finmarchicus improves the cardiometabolic phenotype of diet-induced obese mice. Br. J. Nutr. 2013, 110, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Höper, A.C.; Salma, W.; Sollie, S.J.; Hafstad, A.D.; Lund, J.; Khalid, A.M.; Raa, J.; Aasum, E.; Larsen, T.S. Wax esters from the marine copepod Calanus finmarchicus reduce diet-induced obesity and obesity-related metabolic disorders in mice. J. Nutr. 2014, 144, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Abdjul, D.B.; Kanno, S.; Yamazaki, H.; Ukai, K.; Namikoshi, M.A. Dimeric urea of the bisabolene sesquiterpene from the Okinawan marine sponge Axinyssa sp. inhibits protein tyrosine phosphatase 1B activity in Huh-7 human hepatoma cells. Bioorg. Med. Chem. Lett. 2016, 26, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Takahashi, O.; Kanno, S.; Nakazawa, T.; Takahashi, S.; Ukai, K.; Sumilat, D.A.; Ishikawa, M.; Namikoshi, M. Absolute structures and bioactivities of euryspongins and eurydiene obtained from the marine sponge Euryspongia sp. collected at Iriomote Island. Bioorg. Med. Chem. 2015, 23, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.F.; Wang, T.; Cai, Y.S.; He, W.F.; Sun, P.; Li, Y.F.; Huang, Q.; Taglialatela-Scafati, O.; Wang, H.Y.; Guo, Y.W. Brominated polyunsaturated lipids from the Chinese sponge Xestospongia testudinaria as a new class of pancreatic lipase inhibitors. Eur. J. Med. Chem. 2014, 79, 290–297. [Google Scholar] [CrossRef] [PubMed]
- He, W.F.; Xue, D.Q.; Yao, L.G.; Li, J.; Liu, H.L.; Guo, Y.W. A new bioactive steroidal ketone from the South China Sea sponge Xestospongia testudinaria. J. Asian Nat. Prod. Res. 2016, 18, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.A.; Rae, J.; Fontaine, F.; Conte, M.M.; Khalil, Z.; Martin, S.; Parton, R.G.; Capon, R.J. Heterofibrins: Inhibitors of lipid droplet formation from a deep-water southern Australian marine sponge, Spongia (Heterofibria) sp. Org. Biomol. Chem. 2010, 8, 3188–3194. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, Q.; Yu, Z.; Zhang, Y.; Guo, Y.; Chen, K.; Shen, X.; Jiang, H. Hyrtiosal, a PTP1B inhibitor from the marine sponge Hyrtios erectus, shows extensive cellular effects on PI3K/AKT activation, glucose transport, and TGFbeta/Smad2 signaling. ChemBioChem 2007, 8, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Byun, M.R.; Lee, C.H.; Hwang, J.H.; Kim, A.R.; Moon, S.A.; Sung, M.K.; Roh, J.R.; Hwang, E.S.; Hong, J.H. Phorbaketal A inhibits adipogenic differentiation through the suppression of PPARγ-mediated gene transcription by TAZ. Eur. J. Pharmacol. 2013, 718, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Lee, K.T.; Rho, J.R.; Choi, J.H. Phorbaketal A, Isolated from the Marine Sponge Phorbas sp., Exerts Its Anti-Inflammatory Effects via NF-κB Inhibition and Heme Oxygenase-1 Activation in Lipopolysaccharide-Stimulated Macrophages. Mar. Drugs 2015, 13, 7005–7019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Guo, Y.; Jiang, H.; Shen, X. A sesquiterpenequinone, dysidine, from the sponge Hyrtios erectus, activates the insulin pathway through inhibition of PTPases. Acta Pharmacol. Sin. 2009, 30, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Sepe, V.; D’Amore, C.; Ummarino, R.; Renga, B.; D’Auria, M.V.; Novellino, E.; Sinisi, A.; Taglialatela-Scafati, O.; Nakao, Y.; Limongelli, V.; et al. Insights on pregnane-X-receptor modulation. Natural and semisynthetic steroids from Theonella marine sponges. Eur. J. Med. Chem. 2014, 73, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, T.; Choi, J.; Sukbong, V.; Lee, J.; Park, J.; Jang, B. Inhibition of adipogenesis and leptin production in 3T3-L1 adipocytes by a derivative of meridianin C. Biochem. Biophys. Res. Commun. 2014, 452, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Kim, S.M. α-Glucosidase inhibitory activities of fatty acids purified from the internal organ of sea cucumber Stichopus japonicas. J. Food Sci. 2015, 80, H841–H847. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Nishikawa, S.; Beppu, F.; Tsukui, T.; Abe, M.; Hosokawa, M. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J. Sci. Food Agric. 2011, 91, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch. Biochem. Biophys. 2010, 504, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Hosokawa, M.; Yim, M.J.; Shinoda, T.; Miyashita, K. Down-regulation of hepatic stearoyl-CoA desaturase-1 expression by fucoxanthin via leptin signaling in diabetic/obese KK-A(y) mice. Lipids 2013, 48, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hosokawa, M.; Matsukawa, N.; Hagio, M.; Shinoki, A.; Nishimukai, M.; Miyashita, K.; Yajima, T.; Hara, H. Suppressive effects of the marine carotenoids.; fucoxanthin and fucoxanthinol on triglyceride absorption in lymph duct-cannulated rats. Eur. J. Nutr. 2010, 49, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Kanno, S.; Kodate, M.; Hosokawa, M.; Miyashita, K. Fucoxanthinol, Metabolite of Fucoxanthin, Improves Obesity-Induced Inflammation in Adipocyte Cells. Mar. Drugs 2015, 13, 4799–4813. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Kim, M.S.; Choi, J.S. Anti-adipogenic activity of the edible brown alga Ecklonia stolonifera and its constituent fucosterol in 3T3-L1 adipocytes. Arch. Pharm. Res. 2014, 37, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.H.; Wu, Y.X.; Kim, J.S.; Woo, J.H.; Park, K.T.; Kwon, O.; Seo, H.J.; Kim, T.; Park, N.H. 6,6′-Bieckol inhibits adipocyte differentiation through downregulation of adipogenesis and lipogenesis in 3T3-L1 cells. J. Sci. Food Agric. 2015, 95, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- BelHadj, S.; Hentati, O.; Elfeki, A.; Hamden, K. Inhibitory activities of Ulva lactuca polysaccharides on digestive enzymes related to diabetes and obesity. Arch. Physiol. Biochem. 2013, 119, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.M.; Cha, K.H. Evaluation of the anti-obesity effect of the microalga Phaeodactylum tricornutum. Appl. Biol. Chem. 2016, 59, 283. [Google Scholar] [CrossRef]
- Arunkumar, E.; Bhuvaneswari, S.; Anuradha, C.V. An intervention study in obese mice with astaxanthin, a marine carotenoid-effects on insulin signaling and pro-inflammatory cytokines. Food Funct. 2012, 3, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; De Lutiis, M.A.; Patruno, A.; Grilli, A.; Felaco, M.; Speranza, L. Astaxanthin treatment confers protection against oxidative stress in U937 cells stimulated with lipopolysaccharide reducing O2− production. PLoS ONE 2014, 9, e88359. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of astaxanthin in obese mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2007, 71, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.H.; Lee, Y.R.; Lee, D.S.; Kim, Y.C.; Oh, H. PTP1B inhibitory secondary metabolites from marine-derived fungal strains Penicillium spp. and Eurotium sp. J. Microbiol. Biotechnol. 2013, 23, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Kawazoe, Y.; Iwasaki, A.; Ohno, O.; Suenaga, K.; Uemura, D. Anti-obesity activities of the yoshinone A and therelated marine γ-pyrone compounds. J. Antibiot. 2016, 69, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Thiebaut, P.A.; Besnier, M.; Gomez, E.; Richard, V. Role of protein tyrosine phosphatase 1B in cardiovascular diseases. J. Mol. Cell. Cardiol. 2016, 101, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Breyer, M.K.; Rutten, E.P.; Vernooy, J.H.; Spruit, M.A.; Dentener, M.A.; van der Kallen, C.; van Greevenbroek, M.M.; Wouters, E.F. Gender differences in the adipose secretome system in chronic obstructive pulmonary disease (COPD): A pivotal role of leptin. Respir. Med. 2011, 105, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Breyer, M.K.; Rutten, E.P.; Locantore, N.W.; Watkins, M.L.; Miller, B.E.; Wouters, E.F.; ECLIPSE Investigators (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints). Dysregulated adipokine metabolism in chronic obstructive pulmonary disease. Eur. J. Clin. Investig. 2012, 42, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Lococo, F.; Cesario, A.; Del Bufalo, A.; Ciarrocchi, A.; Prinzi, G.; Mina, M.; Bonassi, S.; Russo, P. Novel therapeutic strategy in the management of COPD: A systems medicine approach. Curr. Med. Chem. 2015, 22, 3655–3675. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrero, D.; Menche, J.; Vargas, C.; Cano, I.; Maier, D.; Barabási, A.L.; Tegnér, J.; Roca, J.; Synergy-COPD Consortia. From comorbidities of chronic obstructive pulmonary disease to identification of shared molecular mechanisms by data integration. BMC Bioinform. 2016, 17 (Suppl. 15), 441. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, A.J.; Sapey, E. Oxidative Stress in COPD. Markers and Potential Mechanisms. J. Clin. Med. 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- McMurray, F.; Patten, D.A.; Harper, M.E. Reactive Oxygen Species and Oxidative Stress in Obesity-Recent Findings and Empirical Approaches. Obesity 2016, 24, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Hallman, A.H.; Roberts, W.J.; Wagner, P.D.; Hogan, M.C. Superoxide release from contracting skeletal muscle in pulmonary TNF-α overexpression mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R75–R81. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Shiah, A.; Roberts, W.J.; Chien, M.T.; Wagner, P.D.; Hogan, M.C. Low Po2 conditions induce reactive oxygen species formation during contractions in single skeletal muscle fibers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R1009–R1016. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.G.; Provoost, S.; Bracke, K.R.; Kuchmiy, A.; Lamkanfi, M. Inflammasomes in respiratory disease: From bench to bedside. Chest 2014, 145, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Robbins, G.R.; Wen, H.; Ting, J.P. Inflammasomes and metabolic disorders: Old genes in modern diseases. Mol. Cell 2014, 54, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Stehlik, C.; Lee, S.H.; Dorfleutner, A.; Stassinopoulos, A.; Sagara, J.; Reed, J.C. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J. Immunol. 2003, 171, 6154–6163. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; O’Neill, L.; Schroder, K. Questions and controversies in innate immune research: What is the physiological role of NLRP3? Cell Death Discov. 2016, 2, 16019. [Google Scholar] [CrossRef] [PubMed]
- Traba, J.; Sack, M.N. The role of caloric load and mitochondrial homeostasis in the regulation of the NLRP3 inflammasome. Cell. Mol. Life Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kursawe, R.; Dixit, V.D.; Scherer, P.E.; Santoro, N.; Narayan, D.; Gordillo, R.; Giannini, C.; Lopez, X.; Pierpont, B.; Nouws, J.; et al. A Role of the Inflammasome in the Low Storage Capacity of the Abdominal Subcutaneous Adipose Tissue in Obese Adolescents. Diabetes 2016, 65, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.K.; Gibson, P.G.; Simpson, J.L.; McDonald, V.M.; Wood, L.G.; Baines, K.J. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology 2016, 21, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.D.; van Geffen, W.H.; Jonker, M.R.; Kerstjens, H.A.; Nawijn, M.C.; Heijink, I.H. Increased neutrophil expression of pattern recognition receptors during COPD exacerbations. Respirology 2017, 22, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; LeVan, T. Obesity and chronic obstructive pulmonary disease: Recent knowledge and future directions. Curr. Opin. Pulm. Med. 2017, 23, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Dreamstime. Available online: https://www.dreamstime.com/photos-images/safe.html (accessed on 10 February 2017).
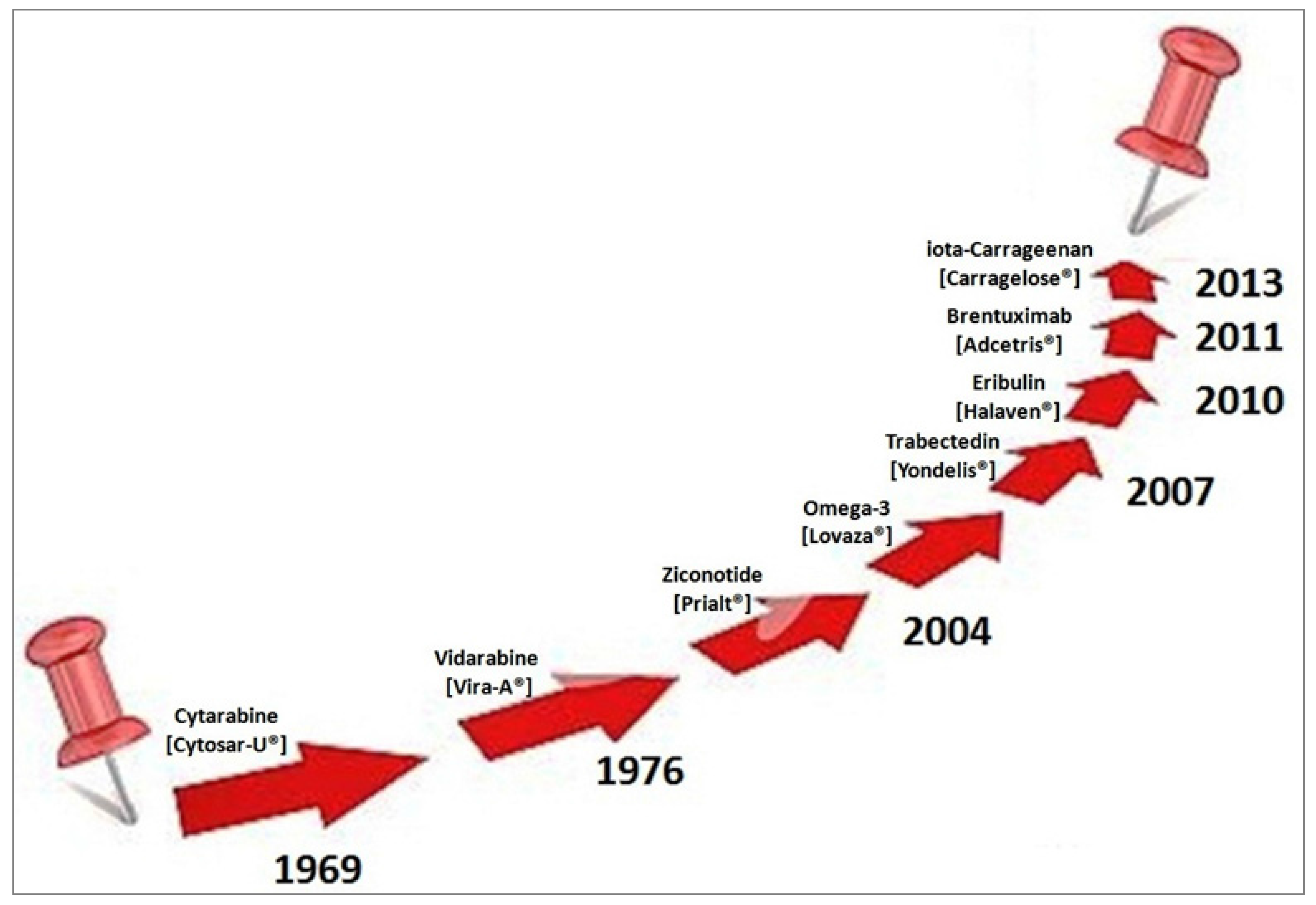








| Drug | Systematic (IUPAC) Name | Indication/Mechanism |
|---|---|---|
| Cytarabine [Cytosar-U®] ATC code: L01BC01 Source: Cryptothecacrypta Phylum: Bryophyta Class:Bryopsida | 4-amino-1-[(2R,3S,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl] pyrimidin-2-one [C9H13N3O5] | Anticancer DNA synthesis interference |
| Eribulin [Halaven®] ATC code: L01XX41 Synthetic macrocyclic analogue of halichondrin B Source: Halichondria okadai Phylum: Porifera Class: Demospongiae | 2-(3-Amino-2-hydroxypropyl)hexacosahydro-3-methoxy-26-methyl-20,27-bis(methylene)11,15-18,21-24,28-triepoxy-7,9-ethano-12,15-methano-9H,15H-furo(3,2-i)furo(2′,3′-5,6) pyrano(4,3-b)(1,4)dioxacyclopentacosin-5-(4H)-one [C40H59NO11] | Anticancer Microtubule dynamics inhibitor |
| Trabectedin [Yondelis®] ATC code: L01CX01 Source: Ecteinascidia turbinate Phylum: Chordata Class: Ascidiacea | (1′R,6R,6aR,7R,13S,14S,16R)-6′,8,14-trihydroxy-7′,9-dimethoxy-4,10,23-trimethyl-19-oxo-3′,4′,6,7,12,13,14,16-octahydrospiro[6,16-(epithiopropano-oxymethano)-7,13-imino-6aH-1,3-dioxolo[7,8]isoquino[3,2-b][3]benzazocine-20,1′(2′H)-isoquinolin]-5-yl acetate [C39H43N3O11S] | Anticancer DNA binding and alkylation at the N2 position of G causing DNA bending toward the major groove. Interfering activated transcription, transcription-coupled nucleotide excision repair (TCR) complex poisoning, RNA polymerase degradation, DNA double-strand breaks generation |
| Brentuximab [Adcetris®] ATC code: L01XC12 synthetic dolastatin 10 Source: Dolabella auricularia Phylum: Mollusca Class: Gastropoda | Antibody-monomethyl auristatin Econjugate [C6476H9930N1690O2030S40 (C68H105N11O15)3–5] | Anticancer Tubulin polymerizationblock |
| Ziconotide [Prialt®] ATC code: N02BG08 Source: Conus magus. Phylum: Mollusca Class: Gastropoda | Peptide: H-Cys-Lys-Gly-Lys-Gly-Ala-Lys-Cys-Ser-Arg-Leu-Met-Tyr-Asp-Cys-Cys-Thr-Gly-Ser-Cys-Arg-Ser-Gly-Lys-Cys-NH2 [C102H172N36O32S7] | Anti-pain Selective N-type voltage-gated calcium channel blocker |
| Vidarabine [Vira-A®] ATC code: J05AB03 Source: Tectitethya crypta Phylum: Porifera Class: Demospongiae | (2R,3S,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol hydrate [C10H15N5O5] | Antiviral Viral DNA polymerase inhibitor/substrate |
| iota-Carrageenan [Carragelose®] Source: Eucheuma denticulatum Phylum: Rhodophyta Class: Florideophyceae | A family of linear sulfated polysaccharides | Antiviral |
| Omega-3[Lovaza®] Source: oil of several fish sources | Omega-3-acid ethyl esters (ethyl esters of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) EPA ethyl ester: [C22H34O2] DHA ethyl ester: [C24H36O2] | Hypertriglyceridemia Adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia (HTG). Increased breakdown of fatty acids; inhibition of diglyceride acyltransferase which is involved in biosynthesis of triglycerides in the liver; and increased activity of lipoprotein lipase in blood |
| CRUSTACEAN Phylum: Arthropoda | |||
| Source | Drug | Target and Activity | Reference |
| Euphausia superb | Eicosapentaenoic acid (EPA) & Docosahexaenoic acid (DHA) (krill oil) | Randomized, double-blind parallel arm trial, overweight and obese men and women (n = 76) were randomly assigned to receive double-blind capsules containing 2 g/day of krill oil, menhaden oil, or control (olive) oil for 4 weeks. Plasma EPA and DHA concentrations increased significantly more in the krill oil groups than in the control group.Well tolerated, with no indication of adverse effects on safety parameters. Reduced body weight gain, abdominal fat, and liver triacylglycerol on diet-induced obese mice | [62,63] |
| Calanus finmarchicus | Wax ester component of Calanus oil = PUFAs | On diet-induced obesity and obesity-related disorders in mice. C57BL/6J mice fed a high-fat diet (HFD, 45% energy from fat) reduced body-weight gain, abdominal fat accumulation and hepatic steatosis and improved glucose tolerance Calanus oil supplementation reduced adipocyte size and increased the mRNA expression of adiponectin in adipose tissue. It also reduced macrophage infiltration accompanied by reduced mRNA expression of pro-inflammatory cytokines (TNF-α, IL-6 and monocyte chemotactic protein-1) | [64,65] |
| SPONGES Phylum: Porifera | |||
| Source | Drug | Target and Activity | Reference |
| Axinyssa sp. | N,N′-bis[(6R,7S)-7-amino-7,8-dihydro-α-bisabolen-7-yl]urea | Protein tyrosine phosphatase 1B (PTP1B) inhibitor. Enhances the insulin-stimulated phosphorylation levels of Akt in Huh-7 human hepatoma cells | [66] |
| Euryspongia sp. | Dehydroeuryspongin A | Protein tyrosine phosphatase 1B (PTP1B) inhibition at IC50 = 3.58 μM | [67] |
| Xestospongia testudinaria | Xestonarienes A-HNew steroidal ketone with an ergosta-22,25-diene side chain | Pancreatic lipase (PL) inhibition IC50 = 3.11 μM. Decrease in the plasma triglyceride level following an oral lipid challenge in C57BLKS/J male mice | [68,69] |
| Protein tyrosine phosphatase 1B (PTP1B) inhibition IC50 value = 4.27 ± 0.55 μM | [70] | ||
| Heterofibria | Fatty acids heterofibrins A1 & B1 possessing a diyne-ene moiety | Lipid droplet formation inhibition in A431 fibroblast cell lines | |
| Hyrtios erectus | Hyrtiosal | Protein tyrosine phosphatase 1B (PTP1B) inhibition with an IC50 = 42 μM in a noncompetitive inhibition mode enhances the membrane translocation of the key glucose transporter Glut4 in PTP1B-overexpressed CHO cells facilitate insulin inhibition of Smad2 activation through the PI3K/AKT pathway | [71] |
| Phorbas sp. | Phorbaketal A (tricyclic sesterterpenoid) | Adipogenic differentiation inhibition as indicated by less fat droplets and decreased expression of adipogenic marker genes. The expression of TAZ (transcriptional coactivator with PDZ-binding motif Phorbaketal A increased the interaction of TAZ and PPARγ to suppress PPARγ (peroxisome proliferator-activated receptor γ) target gene expression | [72] |
| Inhibits the production of inflammatory mediators via down-regulation of the of nuclear factor-kappaB (NF-κB), pathway and up-regulation of the heme oxygenase-1 (HO-1) system in LPS-stimulated RAW 264.7 macrophage cells | [73] | ||
| Dysidea villosa | Dysidine (sesquiterpene quinine) | Differentiated 3T3-L1 cells and resulted in the increased deposition of Glucose transporter type 4 (GLUT4) in the cellular membrane | [74] |
| Theonella sp. | 4-methylenesteroid derivativesconicasteroland heonellasterol) | Pregnane-X-receptor (PXR) modulators PXR is a gene involved in the bilirubin, bile acids, glucose and lipids metabolism | [75] |
| TUNICATES Phylum: Chordata (sub-Phylum: Tunicata) | |||
| Source | Drug | Target and Activity | Reference |
| Aplidium meridianum | Meridianin C derivatives (indole alkaloids) | Inhibition lipid accumulation during 3T3-L1 pre-adipocyte differentiation and lowered leptin expression it influences important differentiation pathways as C/EBP-α, PPARγ and fatty acid synthase | [76] |
| ECHINODERM Phylum: Echinodermata | |||
| Source | Drug | Target and Activity | Reference |
| Stichopus japonicas | 1,3-Dipalmitolein & cis-9-octadecenoic acid | α-Glucosidase inhibitors in Saccharomyces cerevisiae IC50 = 4.45 and 14.87 μM | [77] |
| ALGAE Phylum: Euglenozoa | |||
| Source | Drug | Target and Activity | Reference |
| Undaria pinnati fida, Laminaria japonica (macroalgae, brown seaweeds) & Cylindrotheca closterium (microalgae) | Fucoxanthin | Induces uncoupling protein 1 (UCP1) in abdominal white adipose tissue (WAT) mitochondria, leading to the oxidation of fatty acids and heat production in WAT regulation of cytokine secretions from both abdominal adipose cells and macrophage cells infiltrated into adipose tissue | [78,79] |
| Regulates mRNA expression of inflammatory adipocytokines involved in insulin resistance, iNOS, and COX-2 in WAT and has specific effects on diabetic/obese KK-A(y) mice, but not on lean C57BL/6J mice | [80,81] | ||
| Inhibits lipase activity in the gastrointestinal lumen and suppress triglyceride absorption, and fucoxanthin was converted to fucoxanthinol in the intestine and released into the lymph in conscious rats | [82] | ||
| Fucoxanthin upregulates the expression of uncoupling protein 1 (UCP1) and adipokine mRNA in white adipose tissue (WAT) of diabetic/obese KK-A(y) mice | [83] | ||
| Down-regulates SCD1 expression and alters fatty acid composition of the liver via regulation of leptin signaling in hyperleptinemia KK-A(y) mice but not in leptin-deficient ob/ob mice | [84] | ||
| Ecklonia stolonifera (brown algae) | Fucoxanthinol/Fucoxanthin Metabolite | 3T3-L1 adipocyte cells and a RAW264.7 macrophage cell co-culture system. A diet containing 0.1% Fx was fed to diabetic model KK-Ay mice for three weeks Fx diet significantly improved glucose tolerance compared with the control diet group.In in vitro studies, FxOH showed suppressed tumor necrosis factor-α (TNF-α), and monocyte chemotactic protein-1 (MCP-1) mRNA expression and protein levels in a co-culture of adipocyte and macrophage cells | [85] |
| Inhibits expression of PPARγ and C/EBPα, resulting in a decrease of lipid accumulation in 3T3-L1 pre-adipocytes,3T3-L1 pre-adipocytes differentiation | [86] | ||
| ALGAE Phylum: Euglenozoa | |||
| Source | Drug | Target and Activity | Reference |
| Eisenia bicyclis (brown algae) | 6,6′-bieckol | Decreased lipid accumulation and expression levels of peroxisome proliferator-activated receptor γ (PPARγ), CCATT/enhancer-binding protein α (C/EBPα) and sterol regulatory element binding protein-1c (SREBP-1c) (mRNA and protein), and fatty acid synthase and acyl-coA carboxylase (mRNA). inhibition of differentiation of 3T3-L1 adipocytes | [87] |
| Ulva lactuca | Ulva lactuca polysaccharides (ULPS) | α-amylase and maltase inhibition leading to a significant decrease in blood glucose rate | [88] |
| Phaeodactylum tricornutum | Fucosterol | C57BL/6 mice a high-fat diet supplemented with PT powder (15% or 30% w/v) for 12 weeks, and determined energy intake, weight loss, and lipid profiles each week reduced body weight gain, and epididymal and perirenal adipose tissue weight via activation of AMPK and HMGCR pathways | [89] |
| Hematococcus pluvialis | Astaxanthin | Male Swiss albino mice: starch-based control diet or a high fat-high fructose diet (HFFD). Fifteen days later, mice in each dietary group were divided into two and were treated with either ASX (6 mg·kg−1 per day) in olive oil or olive oil alone. For 60 days ASX treatment reduced lipid levels and oxidative stress in skeletal muscle and adipose tissue and improved insulin signaling by enhancing the autophosphorylation of insulin receptor-β (IR-β), IRS-1 associated PI3-kinase step, phospho-Akt/Akt ratio and GLUT-4 translocation in skeletal muscle | [90] |
| Pre-treatment with ASTA (10 µM) for 1 h attenuates the LPS-induced toxicity and ROS production. In U937 cells stimulated with LPS (10 µg/mL) | [91] | ||
| Astaxanthin inhibited the increases in body weight and weight of adipose tissue that result from feeding mice a high-fat diet, reduced liver weight, liver triglyceride, plasma triglyceride, and total cholesterol | [92] | ||
| FUNGI Phylum: Ascomycota | |||
| Source | Drug | Target and Activity | Reference |
| Penicillium spp. Eurotium sp. | Fructigenine A Cyclopenol Echinulin Flavoglaucin Viridicatol | Selective inhibition of PTP1B fructigenine A in a noncompetitive manner, viridicatol in a competitive manner | [93] |
| CYANOBACTERIA Phylum: Cyanobacteria | |||
| Source | Drug | Target and activity | Reference |
| Leptolyngbya sp. | γ-pyrones yoshinone | Osteoclastogenesis, protein kinase inhibitor. Inhibitory activity against the adipogenic differentiation of 3T3-L1 cells IC50 = 420 nM. Mice at high-fat diet (HFD) for 5 weeks received kalkipyrone at a dosage of 5 mg·kg−1/day showed effective suppression of adipose tissue weight gain in mice | [94] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamonaca, P.; Prinzi, G.; Kisialiou, A.; Cardaci, V.; Fini, M.; Russo, P. Metabolic Disorder in Chronic Obstructive Pulmonary Disease (COPD) Patients: Towards a Personalized Approach Using Marine Drug Derivatives. Mar. Drugs 2017, 15, 81. https://doi.org/10.3390/md15030081
Lamonaca P, Prinzi G, Kisialiou A, Cardaci V, Fini M, Russo P. Metabolic Disorder in Chronic Obstructive Pulmonary Disease (COPD) Patients: Towards a Personalized Approach Using Marine Drug Derivatives. Marine Drugs. 2017; 15(3):81. https://doi.org/10.3390/md15030081
Chicago/Turabian StyleLamonaca, Palma, Giulia Prinzi, Aliaksei Kisialiou, Vittorio Cardaci, Massimo Fini, and Patrizia Russo. 2017. "Metabolic Disorder in Chronic Obstructive Pulmonary Disease (COPD) Patients: Towards a Personalized Approach Using Marine Drug Derivatives" Marine Drugs 15, no. 3: 81. https://doi.org/10.3390/md15030081
APA StyleLamonaca, P., Prinzi, G., Kisialiou, A., Cardaci, V., Fini, M., & Russo, P. (2017). Metabolic Disorder in Chronic Obstructive Pulmonary Disease (COPD) Patients: Towards a Personalized Approach Using Marine Drug Derivatives. Marine Drugs, 15(3), 81. https://doi.org/10.3390/md15030081





