Astaxanthin Inhibits PC-3 Xenograft Prostate Tumor Growth in Nude Mice
Abstract
:1. Introduction
2. Results
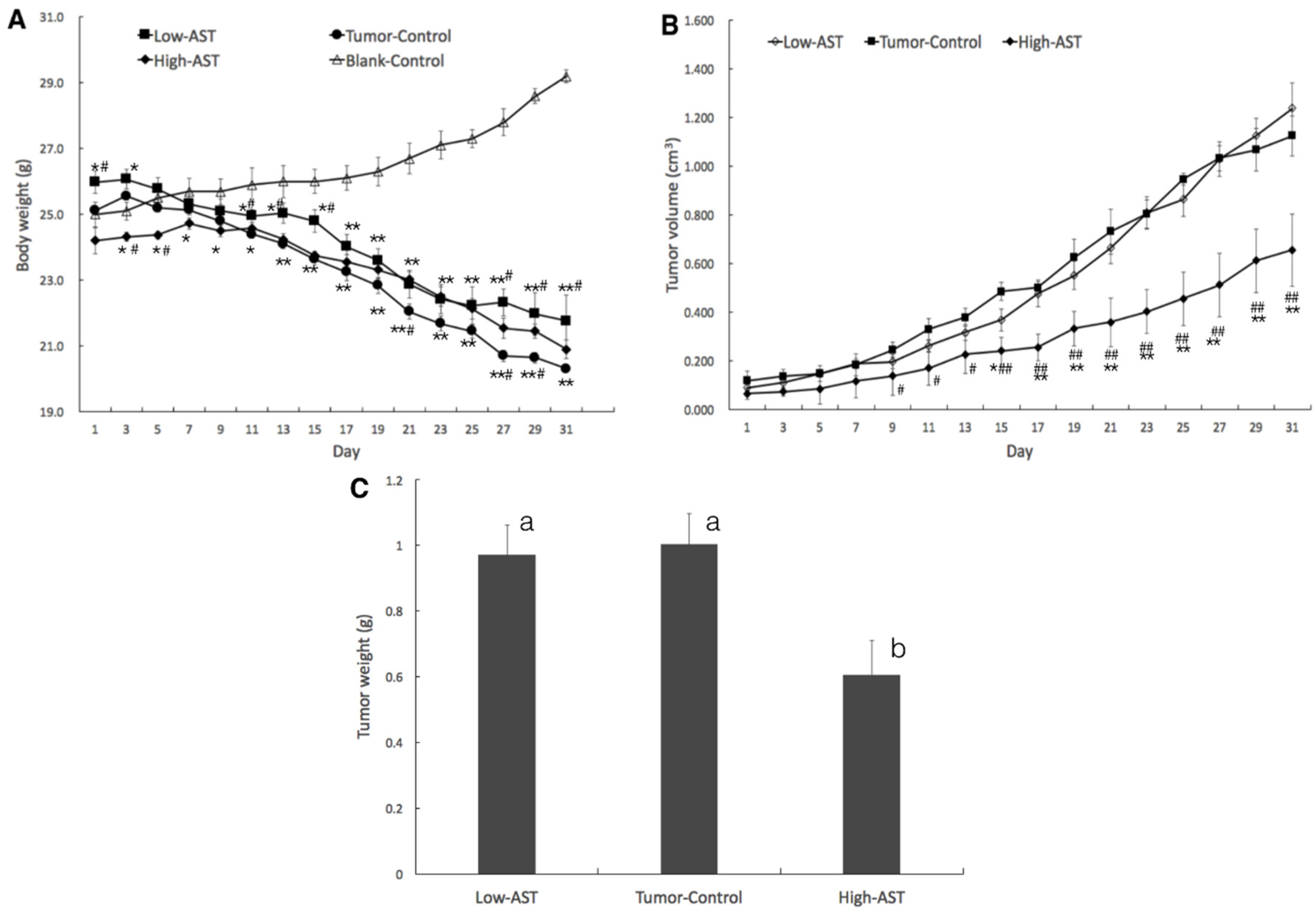
2.1. Suppressed Tumor Growth of PC-3 Xenograft
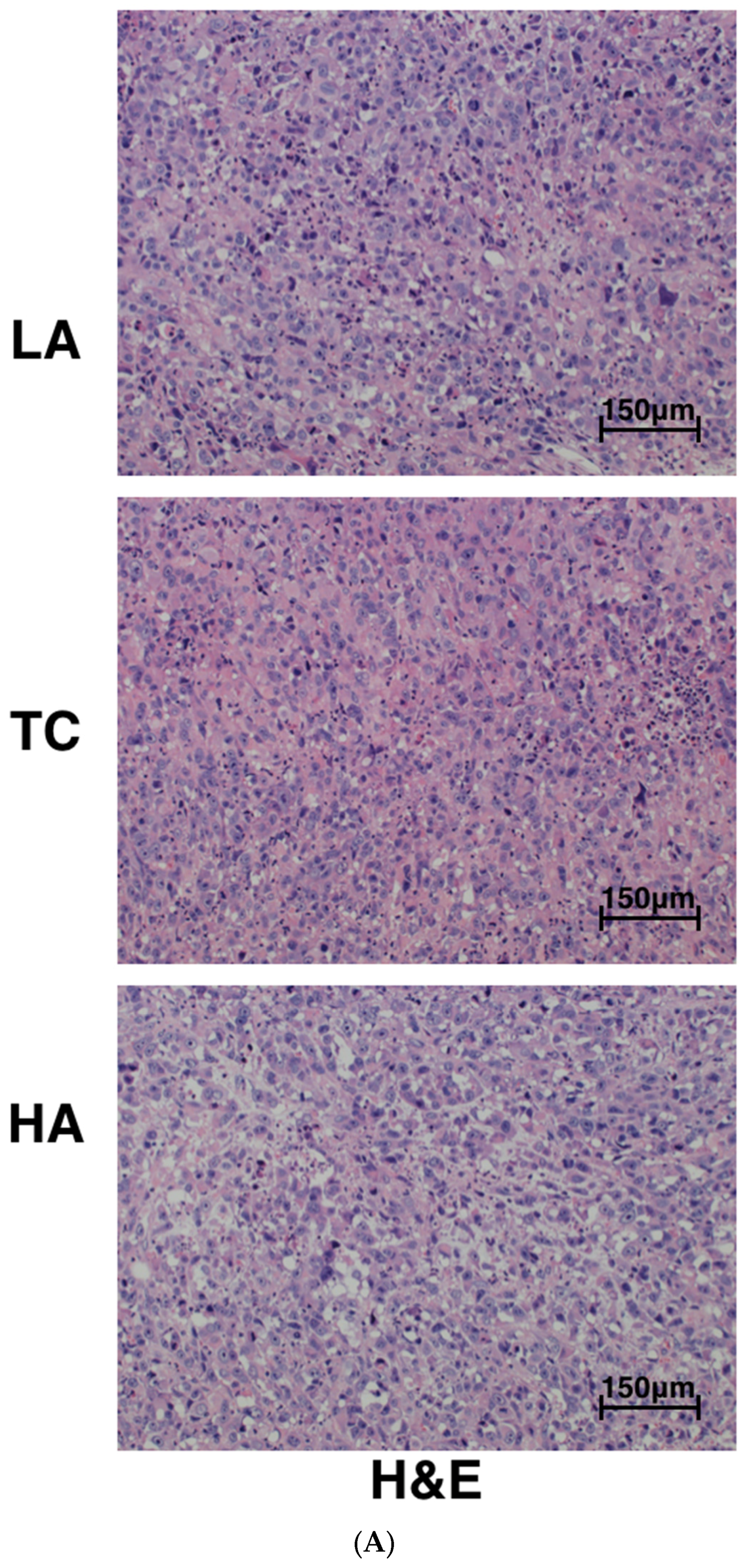
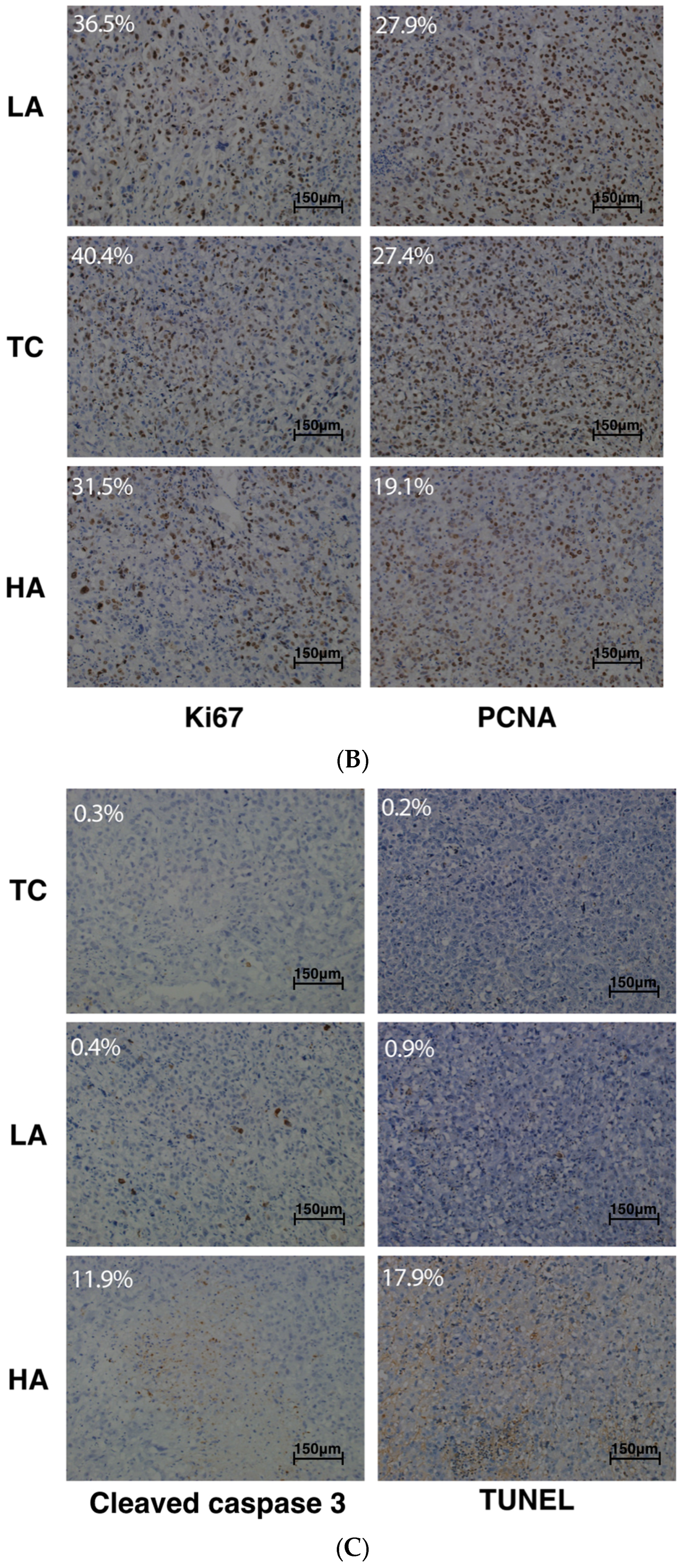
2.2. Decreased Tumor Cell Proliferation and Increased Tumor Cell Apoptosis in Tumors
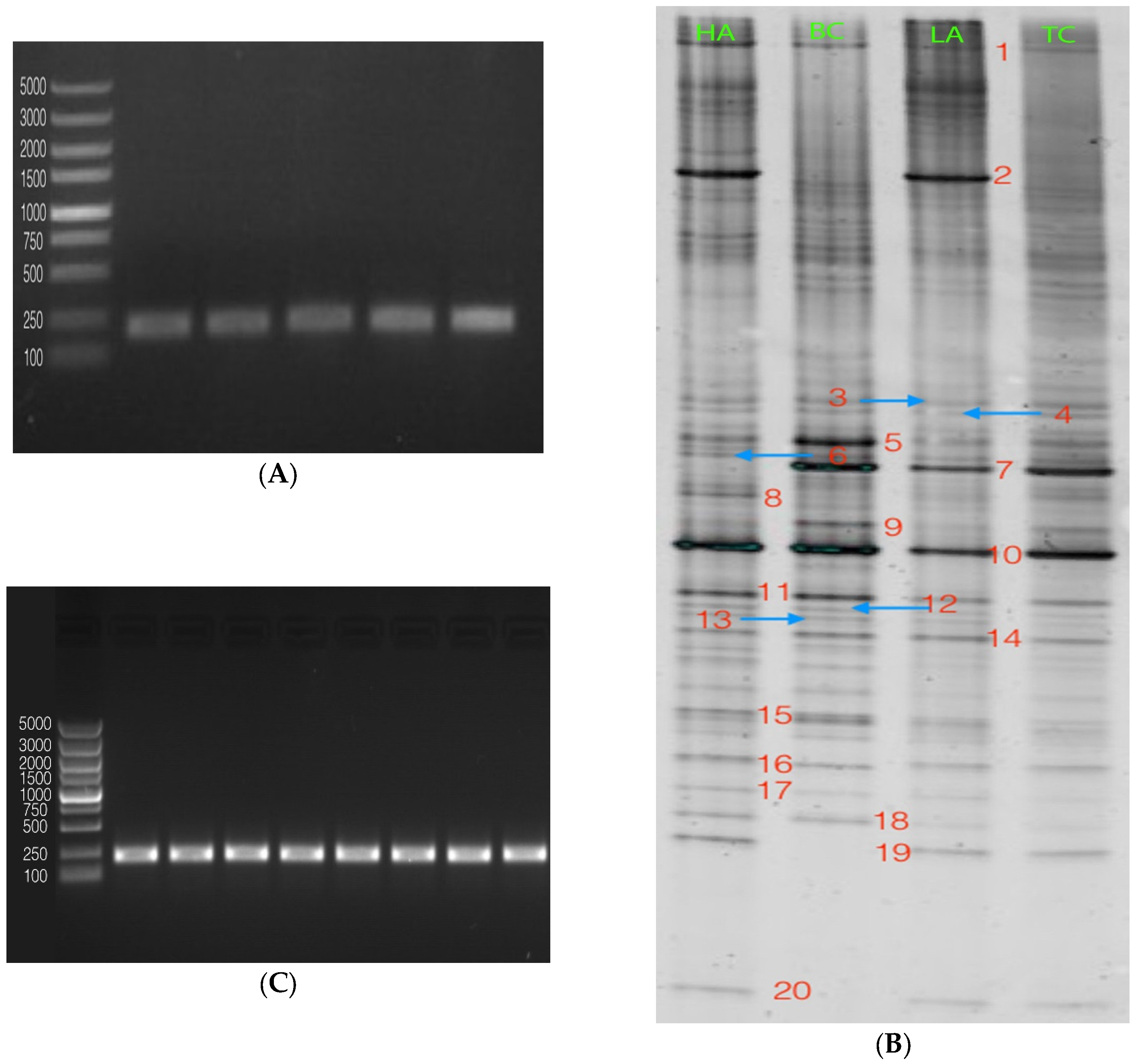
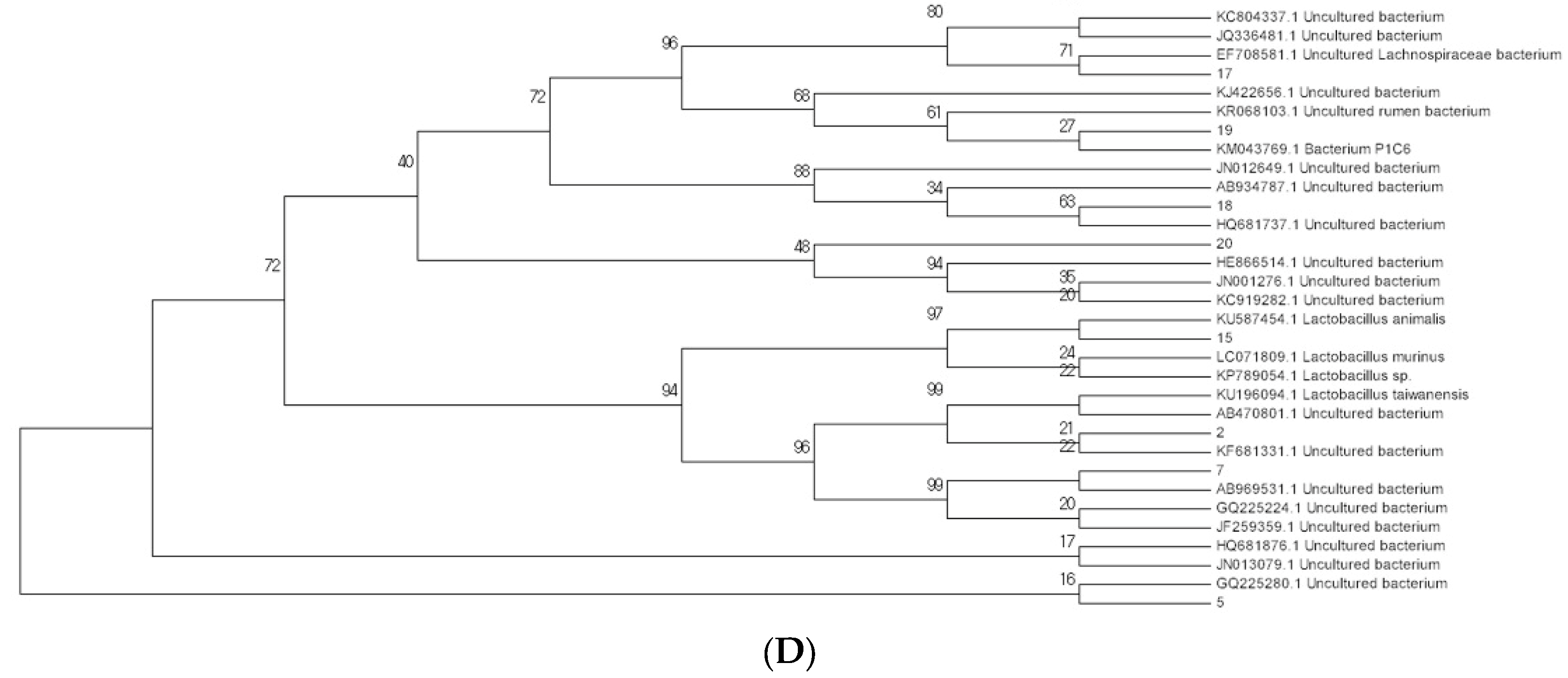
2.3. Modulation of Bacterial Composition in Mice Stools
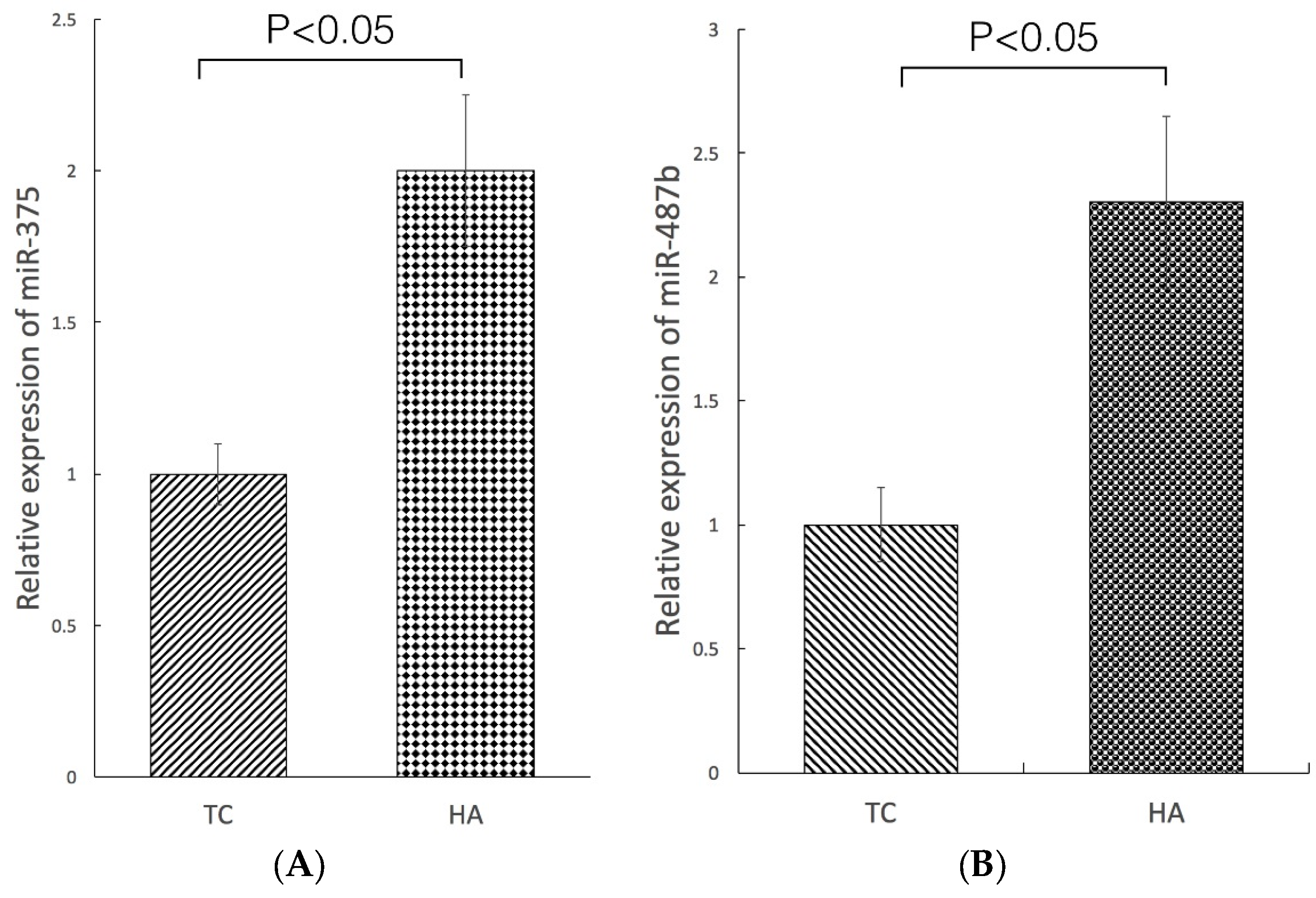
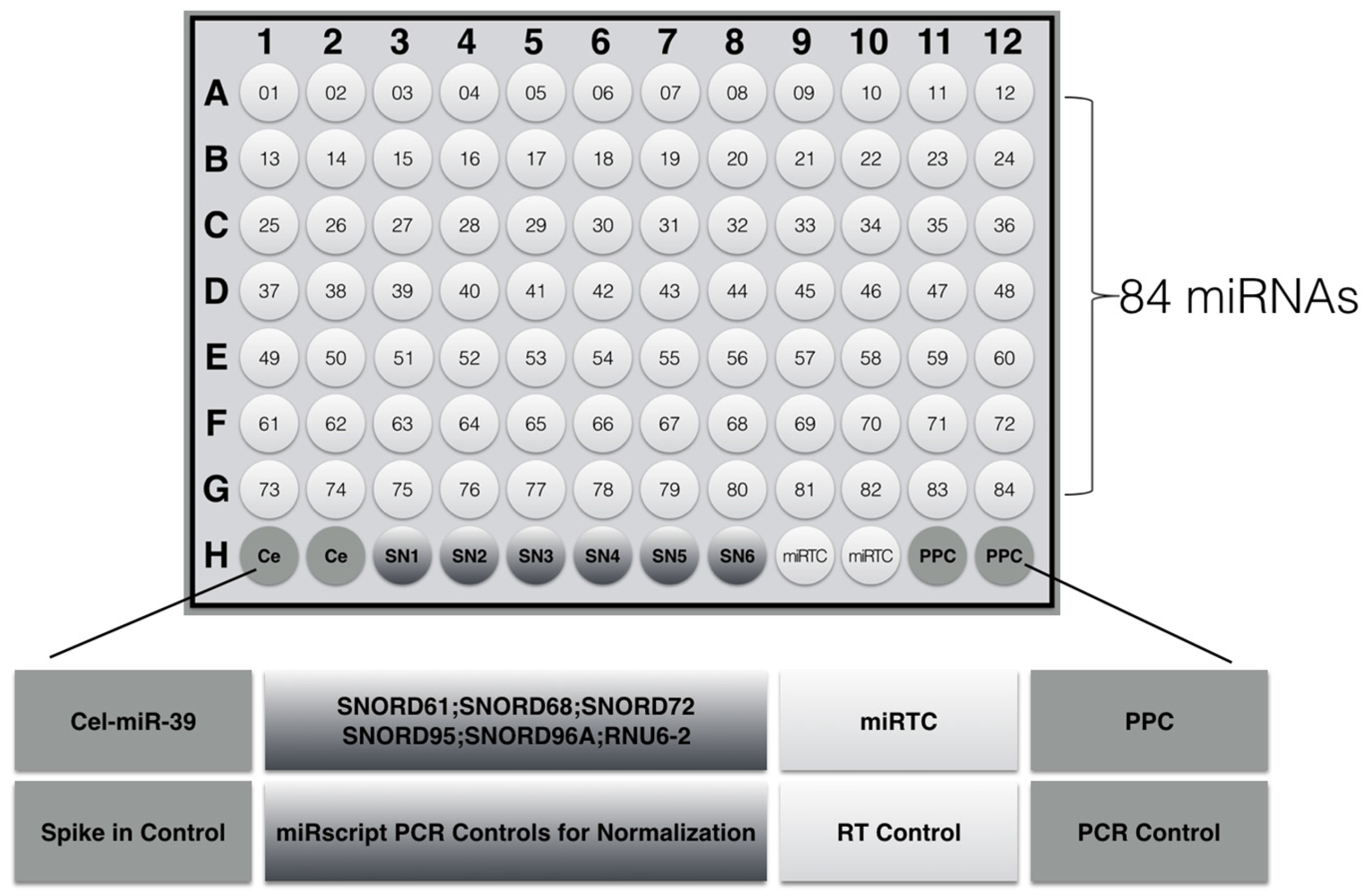
2.4. Modulation of miRNA Expression
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Cell Culture
4.3. Animal Model
4.4. Hematoxylin–Eosin (H&E) Staining and Immunohistochemical (IHC) Analyses
4.5. Polymerase Chain Reaction–Denaturing Gradient Gel Electrophoresis (PCR-DGGE)
4.6. Microarray Array
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA-Cancer J. Clin. 2015, 6, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA-Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.; Lizarrage, T.L.C.; Lazar, A.A. Radical prostatectomy versus radiation and androgen deprivation therapy for clinically localized prostate cancer: How good is the evidence? Int. J. Radiat. Oncol. 2015, 93, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Sydes, M.R.; Clarke, M.D.; Dearnaley, D.P.; Spears, M.R. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomized controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Andriole, G.L.; Bostwick, D.G.; Brawley, O.W.; Gomella, L.G.; Marberger, M.; Montorsi, F.; Pettaway, C.A.; Tammela, T.L.; Teloken, C.; Tindall, D.J.; et al. Effect of dutasteride on the risk of prostate cancer. N. Engl. J. Med. 2010, 362, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.J.; Kahwati, L.C.; Kinsinger, L.S. Knowledge and use of finasteride for the prevention of prostate cancer. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2164–2171. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Miller, G.J.; Ford, L.G.; Lieber, M.M.; Cespedes, R.D.; Atkins, J.N.; Lippman, S.M.; et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 2003, 349, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Van Poppel, H.; Tombal, B. Chemoprevention of prostate cancer with nutrients and supplements. Cancer Manag. Res. 2011, 3, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Agalliu, I.; Kirsh, V.A.; Kreiger, N.; Soskolne, C.L.; Rohan, T.E. Oxidative balance score and risk of prostate cancer: Results from a case-cohort study. Cancer Epidemiol. 2011, 35, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, J.M.; Glynn, R.J.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Sesso, H.D.; Buring, J.E. Vitamins E and C in the prevention of prostate and total cancer in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2009, 301, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Peters, U.; Littman, A.J.; Kristal, A.R.; Patterson, R.E.; Potter, J.D.; White, E. Vitamin E and selenium supplementation and risk of prostate cancer in the Vitamins and lifestyle (VITAL) study cohort. Cancer Causes Control 2008, 19, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol. 2009, 5, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, S58–S68. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Echard, B.; Bagchi, D.; Perricone, N.V.; Yamashita, E. Astaxanthin lowers blood pressure and lessens the activity of the renin-angiotensin system in Zucker Fatty Rats. J. Funct. Foods 2009, 1, 13–22. [Google Scholar] [CrossRef]
- Roche, F. Astaxanthin: Human food safety summary. In Astaxanthin as a Pigmenter in Salmon Feed, Color Additive Petition 7C02 1 1; United States Food and Drug Administration: Silver Spring, MD, USA, 1987; p. 43. [Google Scholar]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–364. [Google Scholar] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Porkka, K.P.; Pfeiffer, M.J.; Waltering, K.K.; Vessella, R.L.; Tammela, T.L.J.; Visakorpi, T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007, 67, 6130–6135. [Google Scholar] [CrossRef] [PubMed]
- Ozen, M.; Creighton, C.J.; Ozdemir, M.; Ittmann, M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene 2008, 27, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, Y.E.; Zhao, C.; Zhou, P.; Yu, L. Analysis and identification of astaxanthin and its carotenoid precursors from Xanthophyllomyces dendrorhous by high-performance liquid chromatography. Z. Naturforsch. C 2010, 65, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lu, M.; Yu, L. Expression of carotenogenic genes and astaxanthin production in Xanthophyllomyces dendrorhous as a function of oxygen tension. Z. Naturforsch. C 2011, 66, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediat. Inflamm. 2013, 2013, 782137. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, Y.; Liu, T.; Wang, J.; Dai, W.; Wang, F.; Zheng, Y.; Chen, K.; Li, S.; Abudumijiti, H.; et al. Protective effects of astaxanthin on ConA-induced autoimmune hepatitis by the JNK/p-JNK pathway-mediated inhibition of autophagy and apoptosis. PLoS ONE 2015, 10, e0120440. [Google Scholar] [CrossRef] [PubMed]
- Campoio, T.R.; Oliveira, F.A.; Otton, R. Oxidative stress in human lymphocytes treated with fatty acid mixture: Role of carotenoid astaxanthin. Toxicol. In Vitro 2011, 25, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Nagendraprabhu, P.; Sudhandiran, G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFkB and COX-2. Investig. New Drug 2011, 29, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Tsuji, S.; Satoh, A.; Ishikura, M.; Shirasawa, T.; Shimizu, T. Protective effects of astaxanthin on 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J. Neurochem. 2008, 107, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Czeczuga-Semeniuk, E.; Wolczynski, S.; Markiewicz, W. Preliminary identification of carotenoids in malignant and benign neoplasms of the breast and surrounding fatty tissue. Neoplasma 2003, 50, 280–286. [Google Scholar] [PubMed]
- Anderson, M.L. A preliminary investigation of the enzymatic inhibition of 5α-reductase and growth of prostatic carcinoma cell line LNCap-FGC by natural astaxanthin and saw palmetto lipid extract in vitro. J. Herb. Pharmacother. 2005, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kiki, T.; Miyazawa, T.; Carpentero, B.G.; Kimura, F.; Satoh, A. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Ji, H.K.; Min, J.C.; Kyu-Youn, Y.; Wan, G.S. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother. Res. 2011, 25, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Clarridge, J.E. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 2004, 17, 840–862. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.A.; Hartley, J.C. Development of broad-range 16S rDNA PCR for use in the routine diagnostic clinical microbiology service. J. Med. Microbiol. 2003, 52, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Leskinen, M.J.; Rantakokko-Jalava, K.; Manninen, R.; Leppilahti, M.; Marttila, T.; Kylmälä, T.; Tammela, T.L. Negative bacterial polymerase chain reaction (PCR) findings in prostate tissue from patients with symptoms of chronic pelvic pain syndrome (CPPS) and localized prostate cancer. Prostate 2003, 55, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Hochreiter, W.W.; Duncan, J.L.; Schaeffer, A.J. Evaluation of the bacterial flora of the prostate using a 16S rRNA gene based polymerase chain reaction. J. Urol. 2000, 163, 127–130. [Google Scholar] [CrossRef]
- Keay, S.; Zhang, C.O.; Baldwin, B.R.; Alexander, R.B. Polymerase chain reaction amplification of bacterial 16s rRNA genes in prostate biopsies from men without chronic prostatitis. Urology 1999, 53, 487–491. [Google Scholar] [CrossRef]
- Krieger, J.N.; Riley, D.E.; Vesella, R.L.; Miner, D.C.; Ross, S.O.; Lange, P.H. Bacterial DNA sequences in prostate tissue from patients with prostate cancer and chronic prostatitis. J. Urol. 2000, 164, 1221–1228. [Google Scholar] [CrossRef]
- Yu, H.N.; Meng, H.Z.; Zhou, F.; Ni, X.F.; Shen, S.R.; Undurti, N.D. Urinary microbiota in patients with prostate cancer and benign prostatic hyperplasia. Arch. Med. Sci. 2015, 11, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.F.; Meng, H.Z.; Zhou, F.; Yu, H.N.; Xiang, J.J.; Shen, S.R. Effect of hypertension on bacteria composition of prostate biopsy in patients with benign prostatic hyperplasia and prostate cancer in PSA grey-zone. Biomed. Rep. 2016, 4, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Okumura, K. Effects of a fermented milk drink containing Lactobacillus casei strains Shirota on the human NK-cell activity. J. Nutr. 2007, 137, 791S–793S. [Google Scholar]
- Takagi, A.; Matsuzaki, T.; Sato, M.; Nomoto, K.; Morotomi, M.; Yokokura, T. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis 2001, 22, 599–1005. [Google Scholar] [CrossRef] [PubMed]
- Horinaka, M.; Yoshida, T.; Kishi, A.; Akatani, K.; Yasuda, T.; Kouhara, J.; Wakada, M.; Sakai, T. Lactobacillus strains induce TRAIL production and facilitate natural killer activity against cancer cells. FEBS Lett. 2010, 584, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Danino, T.; Prindle, A.; Kwong, G.A.; Skalak, M.; Li, H.; Allen, K.; Bhatia, S.N. Programmable probiotics for detection of cancer in urine. Sci. Transl. Med. 2015, 7, 289ra84. [Google Scholar] [CrossRef] [PubMed]
- Davalos, V.; Esteller, M. MicroRNAs and cancer epigenetics: A macrorevolution. Curr. Opin. Oncol. 2010, 22, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C. MicroRNAs: Potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int. J. Biochem. Cell B 2010, 42, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.T.; Kim, W.J. MicroRNAs in prostate cancer. Prostate Int. 2013, 1, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.X.; Gao, W.Q. Roles of microRNAs during prostatic tumorigenesis and tumor progression. Oncogene 2014, 33, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Coppola, V.; De Maria, R.; Bonci, D. MicroRNAs and prostate cancer. Endocr. Relat. Cancer 2010, 17, F1–F7. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jin, J.; Liu, Y.; Huang, Z.; Deng, Y.; You, T.; Zhou, T.; Si, J.; Zhuo, W. Snail-regulated MiR-375 inhibits migration and invasion of gastric cancer cells by targeting JAK2. PLoS ONE 2014, 9, e99516. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Liu, K.; Xie, M.; Xing, Y.; Xi, T. miR-375 inhibits Helicobacter pylori-induced gastric carcinogenesis by blocking JAK2-STAT3 signaling. Cancer Immunol. Immun. 2014, 63, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, Y.; Hoshino, I.; Nohata, N.; Kinoshita, T.; Akutsu, Y.; Hanari, N.; Mori, M.; Yoneyama, Y.; Akanuma, N.; Takeshita, N.; Maruyama, T. Identification of novel molecular targets regulated by tumor suppressive miR-375 induced by histone acetylation in esophageal squamous cell carcinoma. Int. J. Oncol. 2012, 41, 985–994. [Google Scholar] [PubMed]
- Hui, A.B.; Bruce, J.P.; Alajez, N.M.; Shi, W.; Yue, S.; Perez-Ordonez, B.; Xu, W.; O’Sullivan, B.; Waldron, J.; Cummings, B.; Gullane, P. Significance of dysregulated metadherin and microRNA-375 in head and neck cancer. Clin. J. Res. 2011, 17, 7539–7550. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Nohata, N.; Yoshino, H.; Hanazawa, T.; Kikkawa, N.; Fujimura, L.; Chiyomaru, T.; Kawakami, K.; Enokida, H.; Nakagawa, M.; et al. Tumor suppressive microRNA-375 regulates lactate dehydrogenase B in maxillary sinus squamous cell carcinoma. Int. J. Oncol. 2012, 40, 185. [Google Scholar] [PubMed]
- Formosa, A.; Markert, E.K.; Lena, A.M.; Italiano, D.; Finazzi-Agro, E.; Levine, A.J.; Bernardini, S.; Garabadgiu, A.V.; Melino, G.; Candi, E. MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c, miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p, mapped to the 14q32. 31 locus, regulate proliferation, apoptosis, migration and invasion in metastatic prostate cancer cells. Oncogene 2014, 33, 5173–5182. [Google Scholar] [CrossRef] [PubMed]
- Mahenthiralingam, E.; Baldwin, A.; Drevinek, P.; Vanlaere, E.; Vandamme, P.; LiPuma, J.J.; Dowson, C.G. Multilocus sequence typing breathes life into a microbial metagenome. PLoS ONE 2006, 1, e17. [Google Scholar] [CrossRef] [PubMed]
| Tumor-Related miRNAs | ||||||||
|---|---|---|---|---|---|---|---|---|
| miR-1 | miR-149 | miR-200c | miR-32 | miR-376c | miR-487b | miR-556-3p | miR-619 | miR-744 |
| miR-103-1as | miR-15b | miR-203 | miR-320b | miR-377 | miR-490-5p | miR-562 | miR-625 | miR-767-3p |
| miR-122 | miR-154 | miR-21 | miR-320e | miR-378 | miR-495 | miR-571 | miR-626 | miR-885 |
| mir124 | miR-155 | miR-210 | miR-326 | miR-381 | miR-496 | miR-574-3p | miR-627 | miR-936 |
| miR-126 | miR-16 | miR-218 | miR-339-5p | miR-383 | miR-512-3p | miR-575 | miR-628-5p | |
| miR-135a | miR-182 | miR-22 | miR-345 | miR-384 | miR-517b | miR-591 | miR-647 | |
| miR-135b | miR-183 | miR-221 | miR-346 | miR-432 | miR-518c | miR-593 | miR-654-3p | |
| miR-145 | miR-185 | miR-27b | miR-373 | miR-449a | miR-548c-5p | miR-601 | miR-661 | |
| miR-146a | miR-193a-3p | miR-29a | miR-375 | miR-485-3p | miR-551a | miR-608 | miR-664 | |
| miR-147b | miR-197 | miR-299-5p | miR-376a | miR-486-5p | miR-554 | miR-615-3p | miR-671-5p | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, X.; Yu, H.; Wang, S.; Zhang, C.; Shen, S. Astaxanthin Inhibits PC-3 Xenograft Prostate Tumor Growth in Nude Mice. Mar. Drugs 2017, 15, 66. https://doi.org/10.3390/md15030066
Ni X, Yu H, Wang S, Zhang C, Shen S. Astaxanthin Inhibits PC-3 Xenograft Prostate Tumor Growth in Nude Mice. Marine Drugs. 2017; 15(3):66. https://doi.org/10.3390/md15030066
Chicago/Turabian StyleNi, Xiaofeng, Haining Yu, Shanshan Wang, Chengcheng Zhang, and Shengrong Shen. 2017. "Astaxanthin Inhibits PC-3 Xenograft Prostate Tumor Growth in Nude Mice" Marine Drugs 15, no. 3: 66. https://doi.org/10.3390/md15030066
APA StyleNi, X., Yu, H., Wang, S., Zhang, C., & Shen, S. (2017). Astaxanthin Inhibits PC-3 Xenograft Prostate Tumor Growth in Nude Mice. Marine Drugs, 15(3), 66. https://doi.org/10.3390/md15030066





