Hypopigmenting Effects of Brown Algae-Derived Phytochemicals: A Review on Molecular Mechanisms
Abstract
1. Introduction
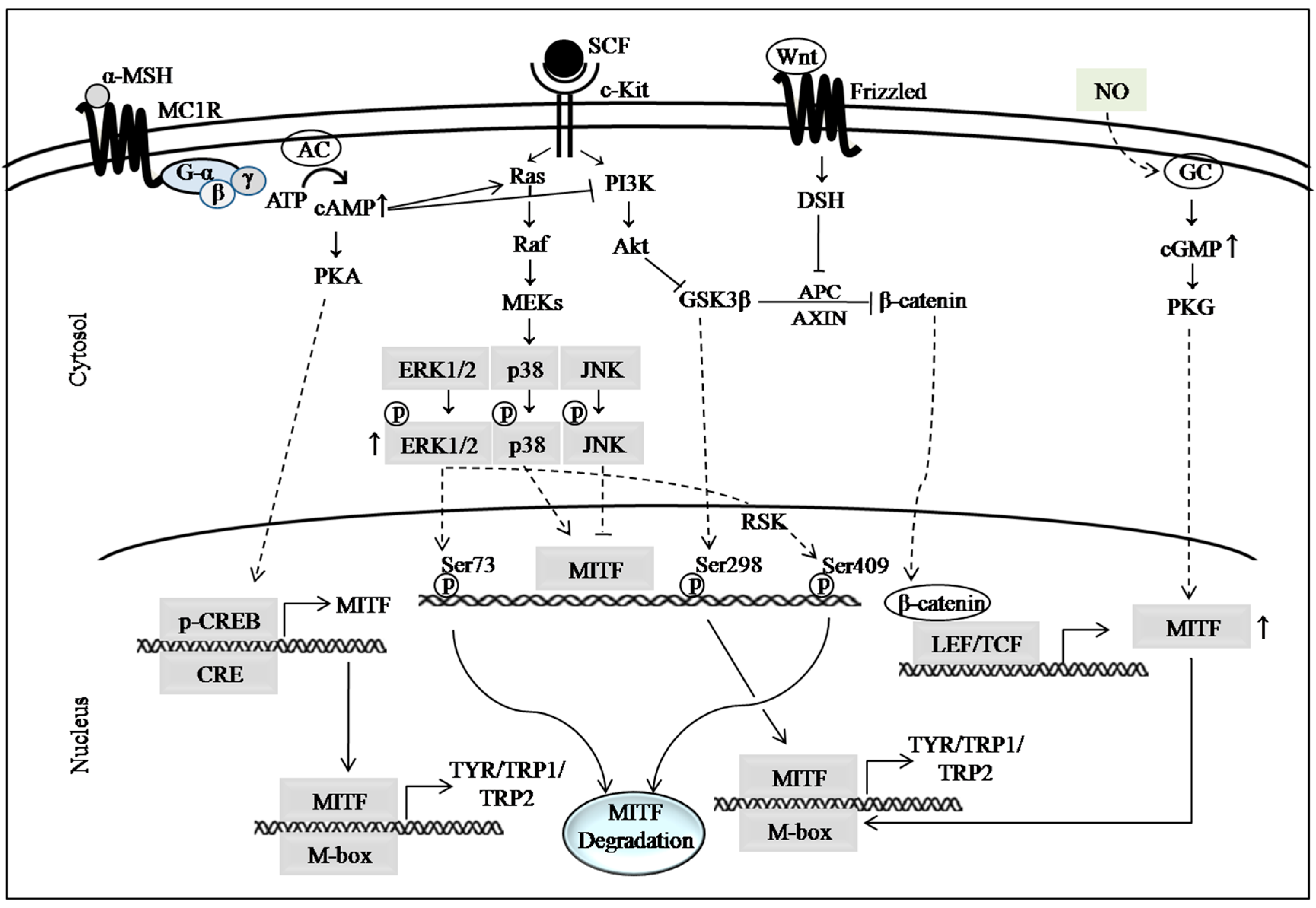
2. Major Pathways Involved in Melanogenesis
2.1. cAMP Signaling Pathway
2.2. MAP Kinase Signaling Pathways
2.3. PI3K/Akt Signaling Pathway
2.4. Wnt/β-Catenin Signaling Pathway
2.5. Autophagy, Nitric Oxide (NO) Signaling and Other Mechanistic Targets of Hypopigmenting Agents
3. Hypopigmenting Effects of Marine Brown Algae-Derived Phenolic Compounds
4. Hypopigmenting Effects of Meroterpenoids
5. Hypopigmenting Effects of Fucoxanthin
6. Hypopigmenting Effects of Non-Phenolic Compounds
7. Current Gaps and Future Directions
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat. Res. 2005, 571, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Goihman-Yahr, M. Skin aging and photoaging: An outlook. Clin. Dermatol. 1996, 14, 153–160. [Google Scholar] [CrossRef]
- Han, E.; Chang, B.; Kim, D.; Cho, H.; Kim, S. Melanogenesis inhibitory effect of aerial part of Pueraria thunbergiana in vitro and in vivo. Arch. Dermatol. Res. 2015, 307, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Yoon, S.J.; Park, Y.J.; Lee, H.B. Inhibitory effect of ephedrannins A and B from roots of Ephedra sinica STAPF on melanogenesis. Biochim. Biophys. Acta 2015, 1850, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Lehraiki, A.; Abbe, P.; Cerezo, M.; Rouaud, F.; Regazzetti, C.; Chignon-Sicard, B.; Passeron, T.; Bertolotto, C.; Ballotti, R.; Rocchi, S. Inhibition of melanogenesis by the antidiabetic metformin. J. Investig. Dermatol. 2014, 134, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Ko, S.C.; Kim, D.; Jeon, Y.J. Screening of marine algae for potential tyrosinase inhibitor: Those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J. Dermatol. 2011, 38, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Curto, E.V.; Kwong, C.; Hermersdorfer, H.; Glatt, H.; Santis, C.; Virador, V.; Hearing, V.J., Jr.; Dooley, T.P. Inhibitors of mammalian melanocyte tyrosinase: In vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem. Pharmacol. 1999, 57, 663–672. [Google Scholar] [CrossRef]
- Gao, X.-H.; Zhang, L.; Wei, H.; Chen, H.-D. Efficacy and safety of innovative cosmeceuticals. Clin. Dermatol. 2008, 26, 367–374. [Google Scholar] [CrossRef] [PubMed]
- DeCaprio, A.P. The toxicology of hydroquinone—Relevance to occupational and environmental exposure. Crit. Rev. Toxicol. 1999, 29, 283–330. [Google Scholar] [CrossRef] [PubMed]
- O‘Donoghue, J.L. Hydroquinone and its analogues in dermatology—A risk-benefit viewpoint. J. Cosmet. Dermatol. 2006, 5, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Serra-Baldrich, E.; Tribo, M.J.; Camarasa, J.G. Allergic contact dermatitis from kojic acid. Contact Dermat. 1998, 39, 86–87. [Google Scholar] [CrossRef]
- Takizawa, T.; Imai, T.; Onose, J.; Ueda, M.; Tamura, T.; Mitsumori, K.; Izumi, K.; Hirose, M. Enhancement of hepatocarcinogenesis by kojic acid in rat two-stage models after initiation with N-bis(2-hydroxypropyl)nitrosamine or N-diethylnitrosamine. Toxicol. Sci. 2004, 81, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Pomponi, S.A. The bioprocess–technological potential of the sea. J. Biotechnol. 1999, 70, 5–13. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol. Bioprocess Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Kim, E.-A.; Son, K.-T.; Jeon, Y.-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Joung, E.-J.; Gwon, W.-G.; Shin, T.; Jung, B.-M.; Choi, J.; Kim, H.-R. Anti-inflammatory action of the ethanolic extract from Sargassum serratifolium on lipopolysaccharide-stimulated mouse peritoneal macrophages and identification of active components. J. Appl. Phycol. 2017, 29, 563–573. [Google Scholar] [CrossRef]
- Seo, Y.; Park, K.E.; Kim, Y.A.; Lee, H.J.; Yoo, J.S.; Ahn, J.W.; Lee, B.J. Isolation of tetraprenyltoluquinols from the brown alga Sargassum thunbergii. Chem. Pharm. Bull. 2006, 54, 1730–1733. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Ahn, B.N.; Kong, C.S.; Kim, S.K. Protective effect of chromene isolated from Sargassum horneri against UV-A-induced damage in skin dermal fibroblasts. Exp. Dermatol. 2012, 21, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Gwon, W.G.; Lee, B.; Joung, E.J.; Choi, M.W.; Yoon, N.; Shin, T.; Oh, C.W.; Kim, H.R. Sargaquinoic Acid Inhibits TNF-alpha-Induced NF-kappaB Signaling, Thereby Contributing to Decreased Monocyte Adhesion to Human Umbilical Vein Endothelial Cells (HUVECs). J. Agric. Food Chem. 2015, 63, 9053–9061. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Choi, H.Y.; Lee, W.; Park, G.M.; Shin, W.S.; Kim, Y.K. Sargaquinoic acid and sargahydroquinoic acid from Sargassum yezoense stimulate adipocyte differentiation through PPARalpha/gamma activation in 3T3-L1 cells. FEBS Lett. 2008, 582, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Lee, W.; Bae, G.U.; Kim, Y.K. Anti-diabetic and hypolipidemic effects of Sargassum yezoense in db/db mice. Biochem. Biophys. Res. Commun. 2012, 424, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Liu-Smith, F.; Meyskens, F.L. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. 2016, 60, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Khaled, M.; Larribere, L.; Bille, K.; Aberdam, E.; Ortonne, J.P.; Ballotti, R.; Bertolotto, C. Glycogen synthase kinase 3beta is activated by cAMP and plays an active role in the regulation of melanogenesis. J. Biol. Chem. 2002, 277, 33690–33697. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.S.; Joung, E.-J.; Choi, J.; Kim, H.-R. Ethanolic extract from Sargassum serratifolium attenuates hyperpigmentation through CREB/ERK signaling pathways in α-MSH-stimulated B16F10 melanoma cells. J. Appl. Phycol. 2017, 29, 2089–2096. [Google Scholar] [CrossRef]
- Garcia-Borron, J.C.; Abdel-Malek, Z.; Jimenez-Cervantes, C. MC1R, the cAMP pathway, and the response to solar UV: Extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 2014, 27, 699–720. [Google Scholar] [CrossRef] [PubMed]
- Widlund, H.R.; Fisher, D.E. Microphthalamia-associated transcription factor: A critical regulator of pigment cell development and survival. Oncogene 2003, 22, 3035–3041. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Dai, R.-Y.; Leu, Y.-L.; Tsai, T.-Y. Effects of the melanogenic inhibitor, uracil, derived from Lactobacillus plantarum TWK10-fermented soy milk on anti-melanogenesis in B16F0 mouse melanoma cells. J. Funct. Foods 2015, 17, 314–327. [Google Scholar] [CrossRef]
- Napolitano, A.; Micillo, R.; Monfrecola, G. Melanin pigmentation control by 1,3-thiazolidines: Does NO scavenging play a critical role? Exp. Dermatol. 2016, 25, 596–597. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cheng, C.Y.; Saldanha, S.A.; Taylor, S.S. PKA-I holoenzyme structure reveals a mechanism for cAMP-dependent activation. Cell 2007, 130, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Yun, C.Y.; Young Yun, J.; Park, D.; Doo Kim, N.; Yeon Hwang, B.; Jung, S.H.; Park, S.K.; Kim, Y.B.; Han, S.B.; et al. cAMP-binding site of PKA as a molecular target of bisabolangelone against melanocyte-specific hyperpigmented disorder. J. Investig. Dermatol. 2013, 133, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Karelina, K.; Obrietan, K. CREB: A multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 2011, 116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Gu, G.E.; Jo, A.R.; Bang, J.S.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Park, K.C.; Kim, D.S. Baicalin-induced Akt activation decreases melanogenesis through downregulation of microphthalmia-associated transcription factor and tyrosinase. Eur. J. Pharmacol. 2015, 761, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.G.; Choi, E.J.; Choi, Y.; Hwang, J.K. 5,7-dimethoxyflavone induces melanogenesis in B16F10 melanoma cells through cAMP-dependent signalling. Exp. Dermatol. 2011, 20, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, C.; Bille, K.; Ortonne, J.P.; Ballotti, R. Regulation of tyrosinase gene expression by cAMP in B16 melanoma cells involves two CATGTG motifs surrounding the TATA box: Implication of the microphthalmia gene product. J. Cell Biol. 1996, 134, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Busca, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000, 13, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Xu, W.; Liang, J.W.; Wang, C.S.; Kang, Y. Effect of fucoidan on b16 murine melanoma cell melanin formation and apoptosis. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Hong, S.D.; Roh, E.; Jung, S.-H.; Cho, W.-J.; Hong Park, S.; Yoon, D.Y.; Ko, S.M.; Hwang, B.Y.; Hong, J.T.; et al. cAMP-dependent activation of protein kinase A as a therapeutic target of skin hyperpigmentation by diphenylmethylene hydrazinecarbothioamide. Br. J. Pharmacol. 2015, 172, 3434–3445. [Google Scholar] [CrossRef] [PubMed]
- Englaro, W.; Bertolotto, C.; Busca, R.; Brunet, A.; Pages, G.; Ortonne, J.P.; Ballotti, R. Inhibition of the mitogen-activated protein kinase pathway triggers B16 melanoma cell differentiation. J. Biol. Chem. 1998, 273, 9966–9970. [Google Scholar] [CrossRef] [PubMed]
- Hemesath, T.J.; Price, E.R.; Takemoto, C.; Badalian, T.; Fisher, D.E. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature 1998, 391, 298–301. [Google Scholar] [PubMed]
- Wu, L.C.; Lin, Y.Y.; Yang, S.Y.; Weng, Y.T.; Tsai, Y.T. Antimelanogenic effect of c-phycocyanin through modulation of tyrosinase expression by upregulation of ERK and downregulation of p38 MAPK signaling pathways. J. Biomed. Sci. 2011, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Hemesath, T.J.; Takemoto, C.M.; Horstmann, M.A.; Wells, A.G.; Price, E.R.; Fisher, D.Z.; Fisher, D.E. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000, 14, 301–312. [Google Scholar] [PubMed]
- Lee, H.J.; Lee, W.J.; Chang, S.E.; Lee, G.Y. Hesperidin, A Popular Antioxidant Inhibits Melanogenesis via Erk1/2 Mediated MITF Degradation. Int. J. Mol. Sci. 2015, 16, 18384–18395. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Park, S.H.; Kwon, S.B.; Kwon, N.S.; Park, K.C. Sphingosylphosphorylcholine inhibits melanin synthesis via pertussis toxin-sensitive MITF degradation. J. Pharm. Pharmacol. 2010, 62, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Englaro, W.; Rezzonico, R.; Durand-Clement, M.; Lallemand, D.; Ortonne, J.P.; Ballotti, R. Mitogen-activated protein kinase pathway and AP-1 are activated during cAMP-induced melanogenesis in B-16 melanoma cells. J. Biol. Chem. 1995, 270, 24315–24320. [Google Scholar] [CrossRef] [PubMed]
- Hirata, N.; Naruto, S.; Ohguchi, K.; Akao, Y.; Nozawa, Y.; Iinuma, M.; Matsuda, H. Mechanism of the melanogenesis stimulation activity of (–)-cubebin in murine B16 melanoma cells. Bioorg. Med. Chem. 2007, 15, 4897–4902. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, J.S.; Woo, J.T.; Lee, I.S.; Cha, B.Y. Hyperpigmentation mechanism of methyl 3,5-di-caffeoylquinate through activation of p38 and MITF induction of tyrosinase. Acta Biochim. Biophys. Sin. 2015, 47, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Ma, P.C.; Chen, Z.Q.; Zhou, W.Q.; Fu, Y.J.; Li, L.J.; Li, C.R. Inhibition of MITF and tyrosinase by paeonol-stimulated JNK/SAPK to reduction of phosphorylated CREB. Am. J. Chin. Med. 2008, 36, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S.; Sung, J.H.; Lee, S.K. Antimelanogenesis Activity of Hydrolyzed Ginseng Extract (GINST) via Inhibition of JNK Mitogen-activated Protein Kinase in B16F10 Cells. J. Food Sci. 2016, 81, H2085–H2092. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Yoon, H.D.; Kim, J.I.; Choi, J.S.; Byun, D.S.; Kim, H.R. Dioxinodehydroeckol inhibits melanin synthesis through PI3K/Akt signalling pathway in alpha-melanocyte-stimulating hormone-treated B16F10 cells. Exp. Dermatol. 2012, 21, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.X.; Lin, M.; Lu, S.S.; Qi, X.Y.; Zhang, R.X.; Zhang, Y.Y. Curcumin inhibits melanogenesis in human melanocytes. Phytother. Res. 2012, 26, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; Andjelkovic, M.; Caudwell, B.; Cron, P.; Morrice, N.; Cohen, P.; Hemmings, B.A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996, 15, 6541–6551. [Google Scholar] [PubMed]
- Cross, D.A.E.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Busca, R.; Bertolotto, C.; Ortonne, J.P.; Ballotti, R. Inhibition of the phosphatidylinositol 3-kinase/p70(S6)-kinase pathway induces B16 melanoma cell differentiation. J. Biol. Chem. 1996, 271, 31824–31830. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.Y.; Yin, W.H.; Wang, M.R.; Dang, Y.Y.; Ye, X.Y. Andrographolide suppresses melanin synthesis through Akt/GSK3beta/beta-catenin signal pathway. J. Dermatol. Sci. 2015, 79, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Ryu, M.; Jeong, Y.; Chung, Y.H.; Kim, D.E.; Cho, H.S.; Kang, S.; Han, J.S.; Chang, M.Y.; Lee, C.K.; et al. Cardamonin suppresses melanogenesis by inhibition of Wnt/beta-catenin signaling. Biochem. Biophys. Res. Commun. 2009, 390, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.J.; Williams, B.O.; Li, Y.; Pavan, W.J. Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development. Proc. Natl. Acad. Sci. USA 2000, 97, 10050–10055. [Google Scholar] [CrossRef] [PubMed]
- Bellei, B.; Pitisci, A.; Catricala, C.; Larue, L.; Picardo, M. Wnt/beta-catenin signaling is stimulated by alpha-melanocyte-stimulating hormone in melanoma and melanocyte cells: Implication in cell differentiation. Pigment Cell Melanoma Res. 2011, 24, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.; Ganesan, A.K. The pleiotropic roles of autophagy regulators in melanogenesis. Pigment Cell Melanoma Res. 2011, 24, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Jo, Y.K.; Park, S.J.; Chang, H.; Shin, J.H.; Choi, E.S.; Kim, J.B.; Seok, S.H.; Kim, J.S.; Oh, J.S.; et al. ARP101 inhibits alpha-MSH-stimulated melanogenesis by regulation of autophagy in melanocytes. FEBS Lett. 2013, 587, 3955–3960. [Google Scholar] [CrossRef] [PubMed]
- Van den Boorn, J.G.; Picavet, D.I.; van Swieten, P.F.; van Veen, H.A.; Konijnenberg, D.; van Veelen, P.A.; van Capel, T.; Jong, E.C.; Reits, E.A.; Drijfhout, J.W.; et al. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase haptenation and melanosome autophagy. J. Investig. Dermatol. 2011, 131, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Romero-Graillet, C.; Aberdam, E.; Clement, M.; Ortonne, J.P.; Ballotti, R. Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. J. Clin. Investig. 1997, 99, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, H.; Cao, J.; Ren, J.; Fan, R.; He, X.; Smith, G.W.; Dong, C. Nitric oxide enhances melanogenesis of alpaca skin melanocytes in vitro by activating the MITF phosphorylation. Mol. Cell. Biochem. 2011, 352, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cao, J.; Wang, H.; Zhang, J.; Zhu, Z.; Bai, R.; Hao, H.; He, X.; Fan, R.; Dong, C. Nitric oxide enhances the sensitivity of alpaca melanocytes to respond to α-melanocyte-stimulating hormone by up-regulating melanocortin-1 receptor. Biochem. Biophys. Res. Commun. 2010, 396, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Esparza, M.; Ferrer, C.; Castells, M.T.; Garcia-Borron, J.C.; Zuasti, A. Transforming growth factor beta1 mediates hypopigmentation of B16 mouse melanoma cells by inhibition of melanin formation and melanosome maturation. Int. J. Biochem. Cell Biol. 2001, 33, 971–983. [Google Scholar] [CrossRef]
- Minwalla, L.; Zhao, Y.; Cornelius, J.; Babcock, G.F.; Wickett, R.R.; Le Poole, I.C.; Boissy, R.E. Inhibition of melanosome transfer from melanocytes to keratinocytes by lectins and neoglycoproteins in an in vitro model system. Pigment Cell Res. 2001, 14, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Yang, H.M.; Kang, S.M.; Ahn, G.N.; Roh, S.W.; Lee, W.; Kim, D.K.; Jeon, Y.J. Whitening Effect of Octaphlorethol A Isolated from Ishige foliacea in an In Vivo Zebrafish Model. J. Microbiol. Biotechnol. 2015, 25, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Joe, M.J.; Kim, S.N.; Choi, H.Y.; Shin, W.S.; Park, G.M.; Kang, D.W.; Kim, Y.K. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Shin, T.; Utsuki, T.; Choi, J.S.; Byun, D.S.; Kim, H.R. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and hepatoprotective properties in tacrine-treated HepG2 cells. J. Agric. Food Chem. 2012, 60, 5340–5349. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.J.; Lee, M.; Shin, T.; Yoon, N.; Kim, J.H.; Kim, H.R. Eckol enhances heme oxygenase-1 expression through activation of Nrf2/JNK pathway in HepG2 cells. Molecules 2014, 19, 15638–15652. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lee, M.S.; Choi, J.W.; Utsuki, T.; Kim, J.I.; Jang, B.C.; Kim, H.R. Phlorofucofuroeckol A suppresses expression of inducible nitric oxide synthase, cyclooxygenase-2, and pro-inflammatory cytokines via inhibition of nuclear factor-kappaB, c-Jun NH2-terminal kinases, and Akt in microglial cells. Inflammation 2013, 36, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.-Y.; Choi, J.-S.; Byun, D.-S. Inhibitory effects of phloroglucinol derivatives isolated from Ecklonia stolonifera on FcεRI expression. Bioorg. Med. Chem. 2009, 17, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch. Pharm. Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Ko, S.-C.; Kang, S.-M.; Cha, S.-H.; Lee, S.-H.; Kang, D.-H.; Jung, W.-K.; Affan, A.; Oh, C.; Jeon, Y.-J. Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food Chem. Toxicol. 2010, 48, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Yang, H.M.; Kang, S.M.; Kim, D.; Ahn, G.; Jeon, Y.J. Octaphlorethol A isolated from Ishige foliacea inhibits alpha-MSH-stimulated induced melanogenesis via ERK pathway in B16F10 melanoma cells. Food Chem. Toxicol. 2013, 59, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.-S.; Park, H.-Y.; Nam, K.-H. Whitening effects of 4-hydroxyphenethyl alcohol isolated from water boiled with Hizikia fusiformis. Food Sci. Biotechnol. 2014, 23, 555–560. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kang, S.-M.; Sok, C.H.; Hong, J.T.; Oh, J.-Y.; Jeon, Y.-J. Cellular activities and docking studies of eckol isolated from Ecklonia cava (Laminariales, Phaeophyceae) as potential tyrosinase inhibitor. Algae 2015, 30, 163–170. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Balcos, M.C.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Kim, M.K.; Kim, D.S. ERK Activation by Fucoidan Leads to Inhibition of Melanogenesis in Mel-Ab Cells. Korean J. Physiol. Pharmacol. 2015, 19, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Tanaka, J.; Shan, S.J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.Y.; Kim, K.H.; Cheah, S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, M.-S.; Lee, B.; Gwon, W.-G.; Joung, E.-J.; Yoon, N.-Y.; Kim, H.-R. Anti-inflammatory effects of sargachromenol-rich ethanolic extract of Myagropsis myagroides on lipopolysaccharide-stimulated BV-2 cells. BMC Complement. Altern. Med. 2014, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Jung, Y.; Kim, M.C.; Kwon, H.C.; Kang, K.S.; Kim, Y.K.; Kim, S.N. Sargahydroquinoic acid inhibits TNFalpha-induced AP-1 and NF-kappaB signaling in HaCaT cells through PPARalpha activation. Biochem. Biophys. Res. Commun. 2014, 450, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Joung, E.J.; Lee, B.; Gwon, W.G.; Shin, T.; Jung, B.M.; Yoon, N.Y.; Choi, J.S.; Oh, C.W.; Kim, H.R. Sargaquinoic acid attenuates inflammatory responses by regulating NF-kappaB and Nrf2 pathways in lipopolysaccharide-stimulated RAW 264.7 cells. Int. Immunopharmacol. 2015, 29, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Joung, E.J.; Kwon, M.S.; Lee, B.; Utsuki, T.; Oh, C.W.; Kim, H.R. Anti-Inflammatory Effect of Ethanolic Extract of Sargassum serratifolium in Lipopolysaccharide-Stimulated BV2 Microglial Cells. J. Med. Food 2016, 19, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Gwon, W.G.; Joung, E.J.; Kwon, M.S.; Lim, S.J.; Utsuki, T.; Kim, H.R. Sargachromenol protects against vascular inflammation by preventing TNF-alpha-induced monocyte adhesion to primary endothelial cells via inhibition of NF-kappaB activation. Int. Immunopharmacol. 2017, 42, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Lim, S.-J.; Lee, B.; Shin, T.; Kim, H.-R. Ethanolic extract of Sargassum serratifolium inhibits adipogenesis in 3T3-L1 preadipocytes by cell cycle arrest. J. Appl. Phycol. 2017, 1–10. [Google Scholar] [CrossRef]
- De la Mare, J.A.; Lawson, J.C.; Chiwakata, M.T.; Beukes, D.R.; Edkins, A.L.; Blatch, G.L. Quinones and halogenated monoterpenes of algal origin show anti-proliferative effects against breast cancer cells in vitro. Investig. New Drugs 2012, 30, 2187–2200. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.W.; Park, M.S.; Kim, N.H.; Lee, J.H.; Oh, C.W.; Kim, H.R.; Kim, G.D. Hexane extract from Sargassum serratifolium inhibits the cell proliferation and metastatic ability of human glioblastoma U87MG cells. Oncol. Rep. 2015, 34, 2602–2608. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.K.; Kamei, Y. Sargaquinoic acid supports the survival of neuronal PC12D cells in a nerve growth factor-independent manner. Eur. J. Pharmacol. 2004, 488, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H.; Dell’Acqua, G.; Gardiner, V.-A.; Karpiniec, S.S.; Stringer, D.N.; Davis, E. Topical Benefits of Two Fucoidan-Rich Extracts from Marine Macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef]
- Wang, Z.J.; Si, Y.X.; Oh, S.; Yang, J.M.; Yin, S.J.; Park, Y.D.; Lee, J.; Qian, G.Y. The effect of fucoidan on tyrosinase: Computational molecular dynamics integrating inhibition kinetics. J. Biomol. Struct. Dyn. 2012, 30, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.V.; Mazurov, A.V.; Preobrazhenskaia, M.E.; Ushakova, N.A.; Mikhailov, V.I.; Berman, A.E.; Usov, A.I.; Nifant’ev, N.E.; Bovin, N.V. Sulfated polysaccharides as inhibitors of receptor activity of P-selectin and P-selectin-dependent inflammation. Vopr. Med. Khimii 1998, 44, 135–144. [Google Scholar] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H. Therapies from Fucoidan; Multifunctional Marine Polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Wichers, H.J.; Recourt, K.; Hendriks, M.; Ebbelaar, C.E.M.; Biancone, G.; Hoeberichts, F.A.; Mooibroek, H.; Soler-Rivas, C. Cloning, expression and characterisation of two tyrosinase cDNAs from Agaricus bisporus. Appl. Microbiol. Biotechnol. 2003, 61, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Seok, J.K.; Suh, H.J.; Choi, Y.H.; Hong, S.S.; Kim, D.S.; Boo, Y.C. Antimelanogenic effects of luteolin 7-sulfate isolated from Phyllospadix iwatensis Makino. Br. J. Dermatol. 2016, 175, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.K.; Subedi, L.; Jeong, M.; Park, Y.U.; Kim, C.Y.; Kim, H.; Kim, S.Y. Gomisin N Inhibits Melanogenesis through Regulating the PI3K/Akt and MAPK/ERK Signaling Pathways in Melanocytes. Int. J. Mol. Sci. 2017, 18, 471. [Google Scholar] [CrossRef] [PubMed]
- Tengamnuay, P.; Pengrungruangwong, K.; Pheansri, I.; Likhitwitayawuid, K. Artocarpus lakoocha heartwood extract as a novel cosmetic ingredient: Evaluation of the in vitro anti-tyrosinase and in vivo skin whitening activities. Int. J. Cosmet. Sci. 2006, 28, 269–276. [Google Scholar] [CrossRef] [PubMed]



| Algae | Compounds/Extract | Type | Action Mechanism | Experimental System | Reference |
|---|---|---|---|---|---|
| Ecklonia stolonifera | Dioxinodehydroeckol | Phlorotannin | PI3K/Akt-mediated downregulation of MITF | B16F10 mouse melanoma cells | [55] |
| Ishige okamurae | Diphlorethohydroxycarmalol | Phlorotannin | Inhibition of mushroom TYR and melanin synthesis | B16F10 cells | [79] |
| E. cava | Eckol | Phlorotannin | Inhibition of cell free TYR (non-competitive) & cellular TYR, TRP1, and TRP2 | B16F10 cells | [82] |
| Dieckol | Inhibition of mushroom TYR & cellular melanin | B16F10 cells | [18] | ||
| Dioxinodehydroeckol | Mushroom TYR inhibition | Cell free | [83] | ||
| 7-phloroeckol | Inhibition of mushroom TYR (non-competitive) & cellular melanin | B16F10 cells | [83] | ||
| I. foliacea | Octaphlorethol A | Phlorotannin | ERK1/2-mediated downregulation of MITF, TYR, TRP1 & TRP2 in B16. Inhibition of in vivo TYR activity and melanin synthesis | B16F10 cells, Zebra fish embryo | [72,80] |
| Hizikia fusiformis | 4-hydroxyphenethyl alcohol | Non-flavonoid phenolic compound | Inhibition of mushroom TYR and melanin synthesis in B16. Reduction of pigmented spots in guinea-pig skin | B16F10 cells, Brown guinea-pig | [81] |
| Fucus vesiculosus | Fucoidan | Fucose-rich sulfated polysaccharide | ERK-mediated downregulation of MITF. | Mel-Ab cells | [84] |
| Inhibition of cellular TYR activity, melanin content & cell proliferation | B16 murine melanoma cells | [42] | |||
| Laminaria Japonica | Fucoxanthin | Carotenoid | Reduced TYR activity in B16 and melanin content in guinea-pigs & mice skin. Suppress PGE2, MSH, TRP1 & melanogenic stimulant receptors, NTR, EP1 & MC1R in vivo | B16 murine melanoma, UVB-induced mice, & guinea-pig | [85] |
| Sargassum serratifolium | Ethanolic extract containing sargaquinoic acid, sargahydroquinoic acid & sargachromenol | Meroterpenoid | cAMP and ERK1/2-mediated downregulation of MITF | B16F10 cells | [30] |
| S. polycystum | Ethanolic extract & its hexane fraction | NR | Inhibition of cellular TYR & melanin production | B16F10 cells | [86] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azam, M.S.; Choi, J.; Lee, M.-S.; Kim, H.-R. Hypopigmenting Effects of Brown Algae-Derived Phytochemicals: A Review on Molecular Mechanisms. Mar. Drugs 2017, 15, 297. https://doi.org/10.3390/md15100297
Azam MS, Choi J, Lee M-S, Kim H-R. Hypopigmenting Effects of Brown Algae-Derived Phytochemicals: A Review on Molecular Mechanisms. Marine Drugs. 2017; 15(10):297. https://doi.org/10.3390/md15100297
Chicago/Turabian StyleAzam, Mohammed Shariful, Jinkyung Choi, Min-Sup Lee, and Hyeung-Rak Kim. 2017. "Hypopigmenting Effects of Brown Algae-Derived Phytochemicals: A Review on Molecular Mechanisms" Marine Drugs 15, no. 10: 297. https://doi.org/10.3390/md15100297
APA StyleAzam, M. S., Choi, J., Lee, M.-S., & Kim, H.-R. (2017). Hypopigmenting Effects of Brown Algae-Derived Phytochemicals: A Review on Molecular Mechanisms. Marine Drugs, 15(10), 297. https://doi.org/10.3390/md15100297






