In vitro Anti-Thrombotic Activity of Extracts from Blacklip Abalone (Haliotis rubra) Processing Waste
Abstract
:1. Introduction
2. Results and Discussion
2.1. Protein and Sulphated Polysaccharide Content of Extracts from Blacklip Abalone Processing Waste
2.2. Separation of Abalone Extracts Using Anion Exchange Chromatography-Fast Performance Liquid Chromatography (AEC-FPLC)
2.3. Anti-Thrombotic Activity Measured through HCII-Mediated Thrombin Inhibition
2.4. Anti-Thrombotic and Anti-Coagulant Activity in Blood and Plasma
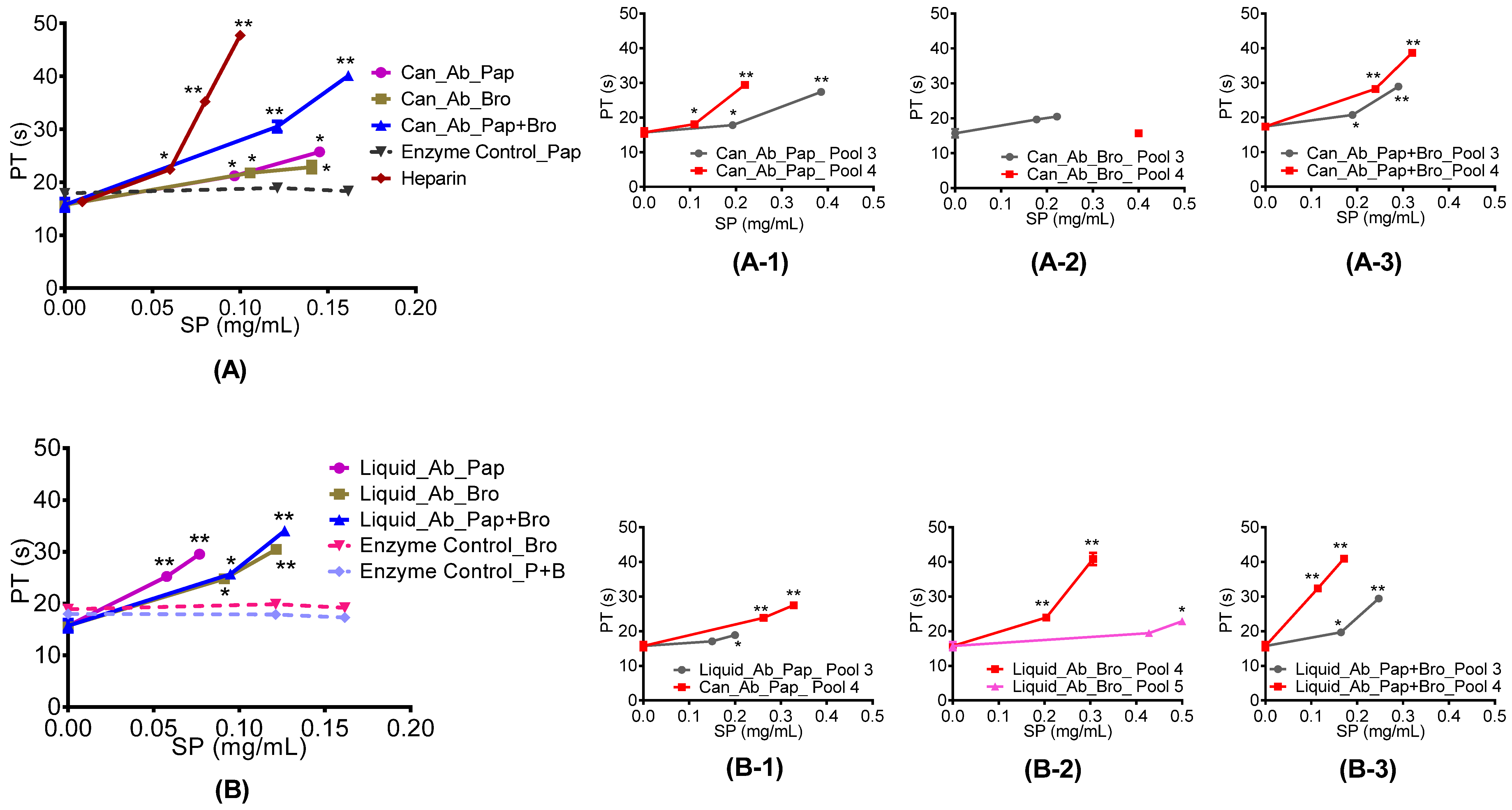
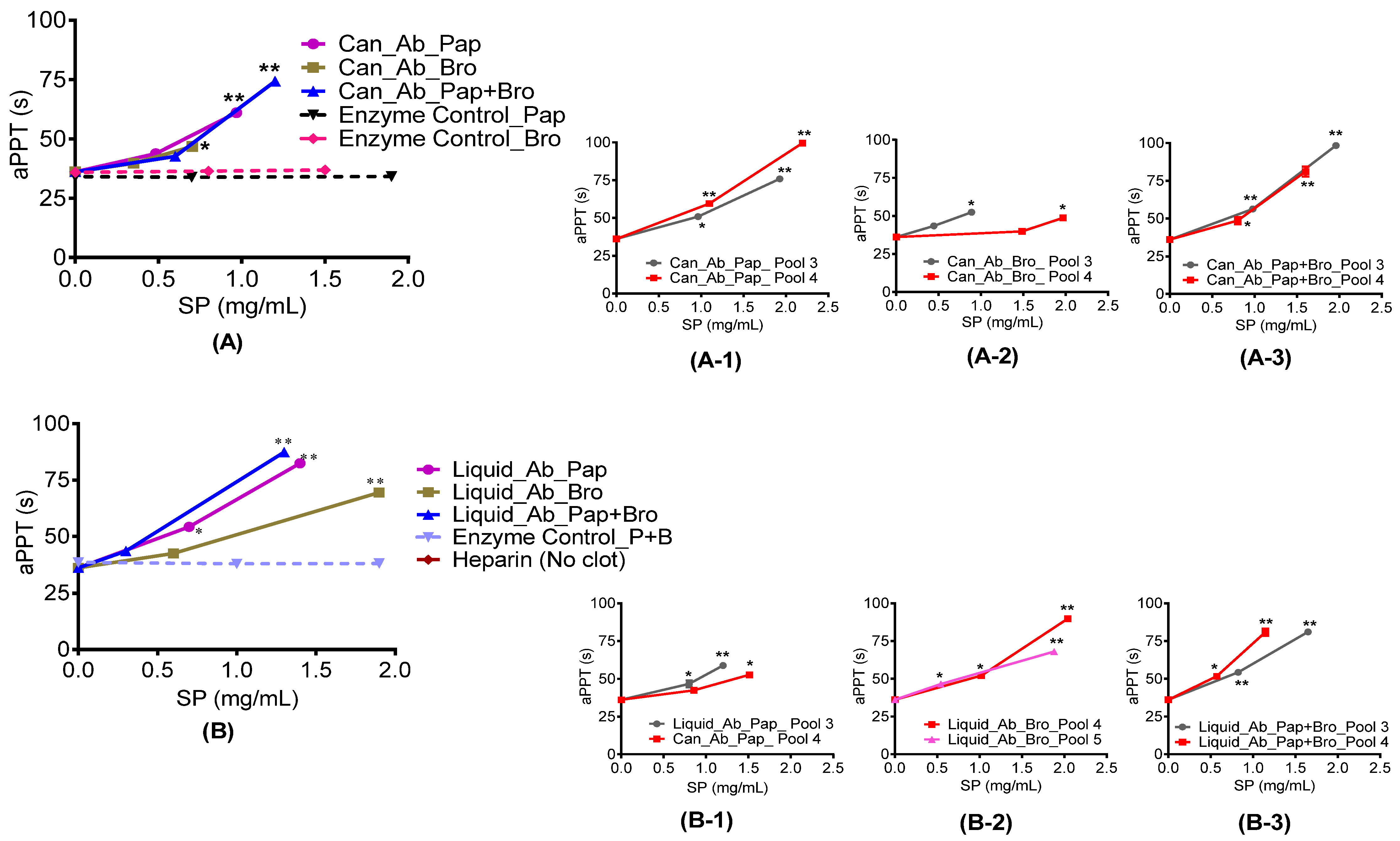
2.4.1. Prothrombin and Activated Partial Thromboplastin Time
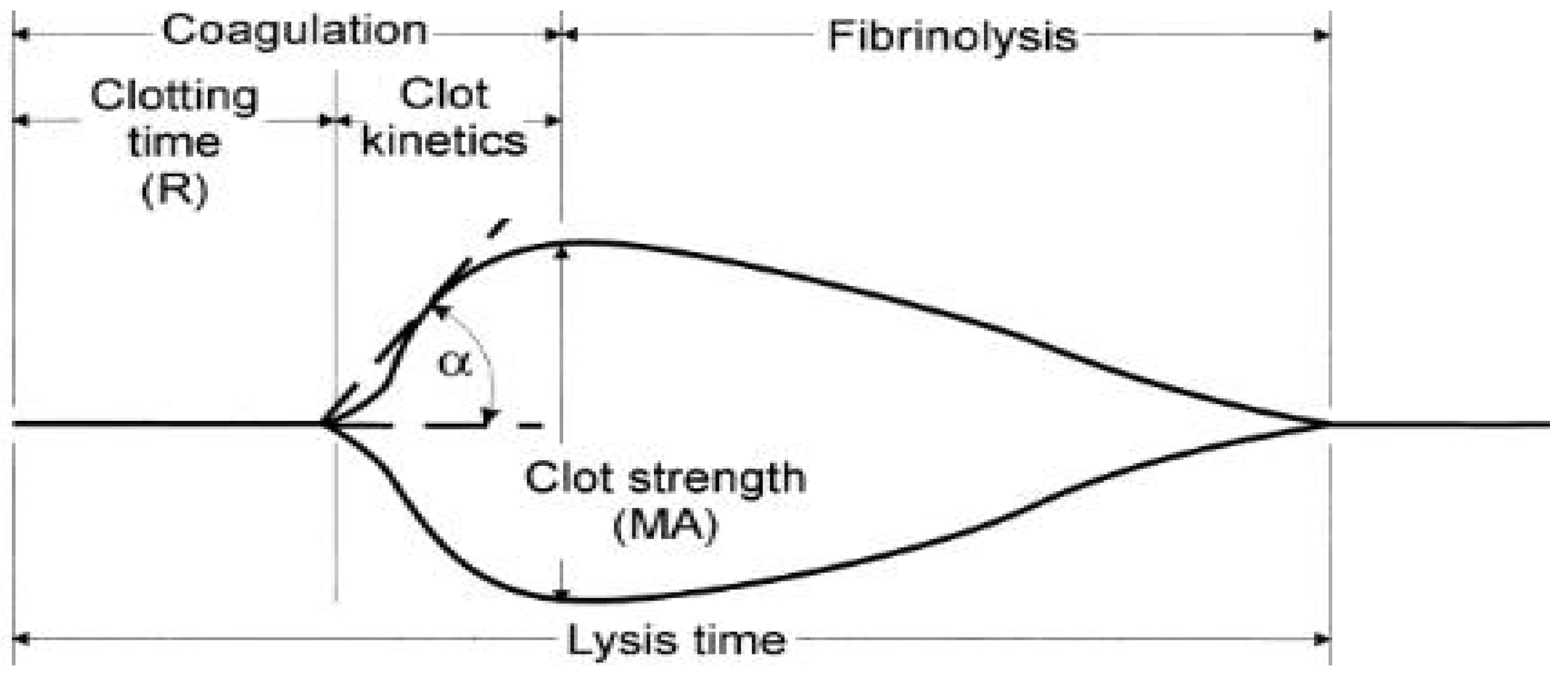
2.4.2. Thromboelastography (TEG)
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Extracts
3.3. Estimation of Sulphated Glycosaminoglycan Content
3.3.1. Dimethyl-Methylene Blue (DMMB) Assay
3.3.2. Blyscan Sulphated Glycosaminoglycan (GAG) Assay
3.4. Estimation of Protein Content
3.5. Separation of Extracts Using Anion Exchange Chromatography-Fast Performance Liquid Chromatography (AEC-FPLC)
3.6. Assessment of Anti-Thrombotic and Anti-Coagulant Activity
3.6.1. Heparin Cofactor II (HCII) Mediated Thrombin Inhibition Assay
3.6.2. Prothrombin Time (PT) Assay
3.6.3. Activated Partial Thromboplastin Time (aPTT) Assay
3.6.4. Thromboelastography (TEG)
3.7. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Wijesekara, I.; Kim, S.K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.M. Extraction and characterization of bioactive carbohydrates with health benefits from marine resources: macro- and microalgae, cyanobacteria, and invertebrates. In Marine Bioactive Compounds: Sources, Characterization and Applications; Hayes, M., Ed.; Springer: Boston, MA, USA, 2012; pp. 159–172. [Google Scholar]
- Suleria, H.A.R.; Masci, P.P.; Gobe, G.C.; Osborne, S.A. Therapeutic potential of abalone and status of bioactive molecules: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gates, K.W. Marine nutraceuticals: prospects and perspectives Edited by Se-Kwon Kim. J. Aquat. Food Prod. Technol. 2014, 23, 522–527. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Leopold, J.A.; Loscalzo, J. Oxidative risk for atherothrombotic cardiovascular disease. Free Radic. Biol. Med. 2009, 47, 1673–1706. [Google Scholar] [CrossRef] [PubMed]
- David, J.C. Pharmacology and Pharmaco-Therapeutics, 7th ed.; Vikas Publishing House: New Delhi, India, 1979. [Google Scholar]
- Franchini, M.; Liumbruno, G.M.; Bonfanti, C.; Lippi, G. The evolution of anticoagulant therapy. Blood Transfus. 2016, 14, 175–184. [Google Scholar] [PubMed]
- Ahmed, I.; Majeed, A.; Powell, R. Heparin induced thrombocytopenia: Diagnosis and management update. Postgrad. Med. J. 2007, 83, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.; Mody, K.H.; Oza, R.M.; Ramavat, B.K. Blood anticoagulant activity of a green marine alga Codium dwarkense (Codiaceae, Chlorophyta) in relation to its growth stages. Indian J. Geo-Mar. Sci. 2001, 30, 49–52. [Google Scholar]
- Kim, Y.S.; Jo, Y.Y.; Chang, I.M.; Toida, T.; Park, Y.; Linhardt, R.J. A new glycosaminoglycan from the giant African snail Achatina fulica. J. Biol. Chem. 1996, 271, 11750–11755. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.C.; Toutain, S.; Walter, B.; Pinck, L.; Otten, L.; Coutos-Thevenot, P.; Barbier, P. High efficiency regeneration of grapevine plants transformed with the GFLV coat protein gene. Plant Sci. 1995, 112, 97–106. [Google Scholar] [CrossRef]
- Santos, J.C.; Mesquita, J.M.F.; Belmiro, C.L.R.; da Silveira, C.B.M.; Viskov, C.; Mourier, P.A.; Pavão, M.S.G. Isolation and characterization of a heparin with low antithrombin activity from the body of Styela plicata (Chordata-Tunicata). Distinct effects on venous and arterial models of thrombosis. Thromb. Res. 2007, 121, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Lima, M.; Ribeiro, I.; Pinto, R.A.; Machado, J. Isolation, purification and characterization of glycosaminoglycans in the fluids of the mollusc Anodonta cygnea. Comp. Biochem. Physiol. A 2005, 141, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Sikka, P.; Bindra, V.K. Newer antithrombotic drugs. Indian journal of critical care medicine: Peer-reviewed. Off. Publ. Indian Soc. Crit. Care Med. 2010, 14, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Chen, S.G.; Wang, Y.M.; Xue, Y.; Chang, Y.G.; Li, Z.J.; Xue, C.H. A novel glycosaminoglycan-like polysaccharide from abalone Haliotis discus hannai Ino: Purification, structure identification and anticoagulant activity. Int. J. Biol. Macromol. 2001, 49, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.Y.; Tang, Y.; Zhu, B.W.; Qin, L.; Li, D.M.; Yang, J.F.; Murata, Y. Antioxidant activity of hydrolysates obtained from scallop (Patinopecten yessoensis) and abalone (Haliotis discus hannai Ino) muscle. Food Chem. 2012, 132, 815–822. [Google Scholar] [CrossRef]
- Kechaou, E.S.; Dumay, J.; Donnay-Moreno, C.; Jaouen, P.; Gouygou, J.P.; Bergé, J.P.; Amar, R.B. Enzymatic hydrolysis of cuttlefish (Sepia officinalis) and sardine (Sardina pilchardus) viscera using commercial proteases: Effects on lipid distribution and amino acid composition. J. Biosci. Bioeng. 2009, 107, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.W.; Zhou, D.Y.; Li, T.; Yan, S.; Yang, J.F.; Li, D.M.; Murata, Y. Chemical composition and free radical scavenging activities of a sulphated polysaccharide extracted from abalone gonad (Haliotis discus hannai Ino). Food Chem. 2010, 121, 712–718. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wu, F.J.; Du, L.; Li, G.Y.; Takahashi, K.; Xue, Y.; Xue, C.H. Effects of polysaccharides from abalone (Haliotis discus hannai Ino) on HepG2 cell proliferation. Int. J. Biol. Macromol. 2014, 66, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.W.; Zhou, D.Y.; Yang, J.F.; Li, D.M.; Yin, H.L.; Tada, M. A neutral polysaccharide from the abalone pleopod, Haliotis discus hannai Ino. Eur. Food Res. Technol. 2008, 228, 591–595. [Google Scholar]
- She, Z.G.; Hu, G.P.; Wu, Y.W. Study on the methanolysis of the sulphated polysaccharide Hal-A from Haliotis diverisicolor Reeve. Chin. J. Org. Chem. 2002, 22, 367–370. [Google Scholar]
- Chen, S.; Xue, C.; Yin, L.A.; Tang, Q.; Yu, G.; Chai, W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Pereira, M.S.; Valente, A.-P.; Tollefsen, D.M.; Pavão, M.S.G.; Mourão, P.A.S. Selective cleavage and anticoagulant activity of a sulfated fucan: Stereospecific removal of a 2-sulfate ester from the polysaccharide by mild acid hydrolysis, preparation of oligosaccharides, and heparin cofactor II–dependent anticoagulant activity. Glycobiology 2005, 15, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.S.; Melo, F.R.; Mourão, P.A.S. Is there a correlation between structure and anticoagulant action of sulfated galactans and sulfated fucans? Glycobiology 2002, 12, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wen, D.; Gao, N.; Xiao, C.; Yang, L.; Xu, L.; Lian, W.; Peng, W.; Jiang, J.; Zhao, J. Anticoagulant and antithrombotic evaluation of native fucosylated chondroitin sulfates and their derivatives as selective inhibitors of intrinsic factor Xase. Eur. J. Med. Chem. 2015, 92, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tong, T.; Ko, D.O.; Kang, S.G. Antithrombotic potential of extracts from abalone, Haliotis discus hannai Ino: and animal studies. Food Sci. Biotechnol. 2013, 22, 471–476. [Google Scholar] [CrossRef]
- Fischer, T.H.; Bode, A.P.; Demcheva, M.; Vournakis, J.N. Hemostatic properties of glucosamine-based materials. J. Biomed. Mater. Res. A 2007, 80, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Dupouy, D.; Sié, P.; Dol, F.; Boneu, B. A simple method to measure dermatan sulfate at sub-microgram concentrations in plasma. Thromb. Haemost. 1988, 60, 236–239. [Google Scholar] [PubMed]
- Hines, B.M.; Suleria, H.A.R.; Osborne, S.A. Automated screening potential thrombin inhibitors using the epMotion® 5075. Eppendorf 2016, 377, 1–6. [Google Scholar]
- Rivard, G.E.; Brummel-Ziedins, K.E.; Mann, K.G.; Fan, L.; Hofer, A.; Cohen, E. Evaluation of the profile of thrombin generation during the process of whole blood clotting as assessed by thromboelastography. J. Thromb. Haemost. 2005, 3, 2039–2043. [Google Scholar] [CrossRef] [PubMed]
 is shown in relation to linear NaCl gradient
is shown in relation to linear NaCl gradient  in canned abalone processing waste digested with (A) papain; (B) bromelain; (C) papain + bromelain, and liquid abalone processing waste digested with (D) papain; (E) bromelain and (F) papain + bromelain.
in canned abalone processing waste digested with (A) papain; (B) bromelain; (C) papain + bromelain, and liquid abalone processing waste digested with (D) papain; (E) bromelain and (F) papain + bromelain.
 is shown in relation to linear NaCl gradient
is shown in relation to linear NaCl gradient  in canned abalone processing waste digested with (A) papain; (B) bromelain; (C) papain + bromelain, and liquid abalone processing waste digested with (D) papain; (E) bromelain and (F) papain + bromelain.
in canned abalone processing waste digested with (A) papain; (B) bromelain; (C) papain + bromelain, and liquid abalone processing waste digested with (D) papain; (E) bromelain and (F) papain + bromelain.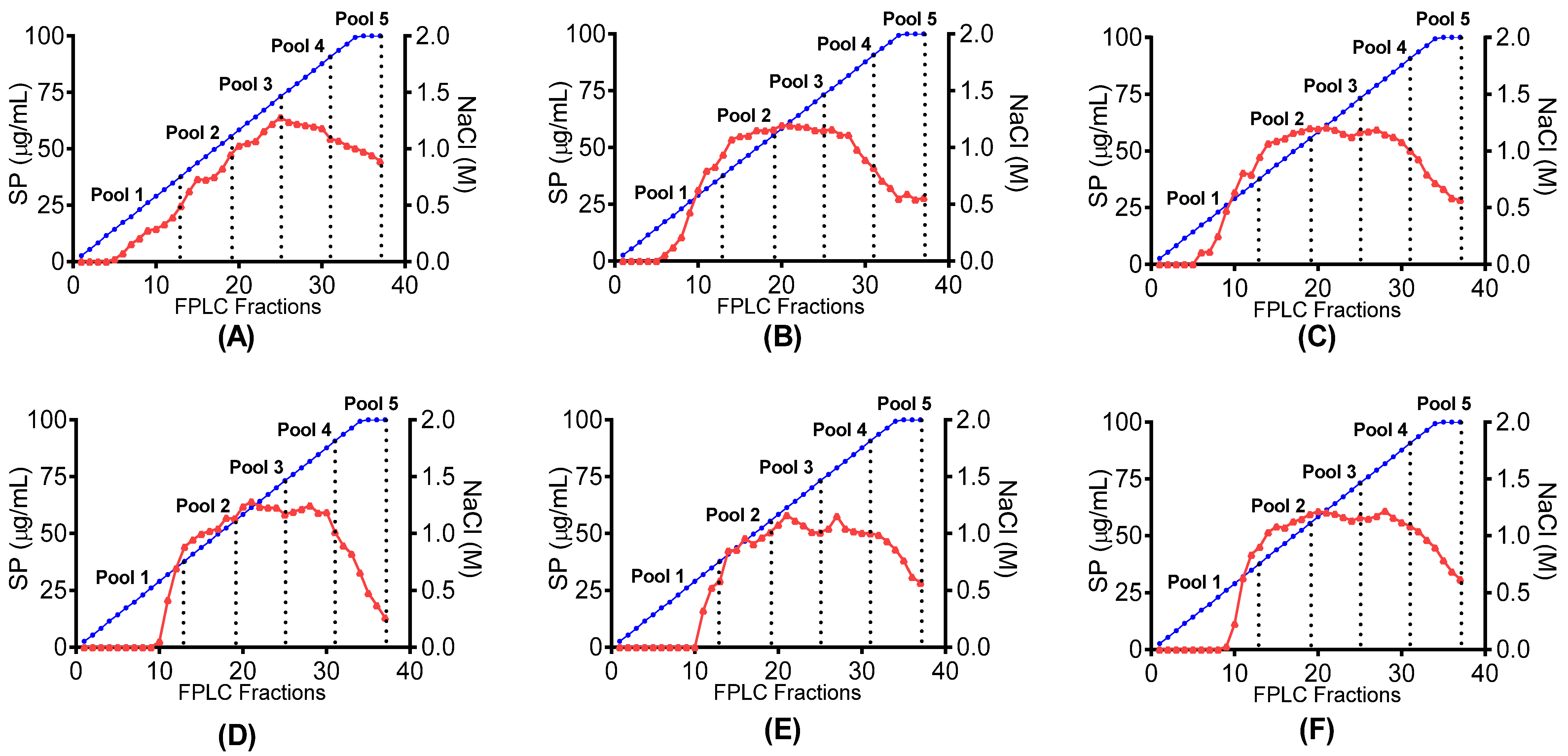
| Abalone Waste | Treatments | Protein (mg/g) | Sulphated Polysaccharides (mg/g) | Anti-Thrombin HCII (% Inhibition) |
|---|---|---|---|---|
| Canned | Papain | 29.95 ± 0.51 b | 1.36 ± 0.09 a | 92.1 ± 1.31 b |
| Canned | Bromelain | 25.06 ± 1.79 c | 1.39 ± 0.91 a | 89.9 ± 2.09 c |
| Canned | Papain + Bromelain | 36.10 ± 0.72 a | 1.46 ± 0.38 a | 96.8 ± 1.12 a |
| Liquid | Papain | 18.82 ± 0.10 e | 1.27 ± 0.82 a | 97.1 ± 0.08 a |
| Liquid | Bromelain | 23.38 ± 2.09 d | 1.03 ± 0.13 a | 95.4 ± 2.13 a |
| Liquid | Papain + Bromelain | 18.90 ± 0.80 e | 1.41 ± 0.68 a | 91.1 ± 0.79 b |
| Sample Descriptions | Protein (mg/mL) | Sulphated Polysaccharides (mg/mL) |
|---|---|---|
| Can_Ab_Pap_Unbound material | 3.40 ± 1.1 | 1.12 ± 0.9 |
| Can_Ab_Pap_AEC Pool 1 | 0.76 ± 0.7 | 0.19 ± 0.4 |
| Can_Ab_Pap_AEC Pool 2 | 0.59 ± 0.4 | 1.04 ± 0.2 |
| Can_Ab_Pap_AEC Pool 3 | 1.87 ± 0.2 | 1.82 ± 1.2 |
| Can_Ab_Pap_AEC Pool 4 | 0.37 ± 0.1 | 1.49 ± 0.2 |
| Can_Ab_Pap_AEC Pool 5 | 0.23 ± 0.9 | 1.15 ± 1.3 |
| Can_Ab_Pap_Final column wash | 0.36 ± 2.3 | 0.03 ± 2.1 |
| Can_Ab_Bro_Unbound material | 5.77 ± 1.4 | 0.56 ± 0.9 |
| Can_Ab_Bro_AEC Pool 1 | 0.74 ± 0.4 | 0.30 ± 0.3 |
| Can_Ab_Bro_AEC Pool 2 | 0.64 ± 0.1 | 1.02 ± 0.8 |
| Can_Ab_Bro_AEC Pool 3 | 0.16 ± 0.2 | 1.11 ± 0.4 |
| Can_Ab_Bro_AEC Pool 4 | 0.11 ± 0.1 | 1.21 ± 0.1 |
| Can_Ab_Bro_AEC Pool 5 | 0.05 ± 1.2 | 0.22 ± 1.1 |
| Can_Ab_Bro_Final column wash | 0.06 ± 2.1 | 0.02 ± 2.1 |
| Can_Ab_Pap+Bro_Unbound material | 8.92 ± 1.2 | 0.65 ± 0.4 |
| Can_Ab_Pap+Bro_AEC Pool 1 | 1.31 ± 0.3 | 0.42 ± 0.2 |
| Can_Ab_Pap+Bro_AEC Pool 2 | 1.90 ± 0.1 | 2.01 ± 1.1 |
| Can_Ab_Pap+Bro_AEC Pool 3 | 0.37 ± 0.7 | 2.45 ± 0.9 |
| Can_Ab_Pap+Bro_AEC Pool 4 | 0.33 ± 0.1 | 2.00 ± 0.1 |
| Can_Ab_Pap+Bro_AEC Pool 5 | 0.05 ± 0.3 | 0.38 ± 0.7 |
| Can_Ab_Pap+Bro_Final column wash | 0.67 ± 0.9 | 0.54 ± 1.2 |
| Liquid_Ab_Pap_Unbound material | 2.52 ± 1.1 | 0.03 ± 0.4 |
| Liquid_Ab_Pap_AEC Pool 1 | 0.17 ± 0.2 | 0.04 ± 0.1 |
| Liquid_Ab_Pap_AEC Pool 2 | 0.44 ± 0.8 | 0.70 ± 0.8 |
| Liquid_Ab_Pap_AEC Pool 3 | 0.33 ± 0.3 | 1.25 ± 0.7 |
| Liquid_Ab_Pap_AEC Pool 4 | 0.13 ± 0.1 | 1.64 ± 0.2 |
| Liquid_Ab_Pap_AEC Pool 5 | 0.02 ± 1.2 | 0.16 ± 0.7 |
| Liquid_Ab_Pap_Final column wash | 0.39 ± 2.1 | 0.01 ± 1.1 |
| Liquid_Ab_Bro_Unbound material | 6.12 ± 1.1 | 0.40 ± 0.9 |
| Liquid_Ab_Bro_AEC Pool 1 | 0.00 ± 2.1 | 0.01 ± 0.1 |
| Liquid_Ab_Bro_AEC Pool 2 | 0.00 ± 0.5 | 0.02 ± 0.9 |
| Liquid_Ab_Bro_AEC Pool 3 | 0.91 ± 0.1 | 1.04 ± 0.2 |
| Liquid_Ab_Bro_AEC Pool 4 | 1.41 ± 0.5 | 3.10 ± 0.7 |
| Liquid_Ab_Bro_AEC Pool 5 | 0.21 ± 1.2 | 0.61 ± 1.1 |
| Liquid_Ab_Bro_Final column wash | 0.11 ± 0.9 | 0.47 ± 1.9 |
| Liquid_Ab_Pap+Bro_Unbound material | 10.34 ± 0.9 | 0.95 ± 0.5 |
| Liquid_Ab_Pap+Bro_AEC Pool 1 | 0.45 ± 0.7 | 0.06 ± 0.6 |
| Liquid_Ab_Pap+Bro_AEC Pool 2 | 0.39 ± 0.9 | 2.07 ± 0.7 |
| Liquid_Ab_Pap+Bro_AEC Pool 3 | 0.56 ± 0.3 | 2.06 ± 0.2 |
| Liquid_Ab_Pap+Bro_AEC Pool 4 | 0.27 ± 0.2 | 1.43 ± 0.1 |
| Liquid_Ab_Pap+Bro_AEC Pool 5 | 0.11 ± 0.1 | 0.53 ± 0.9 |
| Liquid_Ab_Pap+Bro_Final column wash | 0.10 ± 1.1 | 0.05 ± 0.1 |
| Percentage Inhibition of Thrombin Mediated by HCII at 10 min | ||||
|---|---|---|---|---|
| Sample Description | Sulphated Polysaccharide Concentration (μg/mL) | |||
| 100 | 50 | 10 | 1 | |
| Can_Ab_Pap | 93.1 ± 0.8 d | 26 ± 7.5 j | 11.4 ± 10.2 l | 0 |
| Can_Ab_Pap_Unbound material | 56 ± 0.4 k | 26.9 ± 0.8 j | 0 | 0 |
| Can_Ab_Pap_AEC Pool 2 | 8.5 ± 1.9 s | 8.7 ± 3.4 q | 0 | 0 |
| Can_Ab_Pap_AEC Pool 3 | 87.6 ± 3.2 e | 81.8 ± 0.8 f | 13.9 ± 6.6 k | 0 |
| Can_Ab_Pap_AEC Pool 4 | 96.1 ± 0.4b c | 92.7 ± 1.7 c | 43 ± 3.9 f | 9.4 ± 6.6 d |
| Can_Ab_Pap_AEC Pool 5 | 97.5 ± 0.2 ab | 94.6 ± 1.8 b | 78.2 ± 2.7 b | 20.7 ± 4.2 b |
| Can_Ab_Bro | 72.3 ± 1.2 g | 13.2 ± 2.9 ° | 2.4 ± 1.1 p | 0 |
| Can_Ab_Bro_Unbound material | 14.2 ± 5.1 r | 0 | 0 | 0 |
| Can_Ab_Bro_AEC Pool 2 | 56.6 ± 1.9 jk | 38.5 ± 6.1 h | 0 | 0 |
| Can_Ab_Br_AEC Pool 3 | 26.4 ± 3.7 q | 21.5 ± 3.6 m | 3.6 ± 4.5 ° | 0 |
| Can_Ab_Bro_AEC Pool 4 | 93.1 ± 0.5 d | 89.4 ± 0.2 e | 27.7 ± 3.8 g | 0 |
| Can_Ab_Bro_AEC Pool 5 | 45.3 ± 2.1 n | 17.9 ± 3.8 n | 0 | 0 |
| Can_Ab_Pap + Bro | 82.4 ± 0.6 f | 24 ± 2.2 l | 10.3 ± 6.2 m | 0 |
| Can_Ab_Pap+Bro_Unbound material | 61.27 ± 4.8 i | 13.21 ± 3.9 ° | 0 | 0 |
| Can_Ab_Pap+Bro_AEC Pool 2 | 34.3 ± 1.9 ° | 25.8 ± 2.4 kl | 0 | 0 |
| Can_Ab_Pap+Bro_AEC Pool 3 | 93.6 ± 0.3 d | 90.9 ± 0.5 d | 19.4 ± 2 j | 0 |
| Can_Ab_Pap+Bro_AEC Pool 4 | 96.4 ± 0.3b c | 94.9 ± 0.3 b | 56.9 ± 2.1 d | 14.3 ± 4.2 c |
| Can_Ab_Pap+Bro_AEC Pool 5 | 58.45 ± 2.8 j | 21.29 ± 1.2 m | 9.8 ± 2.7 mn | 0 |
| Liquid_Ab_Pap | 93.4 ± 1.1 d | 65.2 ± 2.1 g | 21.4 ± 0.9 i | 5.4 ± 1.1 e |
| Liquid_Ab_Pap_Unbound material | 12.43 ± 1.8 r | 0 | 0 | 0 |
| Liquid_Ab_Pap_AEC Pool 2 | 28.3 ± 0.9 q | 11.2 ± 0.7 p | 0 | 0 |
| Liquid_Ab_Pap_AEC Pool 3 | 94.9 ± 0.1 cd | 88.5 ± 1.8 e | 24.8 ± 2.3 h | 0 |
| Liquid_Ab_Pap_AEC Pool 4 | 98.5 ± 0.1 a | 96.3 ± 0.2 a | 69.1 ± 2.2 c | 13 ± 3.5 c |
| Liquid_Ab_Pap_AEC Pool 5 | 57.12 ± 0.7 jk | 21.23 ± 0.1 m | 10.2 ± 1.1 m | 0 |
| Liquid_Ab_ Bro | 64.32 ± 1.9 h | 21.4 ± 0.4 m | 10.2 ± 1.9 mn | 0 |
| Liquid_Ab_Bro_Unbound material | 14.26 ± 4.9 r | 2.7 ± 1.9 r | 0 | 0 |
| Liquid_Ab_Bro_AEC Pool 3 | 34.68 ± 0.8 ° | 11.2 ± 2.9 p | 0 | 0 |
| Liquid_Ab_Bro_AEC Pool 4 | 52.9 ± 0.7 l | 34.7 ± 1.9 i | 10.21 ± 0.8 mn | 0 |
| Liquid_Ab_Bro_AEC Pool 5 | 64.98 ± 1.8 h | 38.9 ± 0.7 h | 9.7 ± 1.9 n | 1.1 ± 1.8 f |
| Liquid_Ab_Pap+Bro | 93.1 ± 0.8 d | 26 ± 3.5 jk | 11.4 ± 10.2 l | 0 |
| Liquid_Ab_Pap+Bro_AEC Pool 2 | 32.1 ± 0.6 p | 20.6 ± 3.5 m | 3.1 ± 4.1 ° | 0 |
| Liquid_Ab_Pap+Bro_AEC Pool 3 | 95.3 ± 0.3 cd | 93.6 ± 1 c | 47.3 ± 0.8 e | 2.8 ± 2.2 ef |
| Liquid_Ab_Pap+Bro_AEC Pool 4 | 98.4 ± 0.1 a | 96.1 ± 2.2 a | 92.4 ± 1.2 a | 25.7 ± 0.4 a |
| Liquid_Ab_Pap+Bro_AEC Pool 5 | 47.4 ± 2.9 m | 21.49 ± 4.1 m | 1.4 ± 2.9 q | 0 |
| Heparin Standard | 16 | 4 | 2 | 0.5 |
| 91.5 ± 0.6 a | 75.0 ± 1.3 b | 48.0 ± 2.1 c | 27.6 ± 1.2 d | |
| Sample Description | SP Conc. (μg/mL) | R (s) | MA (mm) | α (Degree) |
|---|---|---|---|---|
| Control Saline | 0 | 445 ± 14.5 | 55.2 ± 1.2 | 45.2 ± 0.5 |
| Can_Ab_Pap | 20 | 760 ± 20.5 ** | 37.6 ± 2.4 ** | 12 ± 1.5 ** |
| 80 | 1115 ± 21.8 ** | 33.3 ± 1.7 ** | 22.2 ± 5.4 ** | |
| Can_Ab_Pap_AEC Pool 3 | 20 | 770 ± 8.5 ** | 35.3 ± 2.3 ** | 12 ± 1.7 ** |
| 30 | 1245 ± 12.2 ** | 34.8 ± 0.5 ** | 15.4 ± 0.9 ** | |
| Can_Ab_Pap_AEC Pool 4 | 22 | 930 ± 10.6 ** | 34.4 ± 1.9 ** | 12.8 ± 0.8 ** |
| 34 | 1475 ± 25.5 ** | 24.9 ± 1.5 ** | 10 ± 0.9 ** | |
| Can_Ab_Bro | 10 | 635 ± 10.2 ** | 49.1 ± 2.5 ** | 23.1 ± 3.7 ** |
| 60 | 1010 ± 24.7 ** | 29.8 ± 1.9 ** | 5.6 ± 4.1 ** | |
| Can_Ab_Bro_AEC Pool 3 | 7 | 515 ± 15.6 ** | 40.2 ± 3.6 ** | 31.7 ± 2.1 ** |
| 35 | 915 ± 20.3 ** | 33.4 ± 2.8 ** | 12.5 ± 1.7 ** | |
| Can_Ab_Bro_AEC Pool 4 | 8 | 495 ± 4.9 ** | 27 ± 0.5 ** | 18.1 ± 0.6 ** |
| 38 | 645 ± 15.6 ** | 33.6 ± 1.2 ** | 26 ± 0.9 ** | |
| Can_Ab_Pap+Bro | 20 | 845 ± 12.8 ** | 41.6 ± 1.4 ** | 19 ± 2.1 ** |
| 70 | 1340 ± 24.7 ** | 37.3 ± 2.5 ** | 14.3 ± 1.9 ** | |
| Can_Ab_Pap+Bro_AEC Pool 3 | 15 | 810 ± 20.3 ** | 37.3 ± 4.9 ** | 17.4 ± 1.1 ** |
| 31 | 1180 ± 25.9 ** | 35.6 ± 1.6 ** | 16.6 ± 0.4 ** | |
| Can_Ab_Pap+Bro_AEC Pool 4 | 13 | 690 ± 6.5 ** | 28.5 ± 1.4 ** | 17.2 ± 0.2 ** |
| 25 | 855 ± 12.5 ** | 21.7 ± 1.8 ** | 20.8 ± 0.4 ** | |
| Liquid_Ab_Pap | 30 | 940 ± 16.5 ** | 34.5 ± 4.4 ** | 12.5 ± 4.7 ** |
| 50 | 1410 ± 35.2 ** | 31.2 ± 1.2 ** | 14.9 ± 1.2 ** | |
| Liquid_Ab_Pap_AEC Pool 3 | 8 | 670 ± 14.7 ** | 46.3 ± 6.5 ** | 26.9 ± 4.9 ** |
| 39 | 1540 ± 23.6 ** | 33.6 ± 3.9 ** | 13.6 ± 3.4 ** | |
| Liquid_Ab_Pap_AEC Pool 4 | 10 | 500 ± 6.9 ** | 33.8 ± 2.1 ** | 28.8 ± 0.2 ** |
| 51 | 705 ± 11.8 ** | 25.1 ± 0.5 ** | 10.2 ± 0.9 ** | |
| Liquid_Ab_Bro | 40 | 710 ± 7.5 ** | 38.9 ± 4.7 ** | 25.9 ± 2.8 ** |
| 90 | 1075 ± 12.5 ** | 31.5 ± 1.2 ** | 12.2 ± 3.1 ** | |
| Liquid_Ab_Bro_AEC Pool 4 | 32 | 990 ± 4.2 ** | 36.1 ± 1.9 ** | 35.7 ± 1.7 ** |
| 64 | 1295 ± 10.9 ** | 26.9 ± 2.9 ** | 17.5 ± 2.1 ** | |
| Liquid_Ab_Bro_AEC Pool 5 | 4 | 620 ± 27.1 ** | 36.7 ± 5.9 ** | 32.3 ± 3.5 ** |
| 19 | 1115 ± 12.8 ** | 33.8 ± 7.6 ** | 13.7 ± 4.7 ** | |
| Liquid_Ab_Pap+Bro | 20 | 890 ± 8.5 ** | 39.6 ± 3.2 ** | 23.5 ± 1.9 ** |
| 40 | 1495 ± 24.5 ** | 36.1 ± 1.9 ** | 12.4 ± 2.2 ** | |
| Liquid_Ab_Pap+Bro_AEC Pool 3 | 13 | 685 ± 14.3 ** | 43 ± 2.9 ** | 29.6 ± 4.1 ** |
| 26 | 1865 ± 20.5 ** | 31.8 ± 3.9 ** | 8.5 ± 3.2 ** | |
| Liquid_Ab_Pap+Bro_AEC Pool 4 | 9 | 650 ± 10.3 ** | 27.3 ± 1.2 ** | 27.7 ± 0.8 ** |
| 45 | 1420 ± 15.8 ** | 35.6 ± 0.9 ** | 15.2 ± 0.1 ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suleria, H.A.R.; Hines, B.M.; Addepalli, R.; Chen, W.; Masci, P.; Gobe, G.; Osborne, S.A. In vitro Anti-Thrombotic Activity of Extracts from Blacklip Abalone (Haliotis rubra) Processing Waste. Mar. Drugs 2017, 15, 8. https://doi.org/10.3390/md15010008
Suleria HAR, Hines BM, Addepalli R, Chen W, Masci P, Gobe G, Osborne SA. In vitro Anti-Thrombotic Activity of Extracts from Blacklip Abalone (Haliotis rubra) Processing Waste. Marine Drugs. 2017; 15(1):8. https://doi.org/10.3390/md15010008
Chicago/Turabian StyleSuleria, Hafiz Ansar Rasul, Barney M. Hines, Rama Addepalli, Wei Chen, Paul Masci, Glenda Gobe, and Simone A. Osborne. 2017. "In vitro Anti-Thrombotic Activity of Extracts from Blacklip Abalone (Haliotis rubra) Processing Waste" Marine Drugs 15, no. 1: 8. https://doi.org/10.3390/md15010008
APA StyleSuleria, H. A. R., Hines, B. M., Addepalli, R., Chen, W., Masci, P., Gobe, G., & Osborne, S. A. (2017). In vitro Anti-Thrombotic Activity of Extracts from Blacklip Abalone (Haliotis rubra) Processing Waste. Marine Drugs, 15(1), 8. https://doi.org/10.3390/md15010008







