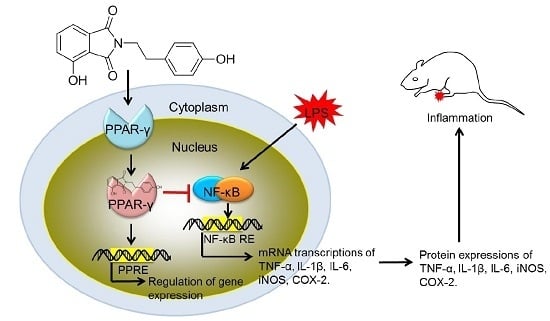
The In Vitro and In Vivo Anti-Inflammatory Effects of a Phthalimide PPAR-γ Agonist
Abstract
:1. Introduction
2. Results and Discussion
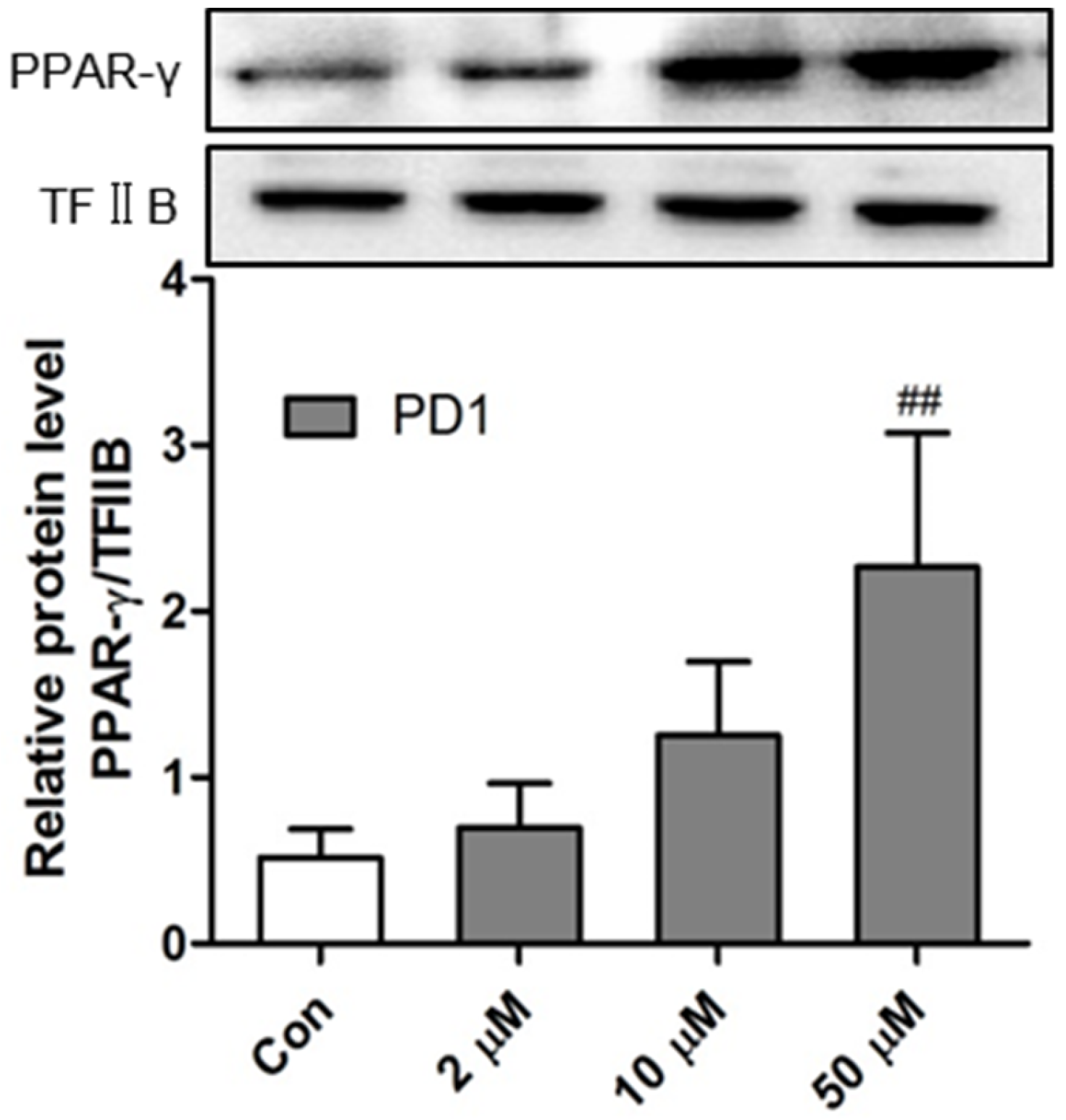
2.1. PD1 Promoted PPAR-γ Translocation to Cell Nuclei
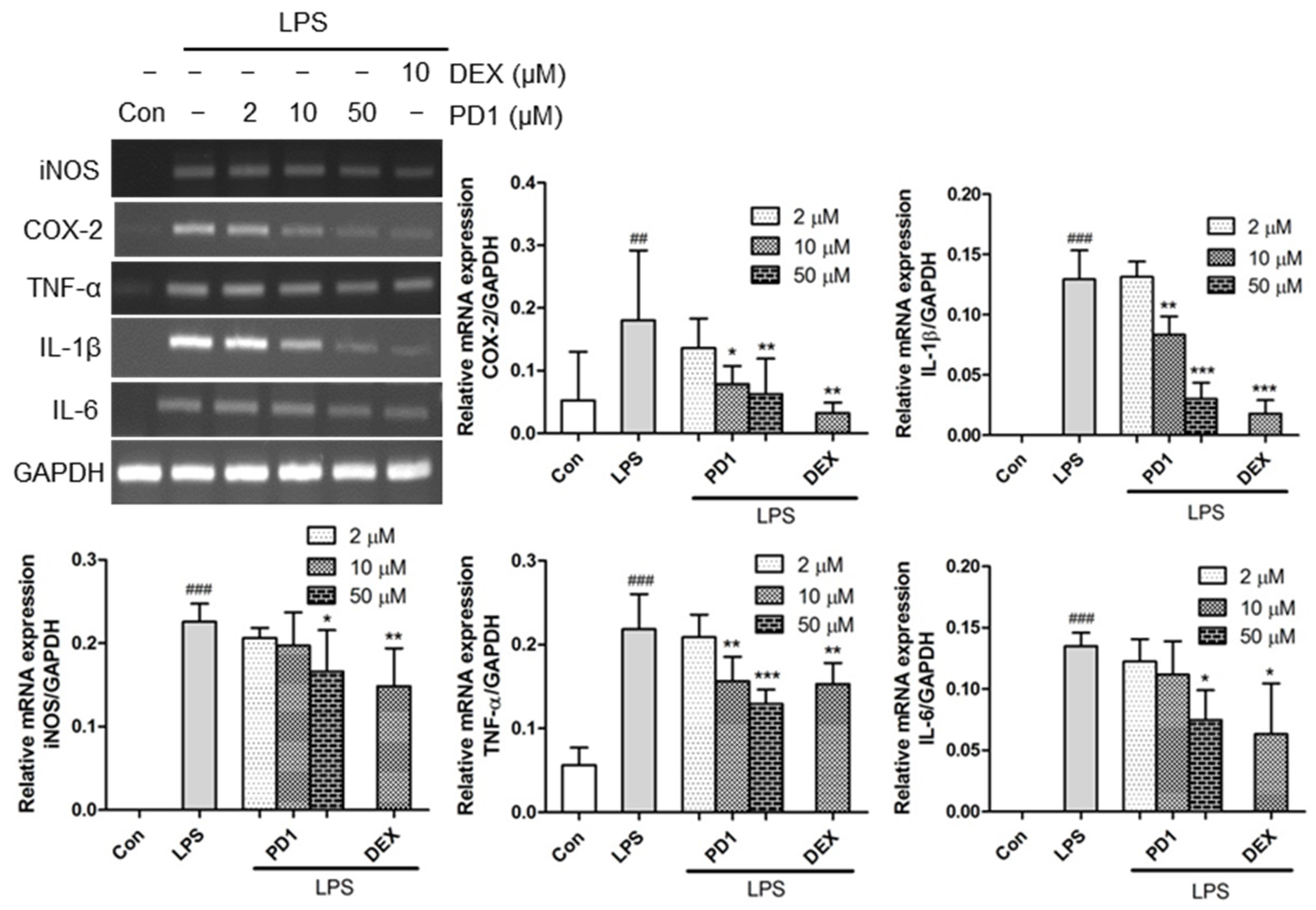
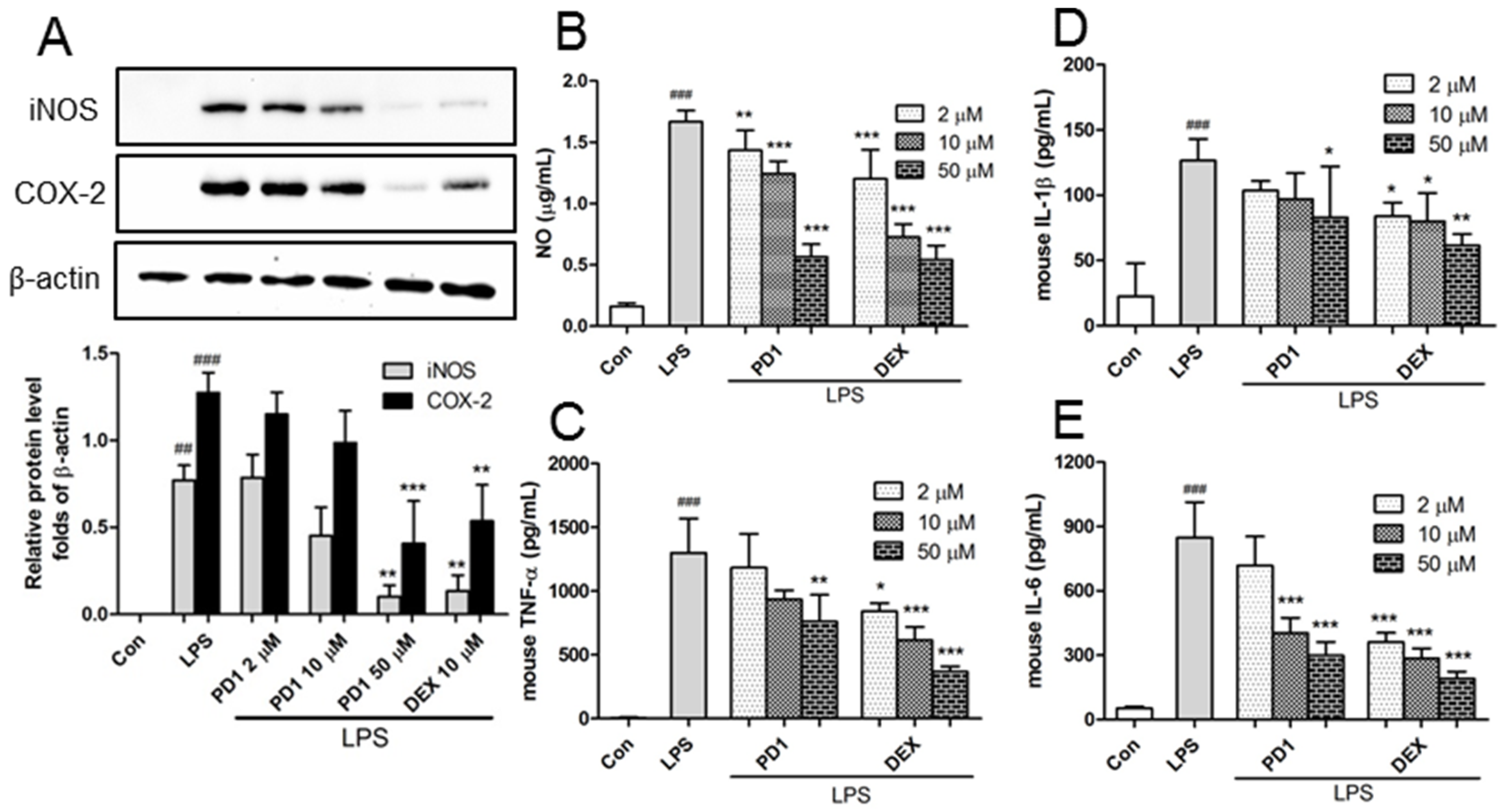
2.2. Decreased Expression of Pro-Inflammatory Factors by PD1
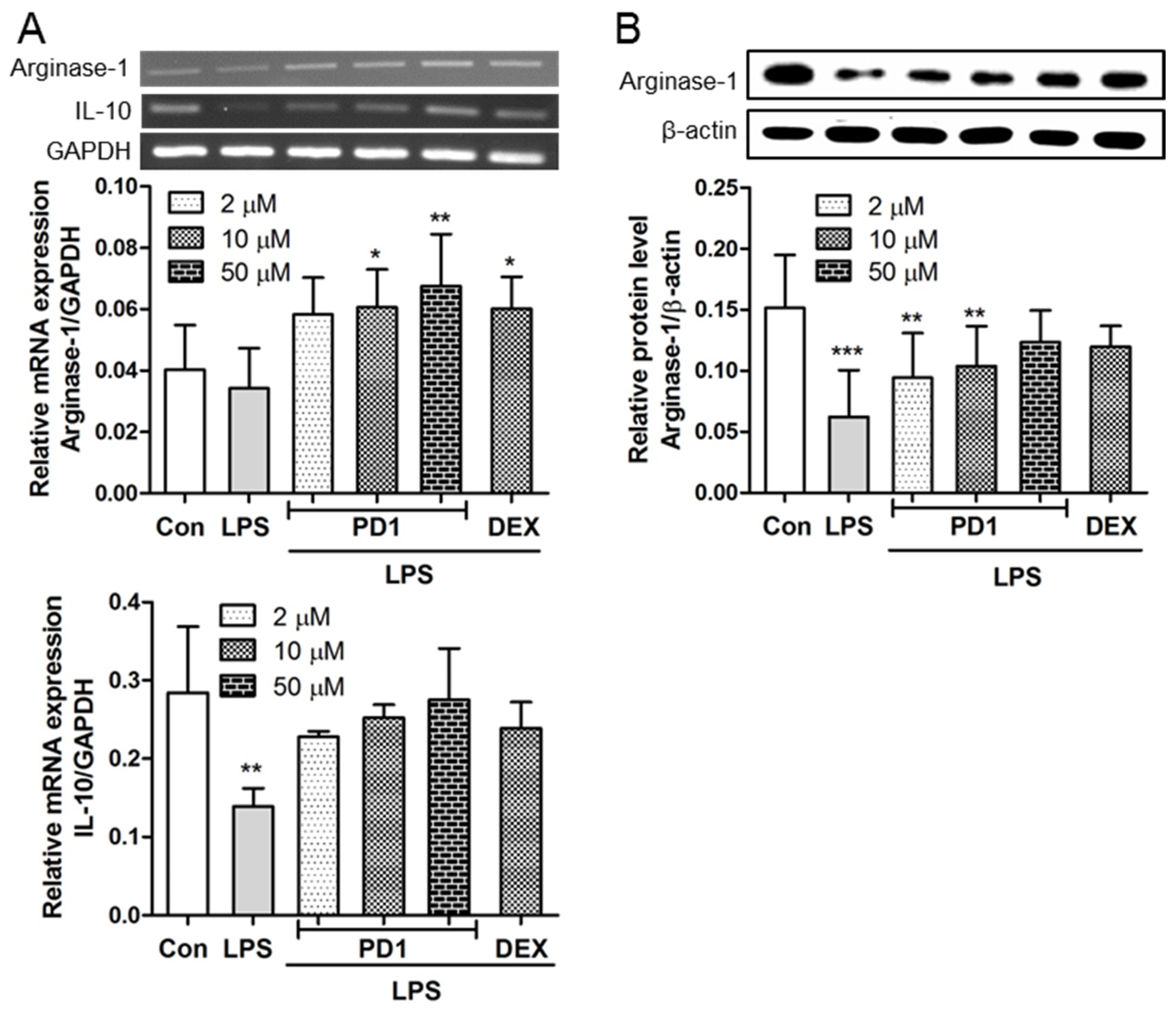
2.3. PD1 Enhanced the Expressions of Anti-Inflammatory Factors
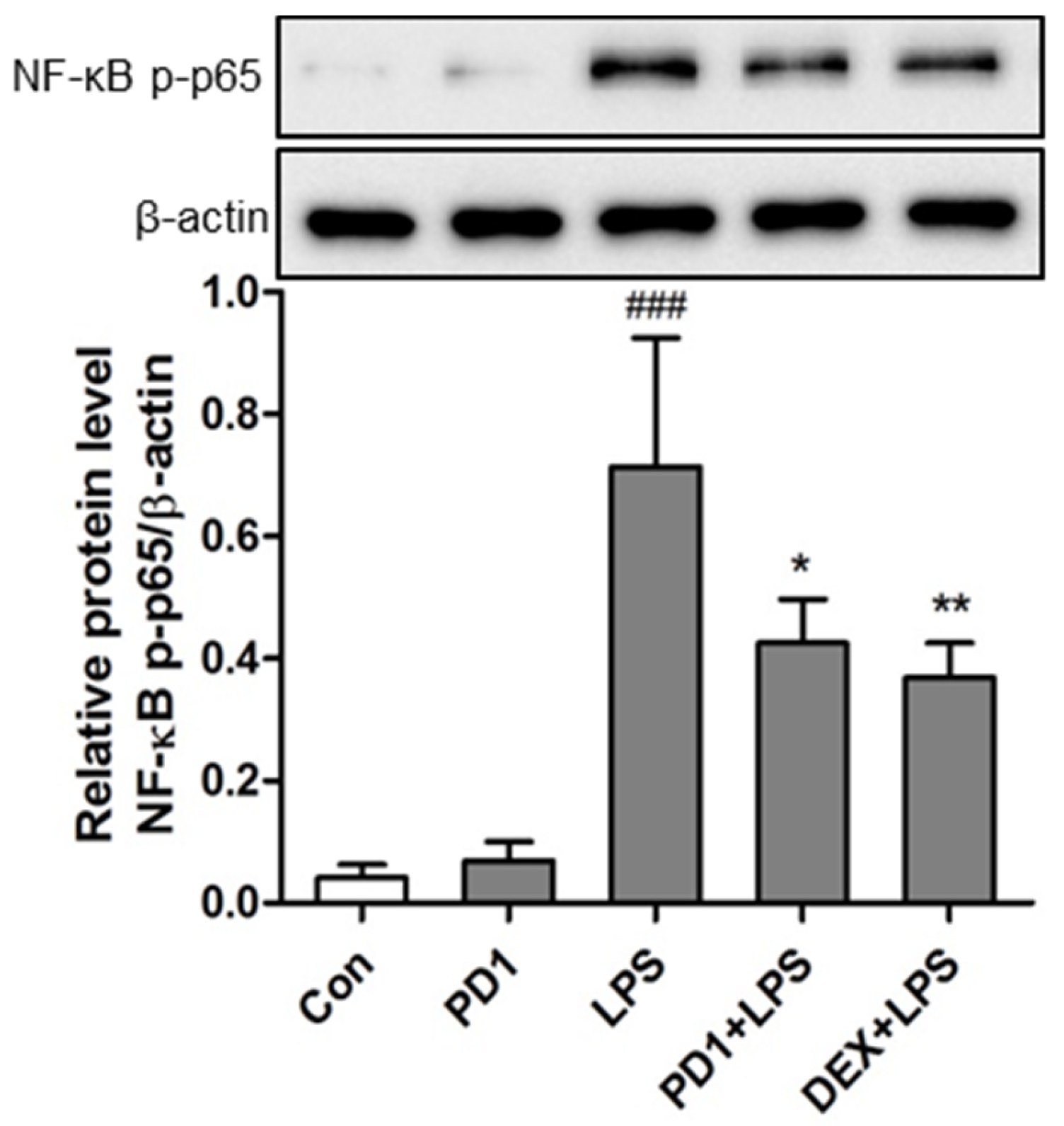
2.4. Modulation of the NF-κB Pathway by PD1
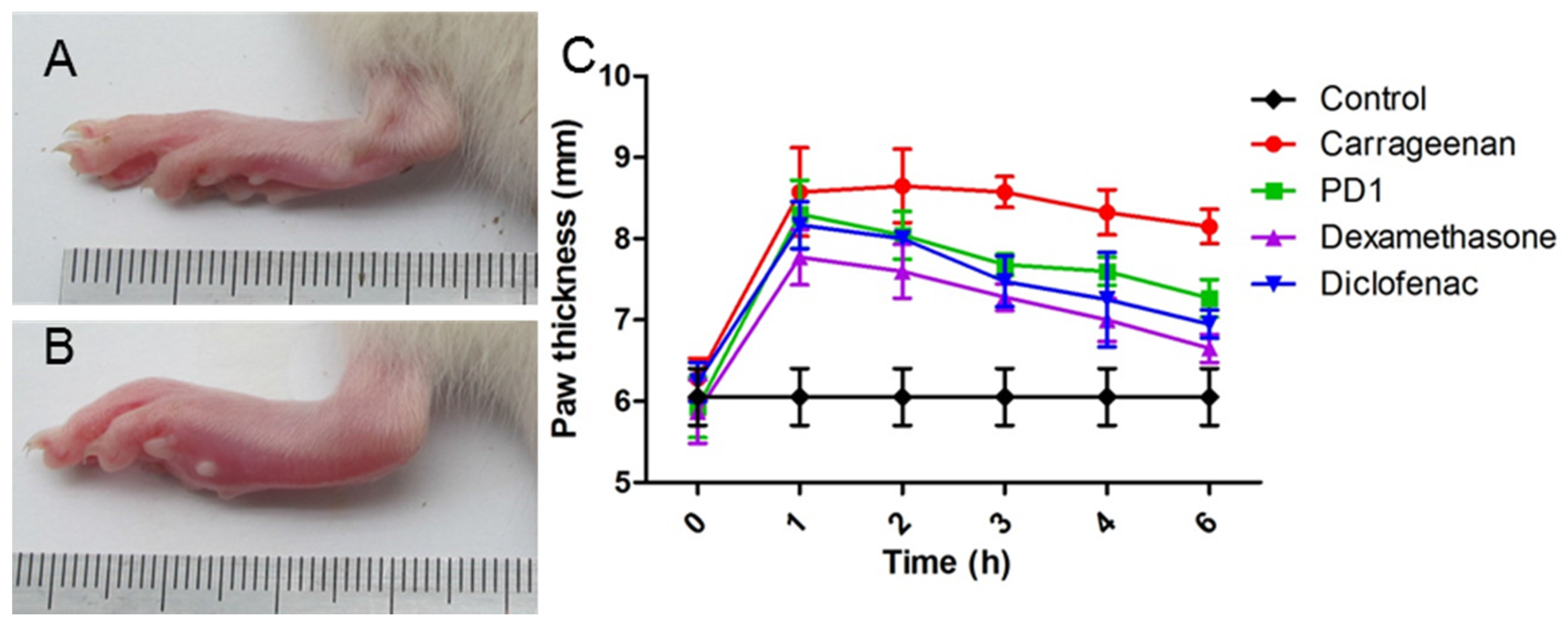
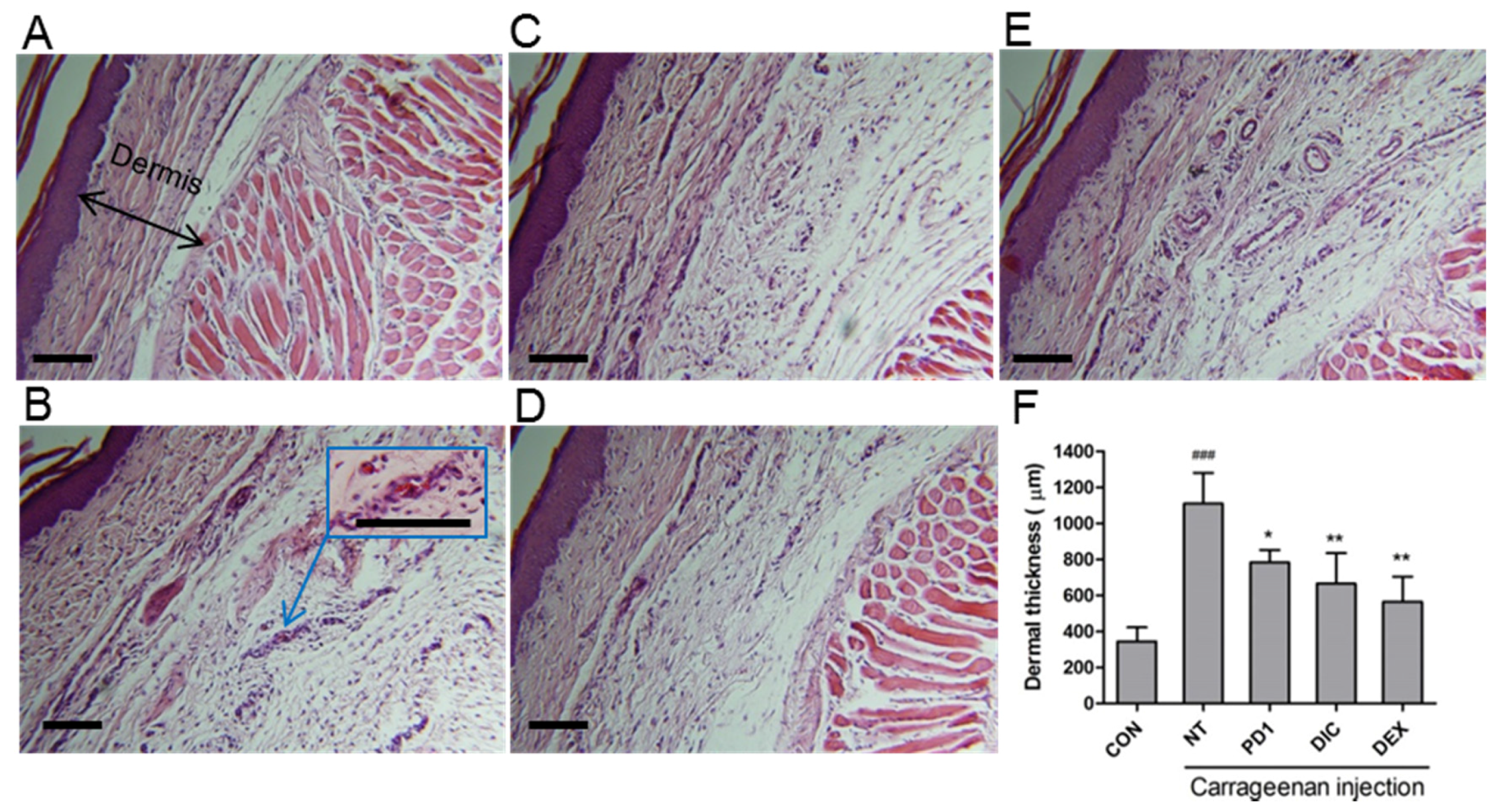
2.5. Anti-Inflammatory Effect of PD1 in a Carrageenan-Induced Paw Edema Model
3. Materials and Methods
3.1. Materials
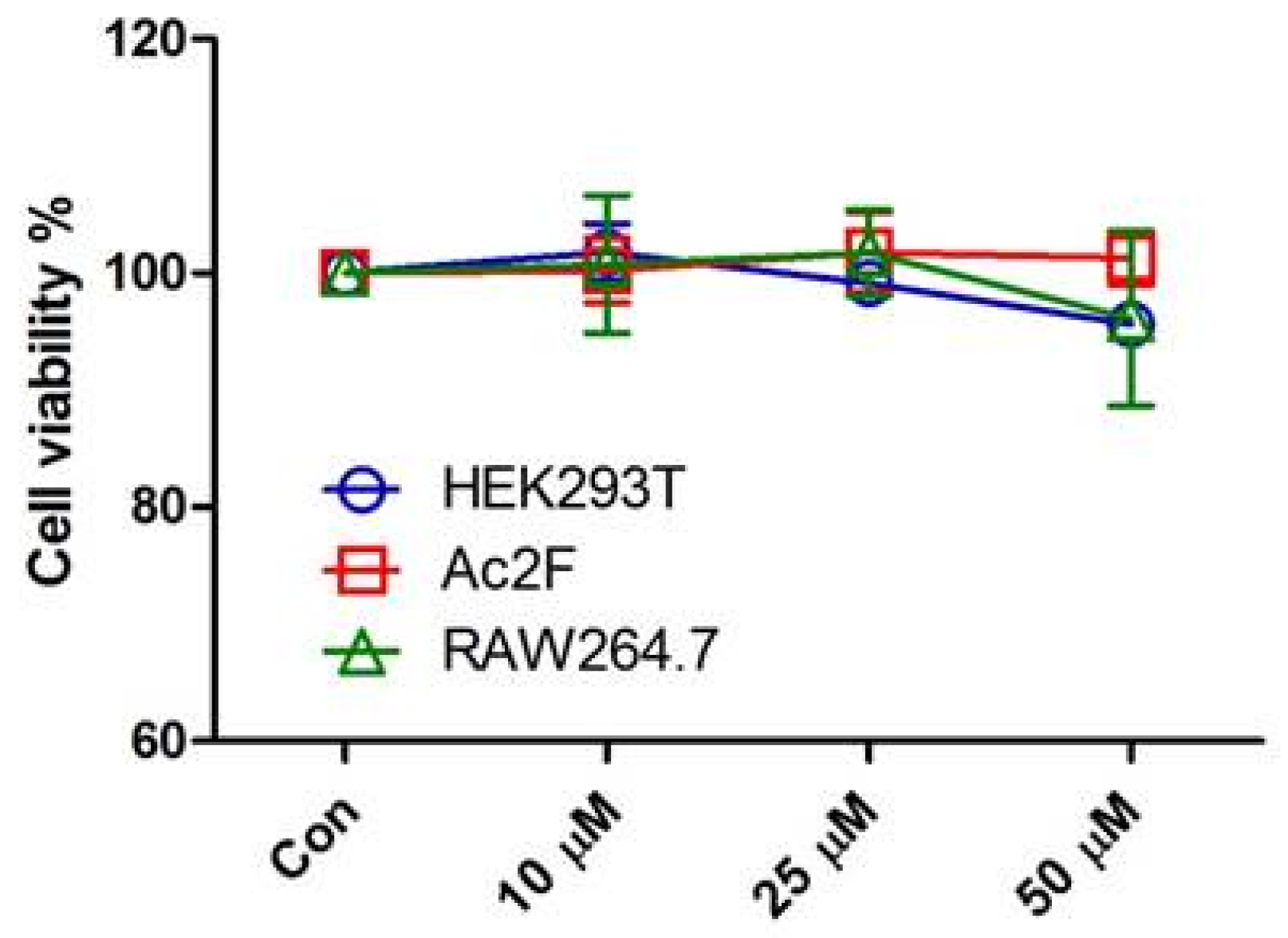
3.2. Cell Culture and Cell Viability
3.3. Reverse Transcriptase-PCR (RT-PCR)
3.4. Western Blot
3.5. Production Levels of NO and Cytokines
3.6. Animals
3.7. Carrageenan-Induced Rat Paw Edema Assay
3.8. Histology
3.9. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kliewer, S.A.; Xu, H.E.; Lambert, M.H.; Willson, T.M. Peroxisome proliferator-activated receptors: From genes to physiology. Recent Prog. Horm. Res. 2000, 56, 239–263. [Google Scholar] [CrossRef]
- Spiegelman, B. PPAR-gamma: Adipogenic regulator and thiazolidinedione receptor. Diabetes 1998, 47, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 99, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, L.; Roszer, T.; Ricote, M. Inflammatory mediators and insulin resistance in obesity: Role of nuclear receptor signaling in macrophages. Mediat. Inflamm. 2010. [Google Scholar] [CrossRef] [PubMed]
- Blanquart, C.; Barbier, O.; Fruchart, J.C.; Staels, B.; Glineur, C. Peroxisome proliferator-activated receptors: Regulation of transcriptional activities and roles in inflammation. J. Steroid Biochem. 2003, 85, 267–273. [Google Scholar] [CrossRef]
- Jiang, C.; Ting, A.T.; Seed, B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 1998, 391, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Ricote, M.; Li, A.C.; Willson, T.M.; Kelly, C.J.; Glass, C.K. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 1998, 391, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Bo, Q.-L.; Chen, Y.-H.; Yu, Z.; Fu, L.; Zhou, Y.; Zhang, G.-B.; Wang, H.; Zhang, Z.-H.; Xu, D.-X. Rosiglitazone pretreatment protects against lipopolysaccharide-induced fetal demise through inhibiting placental inflammation. Mol. Cell. Endocrinol. 2016, 423, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, W.; Kang, K.P.; Moon, S.-O.; Sung, M.J.; Kim, D.H.; Kim, H.J.; Park, S.K. Agonist of peroxisome proliferator-activated receptor-γ, rosiglitazone, reduces renal injury and dysfunction in a murine sepsis model. Nephrol. Dial. Transplant. 2005, 20, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Pisano, B.; Dugo, L.; Ianaro, A.; Maffia, P.; Patel, N.S.; Di Paola, R.; Ialenti, A.; Genovese, T.; Chatterjee, P.K. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-γ, reduces acute inflammation. Eur. J. Pharmacol. 2004, 483, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.; Fernandes, D.; Taylor, C.; Bonacci, J.; Quan, L.; Stewart, A. The PPARγ ligand, rosiglitazone, reduces airways hyperresponsiveness in a murine model of allergen-induced inflammation. Pulm. Pharmacol. Ther. 2006, 19, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Birrell, M.; Patel, H.; McCluskie, K.; Wong, S.; Leonard, T.; Yacoub, M.; Belvisi, M. PPAR-γ agonists as therapy for diseases involving airway neutrophilia. Eur. Respir. J. 2004, 24, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Takano, H.; Zou, Y.; Qin, Y.; Hizukuri, K.; Odaka, K.; Toyozaki, T.; Komuro, I. Pioglitazone, a peroxisome proliferator-activated receptor γ activator, ameliorates experimental autoimmune myocarditis by modulating Th1/Th2 balance. J. Mol. Cell. Cardiol. 2005, 38, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, J.D.; Verstege, M.I.; Thuijls, G.; Te Velde, A.A.; Mensink, R.P.; Plat, J. The PPARγ agonist rosiglitazone impairs colonic inflammation in mice with experimental colitis. J. Clin. Immunol. 2007, 27, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.-I.; Matsui, T.; Nakamura, K.; Takeuchi, M.; Inoue, H. Telmisartan inhibits advanced glycation end products (AGEs)-elicited endothelial cell injury by suppressing AGE receptor (RAGE) expression via peroxisome proliferator-activated receptor-γ activation. Protein Pept. Lett. 2008, 15, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Di, B.B.; Li, H.W.; Li, W.P.; Shen, X.H.; Sun, Z.J.; Wu, X. Pioglitazone inhibits high glucose-induced expression of receptor for advanced glycation end products in coronary artery smooth muscle cells. Mol. Med. Rep. 2015, 11, 2601–2607. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.T.; Lee, H.J.; Yoo, E.S.; Hong, J.; Bao, B.; Choi, J.S.; Jung, J.H. New jasmonate analogues as potential anti-inflammatory agents. Bioorg. Med. Chem. 2008, 16, 10228–10235. [Google Scholar] [CrossRef] [PubMed]
- Choo, J.; Lee, Y.; Yan, X.-J.; Noh, T.H.; Kim, S.J.; Son, S.; Pothoulakis, C.; Moon, H.R.; Jung, J.H.; Im, E. A novel peroxisome proliferator-activated receptor (PPAR) γ agonist 2-hydroxyethyl 5-chloro-4, 5-didehydrojasmonate exerts anti-inflammatory effects in colitis. J. Biol. Chem. 2015, 290, 25609–25619. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, F.; Kim, E.L.; Li, J.L.; Hong, J.; Bae, K.S.; Chung, H.Y.; Kim, H.S.; Jung, J.H. Antibacterial polyketides from the jellyfish-derived fungus Paecilomyces variotii. J. Nat. Prod. 2011, 74, 1826–1829. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Yin, J.; Park, M.; Liu, J.; Li, J.L.; La Kim, E.; Hong, J.; Chung, H.Y.; Jung, J.H. Design and synthesis of marine fungal phthalide derivatives as PPAR-γ agonists. Bioorg. Med. Chem. 2012, 20, 4954–4961. [Google Scholar] [CrossRef] [PubMed]
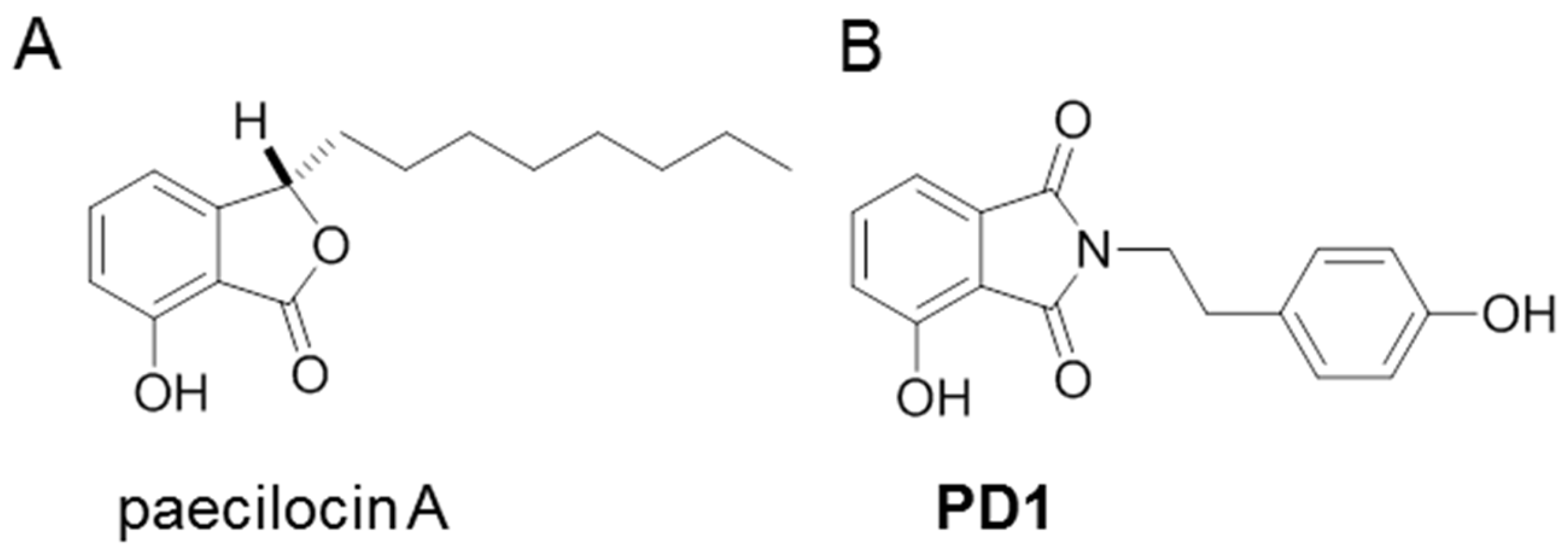
- Xiao, B.; Su, M.; Kim, E.L.; Hong, J.; Chung, H.Y.; Kim, H.S.; Yin, J.; Jung, J.H. Synthesis of PPAR-γ activators inspired by the marine natural product, paecilocin A. Mar. Drugs 2014, 12, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Liu, S.; Su, M.; Noh, T.H.; Hong, J.; Kim, N.D.; Chung, H.Y.; Yang, M.H.; Jung, J.H. Synthesis of phthalimide derivatives as potential PPAR-γ ligands. Mar. Drugs 2016, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Szanto, A.; Nagy, L. The many faces of PPARγ: Anti-inflammatory by any means? Immunobiology 2008, 213, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Oku, T.; Tsuji, T. Platelet supernatant suppresses LPS-induced nitric oxide production from macrophages accompanied by inhibition of NF-κB signaling and increased arginase-1 expression. PLoS ONE 2016, 11, e0162208. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhou, J.; Liu, N.; Wang, L.; Gao, Q.; Wu, Y.; Zhao, Q.; Liu, P.; Wang, S.; Liu, Y. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J. Mol. Cell. Cardiol. 2015, 85, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Csuka, E.; Morganti-Kossmann, M.C.; Lenzlinger, P.M.; Joller, H.; Trentz, O.; Kossmann, T. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: Relationship to IL-6, TNF-α, TGF-β1 and blood–brain barrier function. J. Neuroimmunol. 1999, 101, 211–221. [Google Scholar] [CrossRef]
- Niiro, H.; Otsuka, T.; Kuga, S.; Nemoto, Y.; Abe, M.; Hara, N.; Nakano, T.; Ogo, T.; Niho, Y. IL-10 inhibits prostaglandin E2 production by lipopolysaccharide-stimulated monocytes. Int. Immunol. 1994, 6, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-L.; Chen, S.-L.; Wu, J.-Y.; Ho, T.-C.; Tsao, Y.-P. Pigment epithelium-derived factor induces interleukin-10 expression in human macrophages by induction of PPAR gamma. Life Sci. 2010, 87, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, K.S.; Park, H.S.; Park, S.J.; Min, K.H.; Jin, S.M.; Lee, Y.C. Involvement of IL-10 in peroxisome proliferator-activated receptor γ-mediated anti-inflammatory response in asthma. Mol. Pharmacol. 2005, 68, 1568–1575. [Google Scholar] [PubMed]
- Dang, H.T.; Lee, Y.M.; Kang, G.J.; Yoo, E.S.; Hong, J.; Lee, S.M.; Lee, S.K.; Pyee, Y.; Chung, H.-J.; Moon, H.R. In vitro stability and in vivo anti-inflammatory efficacy of synthetic jasmonates. Bioorg. Med. Chem. 2012, 20, 4109–4116. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Lima, M.; Quintans-Junior, L.J.; de Santana, W.A.; Kaneto, C.M.; Soares, M.B.P.; Villarreal, C.F. Anti-inflammatory effects of carvacrol: Evidence for a key role of interleukin-10. Eur. J. Pharmacol. 2013, 699, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Lee, K.-P.; Kang, S.; Lee, J.; Sato, K.; Chung, H.Y.; Okajima, F.; Im, D.-S. Sphingosine 1-phosphate induced anti-atherogenic and atheroprotective M2 macrophage polarization through IL-4. Cell. Signal. 2014, 26, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, M.; Cao, J.; Huang, J.; Liu, S.; Im, D.S.; Yoo, J.-W.; Jung, J.H. The In Vitro and In Vivo Anti-Inflammatory Effects of a Phthalimide PPAR-γ Agonist. Mar. Drugs 2017, 15, 7. https://doi.org/10.3390/md15010007
Su M, Cao J, Huang J, Liu S, Im DS, Yoo J-W, Jung JH. The In Vitro and In Vivo Anti-Inflammatory Effects of a Phthalimide PPAR-γ Agonist. Marine Drugs. 2017; 15(1):7. https://doi.org/10.3390/md15010007
Chicago/Turabian StyleSu, Mingzhi, Jiafu Cao, Jin Huang, Sen Liu, Dong Soon Im, Jin-Wook Yoo, and Jee H. Jung. 2017. "The In Vitro and In Vivo Anti-Inflammatory Effects of a Phthalimide PPAR-γ Agonist" Marine Drugs 15, no. 1: 7. https://doi.org/10.3390/md15010007
APA StyleSu, M., Cao, J., Huang, J., Liu, S., Im, D. S., Yoo, J.-W., & Jung, J. H. (2017). The In Vitro and In Vivo Anti-Inflammatory Effects of a Phthalimide PPAR-γ Agonist. Marine Drugs, 15(1), 7. https://doi.org/10.3390/md15010007