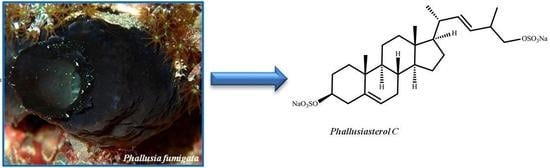
Phallusiasterol C, A New Disulfated Steroid from the Mediterranean Tunicate Phallusia fumigata
Abstract
:1. Introduction
2. Results and Discussion
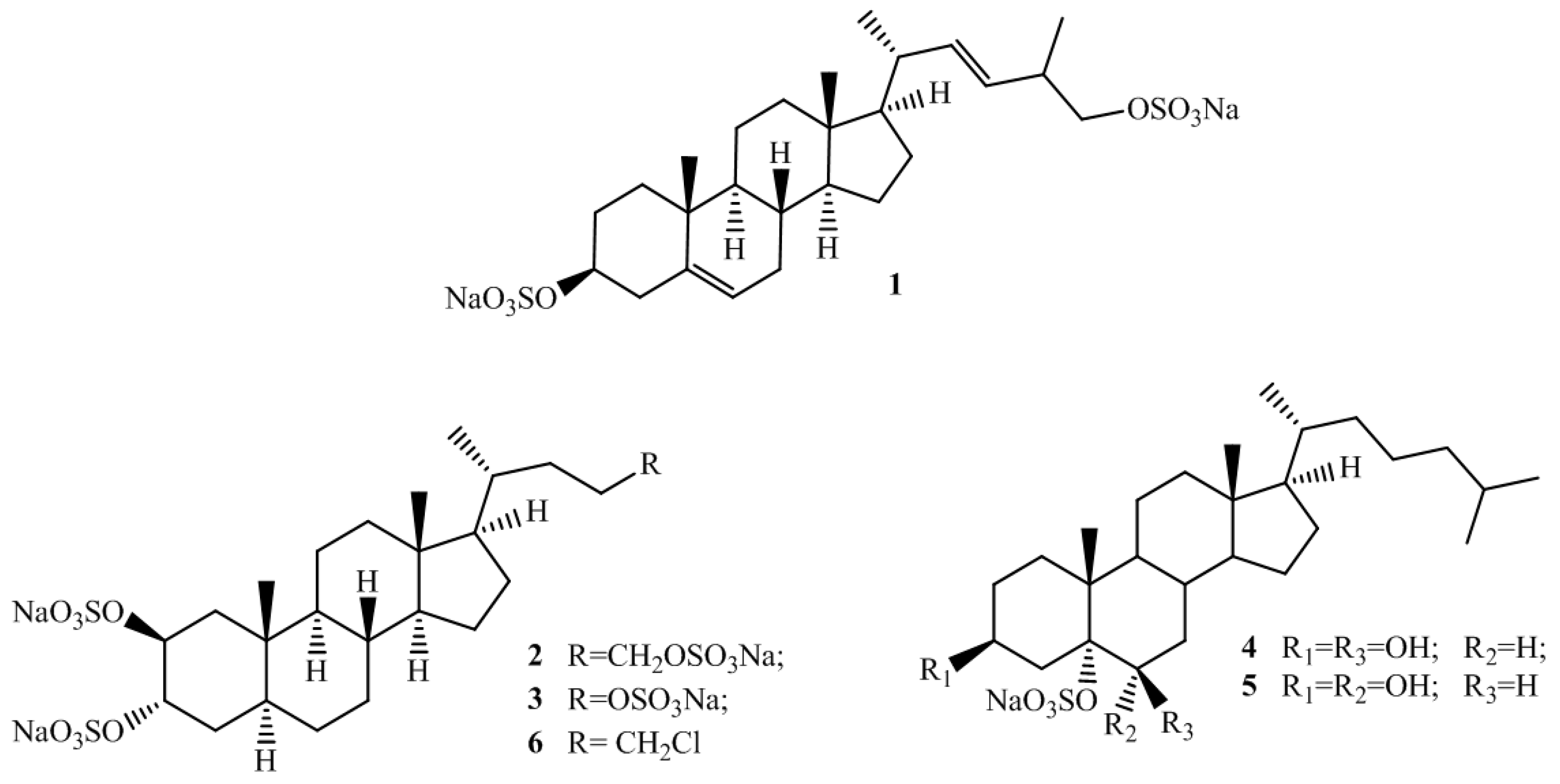
2.1. Isolation and Structure Elucidation
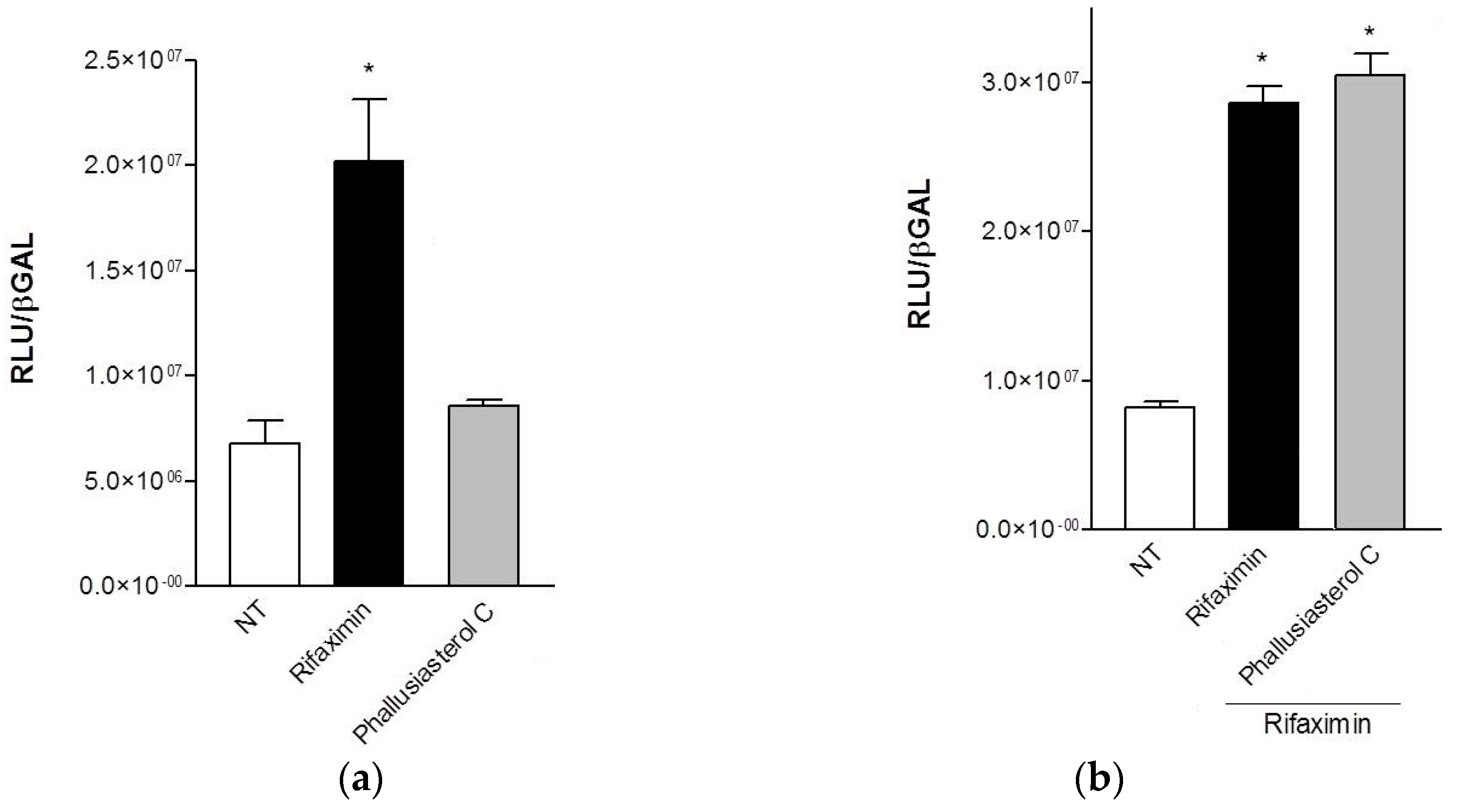
2.2. Biological Evaluation
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Collection, Extraction, and Isolation
3.3. Phallusiasterol C (1)
3.4. Transactivation Experiments
3.5. Cells Culture, RNA Extraction and Real-Time PCR
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PXR | pregnane-X-receptor |
| HCMV | human cytomegalovirus |
| FXR | farnesoid X receptor |
| HepG2 | hepatoma G2 |
| HR ESI | High-resolution electrospray ionisation |
| COSY | Two dimensional 1H correlation |
| HSQC | 1H-detected heteronuclear single-quantum coherence |
| HMBC | 1H-detected heteronuclear multiple-bond correlation |
| ROESY | Rotating-frame Overhauser Spectroscopy |
References
- Lakshmi, V.; Kumar, R. Metabolites from Sinularia species. Nat. Prod. Res. 2009, 23, 801–850. [Google Scholar] [CrossRef] [PubMed]
- Sarma, N.S.; Krishna, M.S.; Pasha, S.G.; Rao, T.S.P.; Venkateswarlu, Y.; Parameswaran, P.S. Marine Metabolites: The Sterols of Soft Coral. Chem. Rev. 2009, 109, 2803–2828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, Y.W.; Gu, Y.C. Secondary metabolites from the South China Sea invertebrates: Chemistry and biological activity. Curr. Med. Chem. 2006, 13, 2041–2090. [Google Scholar] [CrossRef] [PubMed]
- Sica, D.; Musumeci, D. Secosteroids of marine origin. Steroids 2004, 69, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Meng, L.Y.; Tang, H.; Liu, B.S.; Li, L.; Yi, Y.; Zhang, W. Sinularosides A and B, Bioactive 9,11-Secosteroidal Glycosides from the South China Sea Soft Coral Sinularia humilis Ofwegen. J. Nat. Prod. 2012, 75, 1656–1659. [Google Scholar] [CrossRef] [PubMed]
- Goad, L.J. The sterols of marine invertebrates: Composition, biosynthesis and metabolites. In Marine Natural Products, Chemical and Biological Perspectives; Scheuer, P.J., Ed.; Academic Press: New York, NY, USA, 1978; Volume 2, pp. 76–172. [Google Scholar]
- Schmitz, F.J. Uncommon marine steroids. In Marine Natural Products, Chemical and Biological Perspectives; Scheuer, P.J., Ed.; Academic Press: New York, NY, USA, 1978; Volume 1, pp. 241–297. [Google Scholar]
- Yan, X.H.; Lin, L.P.; Ding, J.; Guo, Y.W. Methyl spongoate, a cytotoxic steroid from the Sanya soft coral Spongodes sp. Bioorg. Med. Chem. Lett. 2007, 17, 2661–2663. [Google Scholar] [CrossRef] [PubMed]
- Tillekeratne, L.M.V.; Liyanage, G.K.; Ratnasooriya, W.D.; Ksebati, M.B.; Schmitz, F.J. A new spermatostatic glycoside from the soft coral Sinularia crispa. J. Nat. Prod. 1989, 52, 1143–1145. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shao, C.L.; Qi, X.; Li, X.B.; Li, J.; Sun, L.L.; Wang, C.Y. Polyoxygenated sterols from the South China Sea soft coral Sinularia sp. Mar. Drugs 2012, 10, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Huang, Y.C.; Wen, Z.H.; Hsu, C.H.; Wang, S.K.; Dai, C.F.; Duh, C.Y. New 19-oxygenated and 4-methylated steroids from the Formosan soft coral Nephthea chabroli. Steroids 2009, 74, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Wang, S.K.; Duh, C.Y. Polyhydroxylated Steroids from the Bamboo Coral Isis hippuris. Mar. Drugs 2011, 9, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, B.; Sabbagh, W., Jr.; Juguilon, H.; Bolado, J., Jr.; van Meter, C.M.; Ong, E.S.; Evans, R.M. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998, 12, 3195–3205. [Google Scholar] [CrossRef] [PubMed]
- Festa, C.; de Marino, S.; D’Auria, M.V.; Bifulco, G.; Renga, B.; Fiorucci, S.; Petek, S.; Zampella, A. Solomonsterols A and B from Theonella swinhoei. The first example of C-24 and C-23 sulfated sterols from a marine source endowed with a PXR agonistic activity. J. Med. Chem. 2011, 54, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; D’Amore, C.; Renga, B.; Cipriani, S.; Carino, A.; Sepe, V.; Perissutti, E.; D’Auria, M.V.; Zampella, A.; Distrutti, E.; et al. Solomonsterol A, a marine pregnane-X-receptor agonist, attenuates inflammation and immune dysfunction in a mouse model of arthritis. Mar. Drugs 2013, 12, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, C.; D’Aniello, F.; Aiello, A.; Menna, M.; Fiorucci, S.; D’Amore, C.; Sepe, V. Phallusiasterols A and B: Two new sulfated sterols from the Mediterranean tunicate Phallusia fumigata and their effects as modulators of the PXR receptor. Mar. Drugs 2014, 12, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Fattorusso, E.; Menna, M.; Carnuccio, R.; Iuvone, T. New cytotoxic steroids from the marine sponge Dysidea fragilis coming from the lagoon of Venice. Steroids 1995, 60, 660–673. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Yamada, T.; Ikekawa, N. Pyridine-induced deshielding of 4-methylene protons for the determination of C-6 stereochemistry of sterols having a 5α,6-diol moiety. Revision of the C-6 stereochemistry of marine sterol isolated from a sponge, Dysidea sp. Chem. Pharm. Bull. (Tokyo) 1985, 33, 3129–3133. [Google Scholar] [CrossRef]
- Notaro, G.; Piccialli, V.; Sica, D.; Corriero, G. 3β,5α,6β-Trihydroxylated sterols with a saturated nucleus from two populations of the marine sponge Cliona copiosa. J. Nat. Prod. 1991, 54, 1570–1575. [Google Scholar] [CrossRef]
- Teta, R.; Della Sala, G.; Renga, B.; Mangoni, A.; Fiorucci, S.; Costantino, V. Chalinulasterol, a Chlorinated Steroid Disulfate from the Caribbean Sponge Chalinula molitba. Evaluation of Its Role as PXR Receptor Modulator. Mar. Drugs 2012, 10, 1383–1390. [Google Scholar] [PubMed]
- Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A. Structures of new polar steroids from the Far-Eastern starfish Ctenodiscus crispatus. Russ. Chem. Bull. Int. Ed. 2005, 54, 1266–1271. [Google Scholar] [CrossRef]
- De Marino, S.; Iorizzi, M.; Zollo, F.; Minale, L.; Amsler, C.D.; Baker, B.J.; McClintock, J.B. Isolation, Structure Elucidation, and Biological Activity of the Steroid Oligoglycosides and Polyhydroxysteroids from the Antarctic Starfish Acodontaster conspicuus. J. Nat. Prod. 1997, 60, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Minale, L.; Pizza, C.; Zollo, F.; Riccio, R. Trace Polyhydroxylated steroids from starfish Hacelia attenuata. J. Nat. Prod. 1983, 46, 736–741. [Google Scholar] [CrossRef]
- Finamore, E.; Minale, L.; Riccio, R.; Rinaldo, G.; Zollo, F. Novel marine polyhydroxylated steroids from the starfish Myxoderma platyacanthum. J. Org. Chem. 1991, 56, 1146–1153. [Google Scholar] [CrossRef]
- Wang, W.; Li, F.; Park, Y.; Hong, J.; Lee, C.O.; Kong, J.Y.; Shin, S.; Im, K.S.; Jung, J.H. Bioactive sterols from the starfish Certonardoa semiregularis. J. Nat. Prod. 2003, 66, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, G.; Lu, X.; Wang, H.; Feng, B.; Pei, Y. Three new steroid glycosides from the starfish Asterina pectinifera. Nat. Prod. Res. 2013, 27, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Djerassi, C.; Theobald, N.; Kokke, W.C.M.C.; Pak, C.S.; Carlson, R.M.K. Recent progress in the marine sterol field. Pure Appl. Chem. 1979, 51, 1815–1828. [Google Scholar] [CrossRef]
- Ferezou, J.P.; Devys, M.; Allais, J.P.; Barbier, M. C26 sterols. IX. C26 sterol from the red alga Rhodymenia Palmata. Phytochemistry 1974, 13, 593–598. [Google Scholar] [CrossRef]
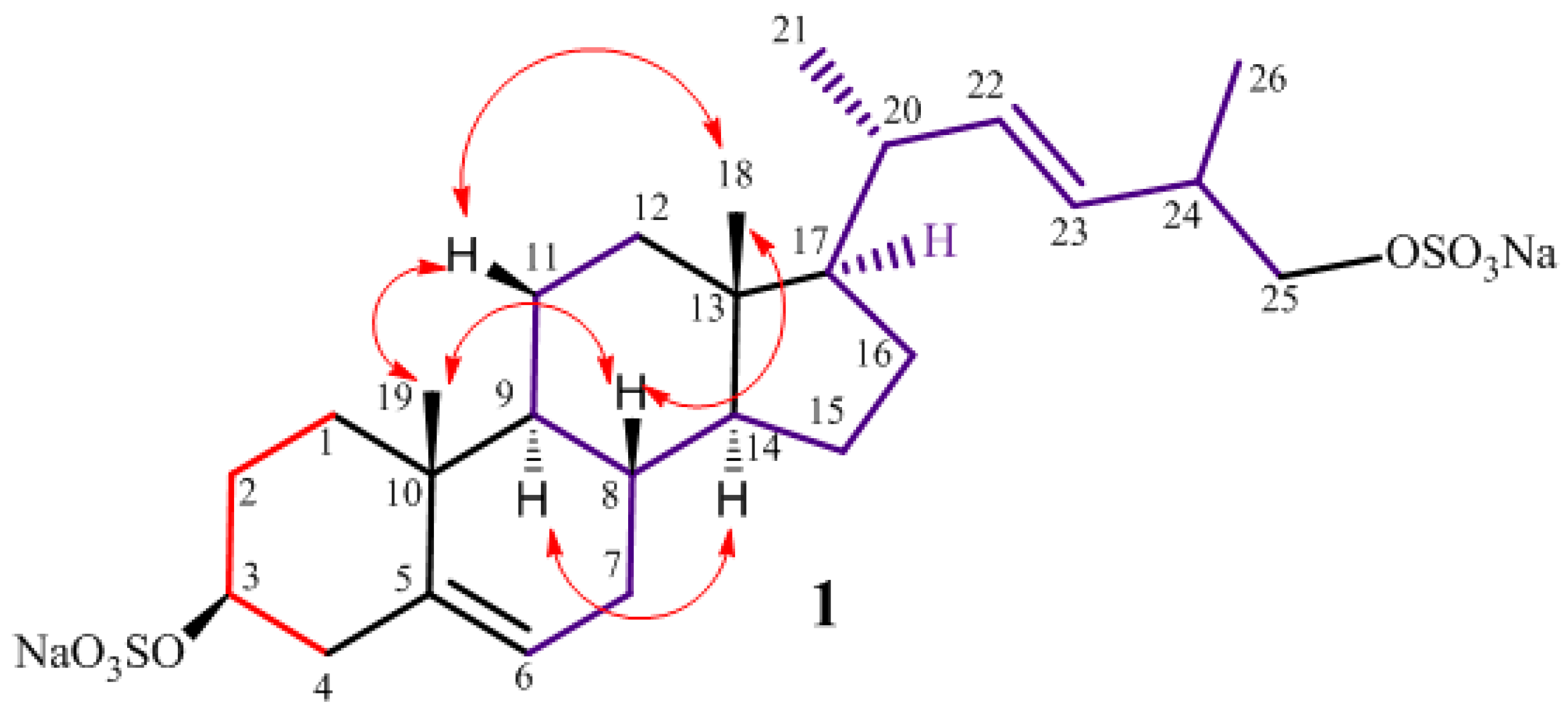
| Position | δH (mult., J in Hz) | δC | HMBC | Pos. | δH (mult., J in Hz) | δC | HMBC |
|---|---|---|---|---|---|---|---|
| 1β | 1.90, dt, (13.5, 3.6) | 38.4 | 2, 5, 10, 19 | - | - | - | - |
| 1α | 1.10, m | 2, 3, 10, 19 | 13 | - | 43.4 | - | |
| 2β | 1.63, m a | 29.9 | 1, 3, 10 | 14α | 1.02 b | 58.2 | 8, 13, 15, 16, 18 |
| 2α | 2.07, m a | 1, 3, 10 | 15β | 1.07, m a | 25.3 | 13, 14, 16, 17 | |
| 3α | 4.14, dddd, (11.4, 11.4, 4.8, 4.8) | 80.0 | 1, 2, 4 | 15α | 1.58 a,b | 8, 14, 16 | |
| 4β | 2.34, dd, (13.2, 11.4) | 40.3 | 2, 3, 5, 6, 10 | 16β | 1.28, m a | 29.7 | 13, 15, 17 |
| 4α | 2.53, ddd, (13.2, 4.8, 2.2) | 2, 3, 5 | 16α | 1.71, m a | 13, 17, 20 | ||
| 5 | - | 141.5 | - | 17 | 1.17, m | 57.2 | 13, 15, 16, 20, 22 |
| 6 | 5.38, dd, (6.9, 3.3) | 123.4 | 4, 7, 8, 10 | 18 | 0.72, s | 12.5 | 12, 13, 14, 17 |
| 7β | 1.98, dt, (13.4, 6.9, 3.3) | 33.0 | 5, 6, 8, 9 | 19 | 1.03, s | 19.7 | 1, 5, 9, 10 |
| 7α | 1.55 b | 5, 8, 14 | 20 | 2.06, m | 41.5 | 17, 21, 22, 23 | |
| 8β | 1.48, m (qd, 10.7, 4.3) | 33.2 | 7, 9, 14 | 21 | 1.03, d, (6.4) | 19.7 | 17, 20, 22 |
| 9α | 0.96, ddd (13.2, 10.7, 4.3) | 51.7 | 8, 10, 11, 19 | 22 | 5.35, dd, (15.3, 8.5) | 138.5 | 20, 21, 23, 24 |
| 10 | - | 37.7 | - | 23 | 5.28, dd, (15.3, 7.0) | 130.2 | 20, 21, 22, 25, 26 |
| 11β | 1.55 b | 22.1 | 9, 10, 12 | 24 | 2.44, m | 37.6 | 22, 23, 25, 26 |
| 11α | 1.51, m | 9, 10, 12 | 25a | 3.88, dd, (9.3, 6.0) | 73.8 | 23, 24, 26 | |
| 12β | 2.01, dt, (12.9, 3.5) | 41.0 | 11, 14 | 25b | 3.74, dd, (9.3, 7.8) | 23, 24, 26 | |
| 12α | 1.18, m | 9, 13, 14 | 26 | 1.02, d, (6.2) | 21.2 | 23, 24, 25 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imperatore, C.; Senese, M.; Aiello, A.; Luciano, P.; Fiorucci, S.; D’Amore, C.; Carino, A.; Menna, M. Phallusiasterol C, A New Disulfated Steroid from the Mediterranean Tunicate Phallusia fumigata. Mar. Drugs 2016, 14, 117. https://doi.org/10.3390/md14060117
Imperatore C, Senese M, Aiello A, Luciano P, Fiorucci S, D’Amore C, Carino A, Menna M. Phallusiasterol C, A New Disulfated Steroid from the Mediterranean Tunicate Phallusia fumigata. Marine Drugs. 2016; 14(6):117. https://doi.org/10.3390/md14060117
Chicago/Turabian StyleImperatore, Concetta, Maria Senese, Anna Aiello, Paolo Luciano, Stefano Fiorucci, Claudio D’Amore, Adriana Carino, and Marialuisa Menna. 2016. "Phallusiasterol C, A New Disulfated Steroid from the Mediterranean Tunicate Phallusia fumigata" Marine Drugs 14, no. 6: 117. https://doi.org/10.3390/md14060117
APA StyleImperatore, C., Senese, M., Aiello, A., Luciano, P., Fiorucci, S., D’Amore, C., Carino, A., & Menna, M. (2016). Phallusiasterol C, A New Disulfated Steroid from the Mediterranean Tunicate Phallusia fumigata. Marine Drugs, 14(6), 117. https://doi.org/10.3390/md14060117