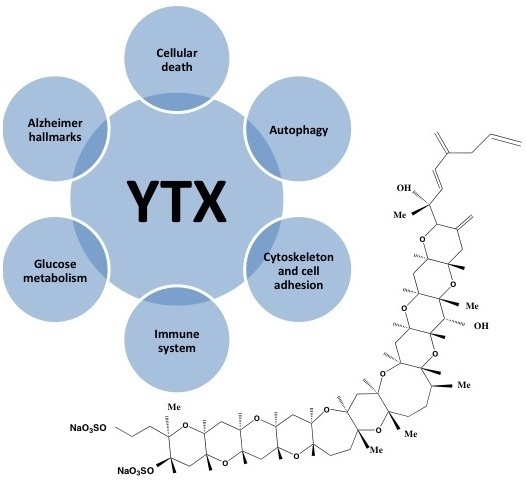
Yessotoxin, a Promising Therapeutic Tool
Abstract
:1. Introduction
2. YTX Origin
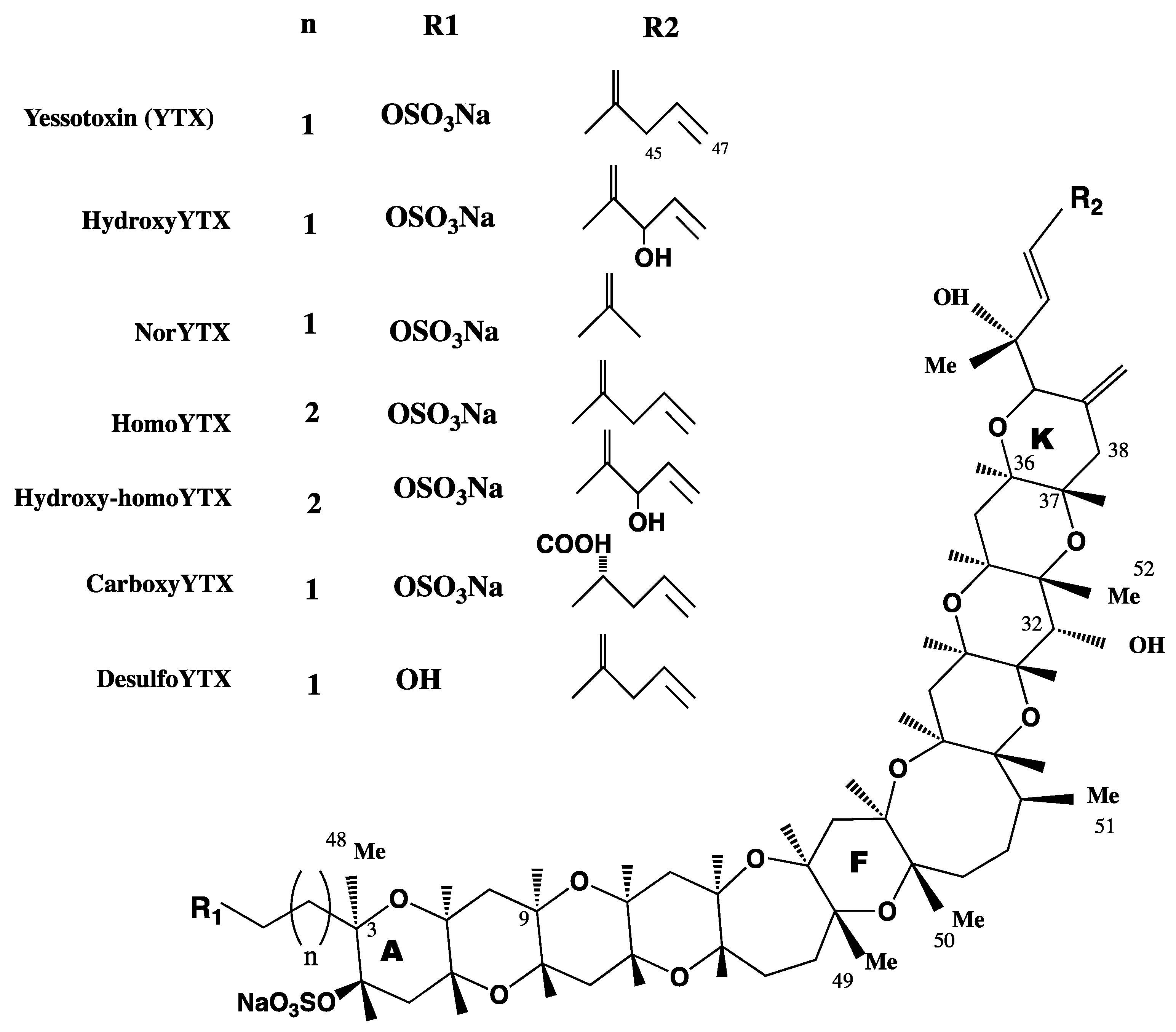
3. YTX Chemistry
4. Mechanism of Action of YTX
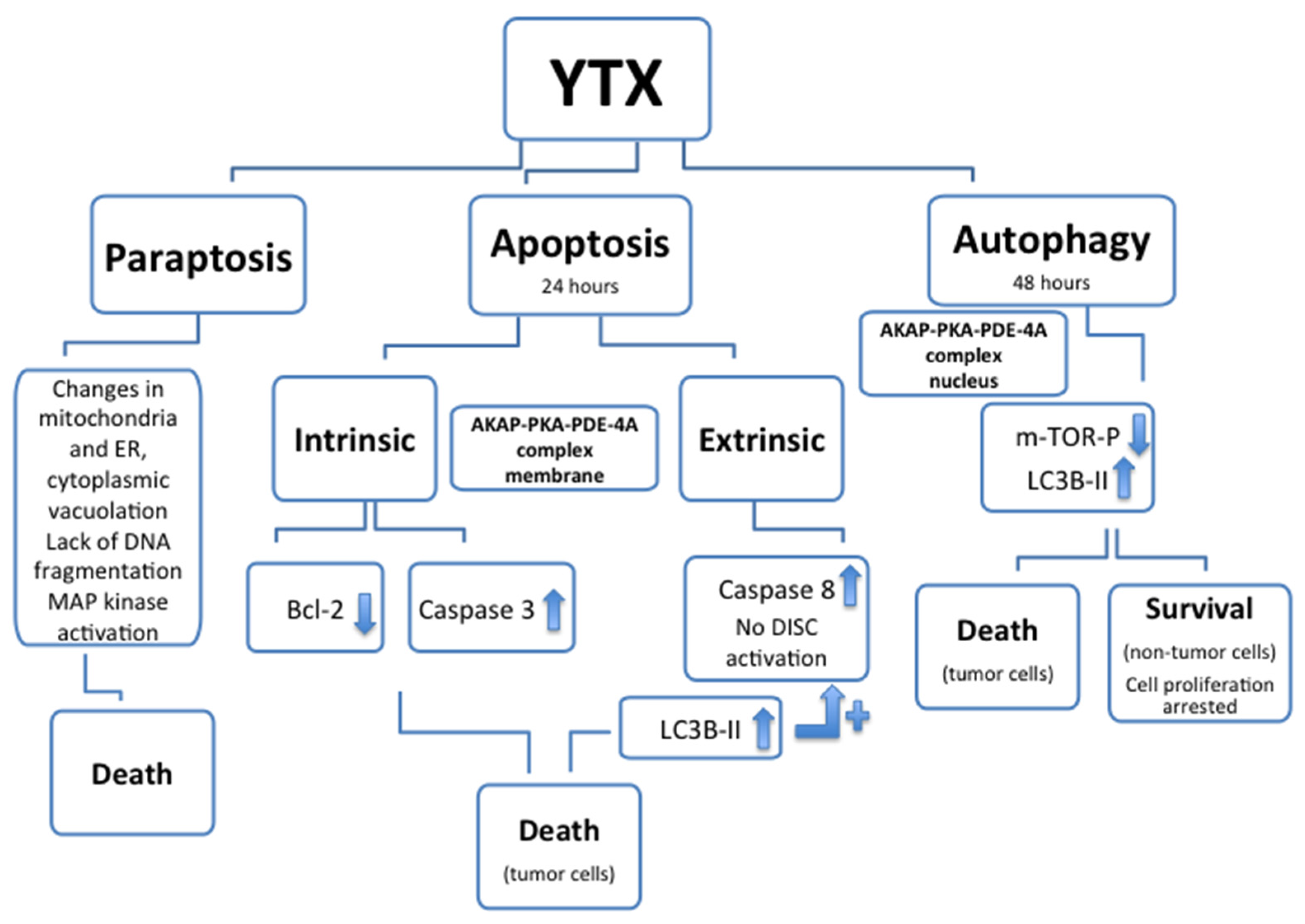
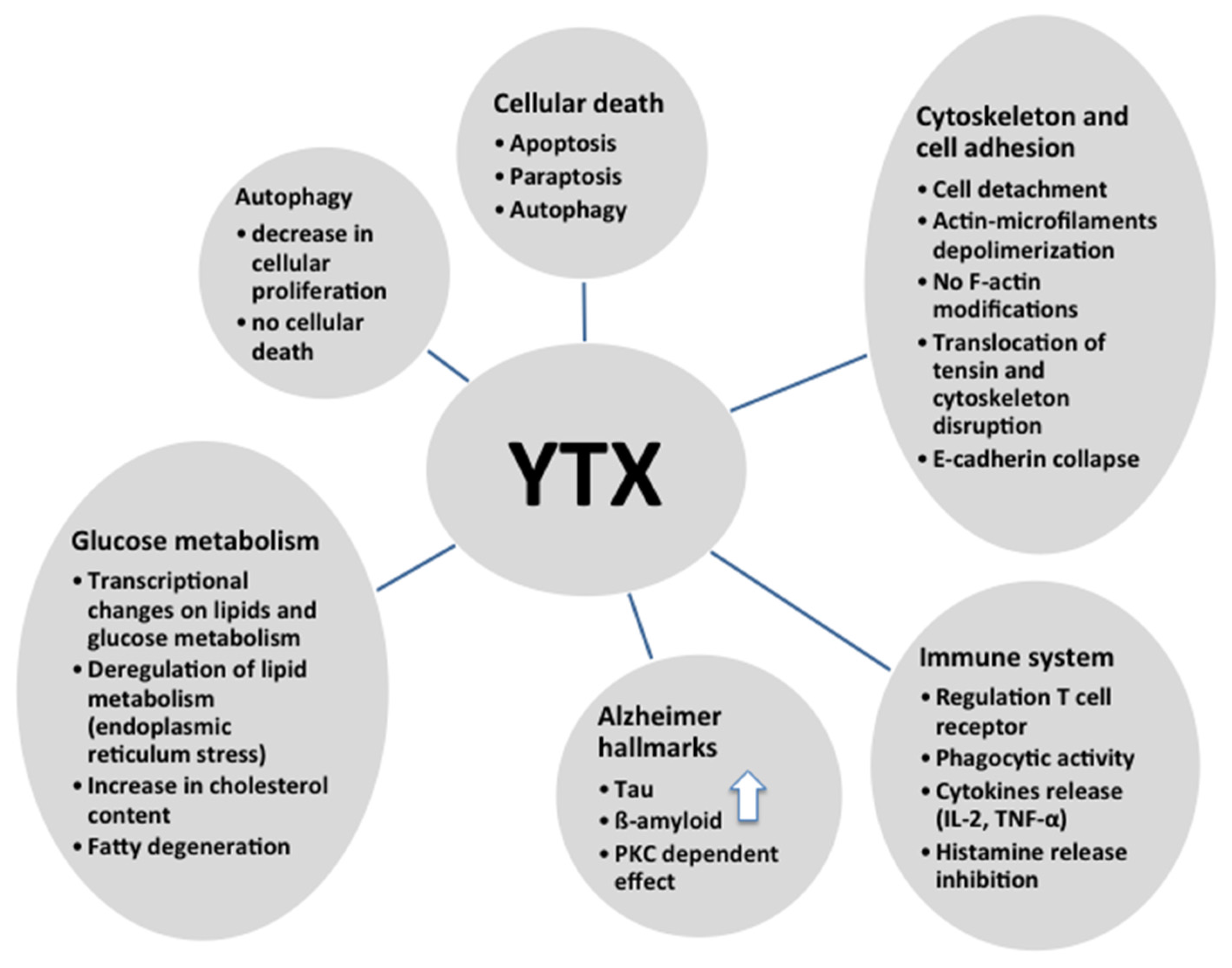
5. Cellular Death and YTX
| Cellular Model | Effect (YTX Concentration and Incubation Time) | Reference |
|---|---|---|
| Fresh enterocytes (rabbit) | No effect on F-actin (1 µM, 4 h) | [74] |
| Long cultured cerebellar neurons (rat) | Actin decrease, Apoptosis (5–150 nM, 48 h) | [39] |
| Primary cardiomyocytes (rat) | Irreversible reduction of cell viability (>10 nM, 48 h) | [40] |
| Primary cerebellar neurons (mouse) | No cellular death (1 µM, 48 h) (50 µM, 48 h, 70% cellular death) | [45] |
| MDCK kidney cells (dog) | Cellular Death Accumulation of E-cadherin fragment ECRA100 (1 nM, 21 h) | [62] |
| Fresh lymphocytes (human) | No effect on cell viability (1 µM, 48 h) | [35] |
| Lymphoblastoid cell line (human) | No effect on cell viability (30 nM, 24 h, no proliferation but no death) | [65] |
| Fresh cortical neurons (mouse) | Cellular death (1–100 nM, 48 h) | [56] |
| Cellular Model | Effect | Reference |
|---|---|---|
| Rat glioma cells | Cell detachment and cytotoxicity | [33] |
| BE(2)-M17 human neuroblastoma cells | Apoptosis | [42] |
| HeLa S3 human cervix adenocarcinoma cells | Cellular death Apoptotic hallmarks | [59] |
| L6 and BC3H1, rat and mouse skeletal myoblasts | Cytoskeleton disruption Apoptosis | [43,60,81] |
| NIH3T3 mouse fibroblasts | Lysosomal damage, which may suggest autophagy | [61] |
| Bel7402 human hepatoma cells | Apoptosis | [36,38] |
| MCF-7 human breast adenocarcinoma cells | Cellular Death Accumulation of E-cadherin fragment ECRA100 | [62,63] |
| A2780 human ovarian carcinoma and HeLa229 human cervix carcinoma cells | Cellular death | [45] |
| Hep G2 human hepatocellular cells | Apoptosis | [64] |
| BC3H1 myoblast cells | Paraptosis | [70] |
| Mouse T-lymphocytes EL-4 cells | Disruption of F-actin cytoskeleton Apoptosis | [82] |
| HL7702 human hepatoma cells | Apoptosis | [37] |
| Human Erythroleukemia K-562 cells | Apoptosis and autophagy | [55,67] |
| Human glioma cells | Autophagy | [68] |
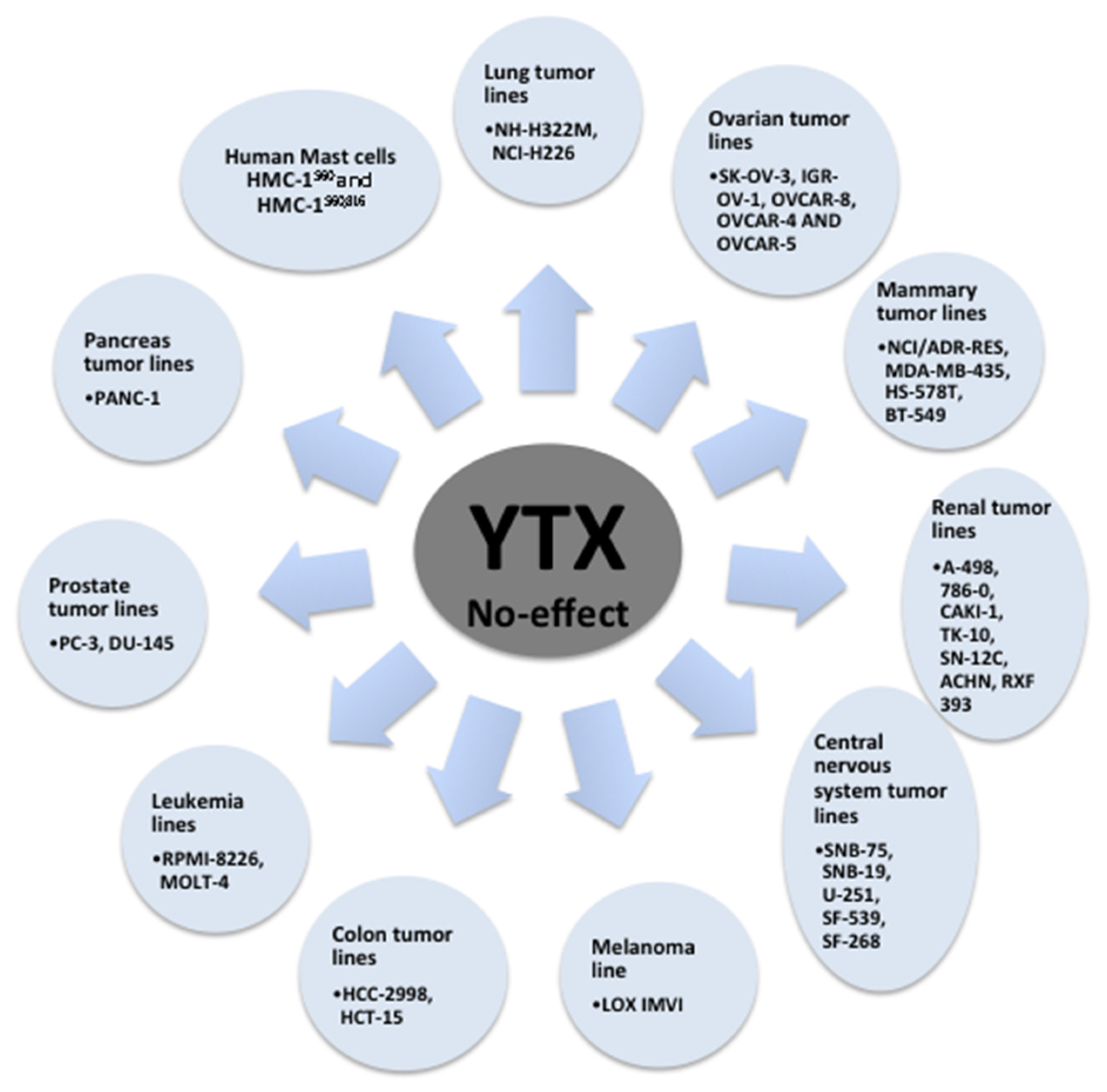
| Mammary tumor lines MDA-MB-231, MCF-7, T-47D | Cellular death | [73] |
| Ovarian tumor lines OVCAR-3 | Cellular death | [73] |
| Lung tumor lines A-549, HOP-92, EKVX, HOP-62, NCI-H23, NCI-H522, NCI-H460, MSTO-211H | Cellular death | [73] |
| Renal tumor lines UO-31 | Cellular death | [73] |
| Central nervous system tumor lines SF-295 | Cellular death | [73] |
| Melanoma line MALME-3M, SK-MEL-28, SK-MEL-2, SK-MEL-5, UACC-257, UACC-62, M-14 | Cellular death | [73] |
| Colon tumor lines KM-12, COLO-205, HT-29, SW-620, HCT-116 | Cellular death | [73] |
| Leukemia lines K-562, SR, CCRF-CEM | Cellular death | [73] |
| Pancreas tumor lines BxPC-3 | Cellular death | [73] |
6. Cytoskeleton and Cell Adhesion and YTX
7. Immune System and YTX
8. Alzheimer’s Disease and YTX
9. Glucose Metabolism and YTX
10. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hess, P.; Aasen, J. Chemistry, origins and distribution of Yessptoxinand its analogues. In Phycotoxins, Chemistry and Biochemistry; Botana, L.M., Ed.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 187–202. [Google Scholar]
- Aune, T.; Sorby, R.; Yasumoto, T.; Ramstad, H.; Landsverk, T. Comparison of oral and intraperitoneal toxicity of yessotoxin towards mice. Toxicon 2002, 40, 77–82. [Google Scholar] [CrossRef]
- Munday, R.; Aune, T.; Rossini, J.P. Toxicology of the yessotoxins. In Seafood and Freshwater Toxins, 2nd ed.; Botana, L.M., Ed.; CRC Press, Taylor and Francis Group: London, UK, 2008; pp. 371–380. [Google Scholar]
- EFSA. Marine biotoxins in shellfish—Yessotoxin group. Scientific opinion of the panel on contaminants in the food chain. Eur. Food Saf. Auth. J. 2008, 907, 1–62. [Google Scholar]
- EU. Commission Regulation (EU) No. 786/2013 of 16 August 2013 amending Annex III to Regulation (EC) No. 853/2004 of the European Parliament and of the Council as regards the permitted limits of yessotoxins in live bivalve molluscs. Off. J. Eur. Union 2013, L220, 14. [Google Scholar]
- Ferreiro, S.F.; Carrera, C.; Vilarino, N.; Louzao, M.C.; Santamarina, G.; Cantalapiedra, A.G.; Botana, L.M. Acute cardiotoxicity evaluation of the marine biotoxins OA, DTX-1 and YTX. Toxins 2015, 7, 1030–1047. [Google Scholar] [CrossRef] [PubMed]
- Tubaro, A.; Giangaspero, A.; Ardizzone, M.; Soranzo, M.R.; Vita, F.; Yasumoto, T.; Maucher, J.M.; Ramsdell, J.S.; Sosa, S. Ultrastructural damage to heart tissue from repeated oral exposure to yessotoxin resolves in 3 months. Toxicon 2008, 51, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Tubaro, A.; Dell’ovo, V.; Sosa, S.; Florio, C. Yessotoxins: A toxicological overview. Toxicon 2010, 56, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Sosa, S.; Ardizzone, M.; Beltramo, D.; Vita, F.; Dell’Ovo, V.; Barreras, A.; Yasumoto, T.; Tubaro, A. Repeated oral co-exposure to yessotoxin and okadaic acid: A short term toxicity study in mice. Toxicon 2013, 76, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Aasen, J.A.; Espenes, A.; Miles, C.O.; Samdal, I.A.; Hess, P.; Aune, T. Combined oral toxicity of azaspiracid-1 and yessotoxin in female NMRI mice. Toxicon 2011, 57, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Tubaro, A.; Sosa, S.; Carbonatto, M.; Altinier, G.; Vita, F.; Melato, M.; Satake, M.; Yasumoto, T. Oral and intraperitoneal acute toxicity studies of yessotoxin and homoyessotoxins in mice. Toxicon 2003, 41, 783–792. [Google Scholar] [CrossRef]
- Franchini, A.; Malagoli, D.; Ottaviani, E. Targets and effects of yessotoxin, okadaic acid and palytoxin: A differential review. Mar. Drugs 2010, 8, 658–677. [Google Scholar] [CrossRef] [PubMed]
- Franchini, A.; Marchesini, E.; Poletti, R.; Ottaviani, E. Lethal and sub-lethal yessotoxin dose-induced morpho-functional alterations in intraperitoneal injected Swiss CD1 mice. Toxicon 2004, 44, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Franchini, A.; Marchesini, E.; Poletti, R.; Ottaviani, E. Acute toxic effect of the algal yessotoxin on Purkinje cells from the cerebellum of Swiss CD1 mice. Toxicon 2004, 43, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Terao, K.; Ito, E.; Oarada, M.; Murata, M.; Yasumoto, T. Histopathological studies on experimental marine toxin poisoning-5. The effects in mice of yessotoxin isolated from Patinopecten yessoensis and of a desulfated derivative. Toxicon 1990, 28, 1095–1104. [Google Scholar] [CrossRef]
- Murata, M.; Kumagai, M.; Lee, J.S.; Yasumoto, T. Isolation and structure of yessotoxin, a novel polyether compound implicated in diarrhetic shellfish poisoning. Tetrahedron Lett. 1987, 28, 5869–5872. [Google Scholar] [CrossRef]
- Miles, C.O.; Wilkins, A.L.; Hawkes, A.D.; Selwood, A.I.; Jensen, D.J.; Munday, R.; Cooney, J.M.; Beuzenberg, V. Polyhydroxylated amide analogs of yessotoxin from Protoceratium reticulatum. Toxicon 2005, 45, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Paz, B.; Riobo, P.; Fernandez, M.L.; Fraga, S.; Franco, J.M. Production and release of yessotoxins by the dinoflagellates Protoceratium reticulatum and Lingulodinium polyedrum in culture. Toxicon 2004, 44, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; McNabb, P.; de Salas, M.; Briggs, L.; Beuzenberg, V.; Gladstone, M. Yessotoxin production by Gonyaulax spinifera. Harmful Algae 2006, 5, 148–155. [Google Scholar] [CrossRef]
- Tubaru, A.; Sidari, L.; Della Loggia, R.; Yasumoto, T. Occurrence of yessotoxin-like toxins in phytoplankton and mussels from Northern Adriatic Sea, Vigo, Spain, 1997. In Proceedings of the VIII International Conference on Harmful Algae, Santiago de Compostela, Spain, 25–29 June 1997; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Santiago de Compostela, Spain, 1998. [Google Scholar]
- Paz, B.; Daranas, A.H.; Norte, M.; Riobo, P.; Franco, J.M.; Fernandez, J.J. Yessotoxins, a group of marine polyether toxins: An overview. Mar. Drugs 2008, 6, 73–102. [Google Scholar] [CrossRef] [PubMed]
- Franchini, A.; Milandri, A.; Poletti, R.; Ottaviani, E. Immunolocalization of yessotoxins in the mussel Mytilus galloprovincialis. Toxicon 2003, 41, 967–970. [Google Scholar] [CrossRef]
- Satake, M.; Terasawa, K.; Kadowaki, Y.; Yasumoto, T. Relative configuration of yessotoxin and isolation of two new analogs from toxic scallops. Tetrahedron Lett. 1996, 37, 5955–5958. [Google Scholar] [CrossRef]
- Takahashi, H.; Kusumi, T.; Kan, Y.; Satake, M.; Yasumoto, T. Determination of the absolute configuration of yessotoxin, a polyether compound implicated in diarrhetic shellfish poisoning, by NMR spectroscopic method using a chiral anisotropic reagent, methoxy-(2-naphthyl)acetic acid. Tetrahedron Lett. 1996, 37, 7087–7090. [Google Scholar] [CrossRef]
- Miles, C.O.; Samdal, I.A.; Aasen, J.; Jensen, D.J.; Quilliam, M.A.; Petersen, D.; Briggs, L.M.; Wilkins, A.L.; Rise, F.; Cooney, J.M.; et al. Evidence for numerous analogs of yessotoxin in Protoceratium reticulatum. Harmful Algae 2005, 4, 1075–1091. [Google Scholar] [CrossRef]
- Rodriguez, I.; Genta-Jouve, G.; Alfonso, C.; Calabro, K.; Alonso, E.; Sanchez, J.A.; Alfonso, A.; Thomas, O.P.; Botana, L.M. Gambierone, a ladder-shaped polyether from the dinoflagellate gambierdiscus belizeanus. Org. Lett. 2015, 17, 2392–2395. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, T.; Takizawa, A. Fluorometric measurement of yessotoxins in shellfish by high-pressure liquid chromatography. Biosci. Biotechnol. Biochem. 1997, 61, 1775–1777. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.O.; Wilkins, A.L.; Jensen, D.J.; Cooney, J.M.; Quilliam, M.A.; Aasen, J.; MacKenzie, A.L. Isolation of 41a-homoyessotoxin and the identification of 9-methyl-41a-homoyessotoxin and nor-ring A-yessotoxin from Protoceratium reticulatum. Chem. Res. Toxicol. 2004, 17, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Tubaro, A.; Lee, J.S.; Yasumoto, T. Two new analogs of yessotoxin, homoyessotoxin and 45-hydroxyhomoyessotoxin, isolated from mussels of the Adriatic Sea. Nat. Toxins 1997, 5, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Aasen, J.; Samdal, I.A.; Miles, C.O.; Dahl, E.; Briggs, L.R.; Aune, T. Yessotoxins in Norwegian blue mussels (Mytilus edulis): Uptake from Protoceratium reticulatum, metabolism and depuration. Toxicon 2005, 45, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Draisci, R.; Lucentini, L.; Mascioni, A. Enteric toxic episodes. Pectenotoxins and yessotoxins: Chemistry, toxicology, pharmacology and analysis. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection; Botana, L.M., Ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 289–324. [Google Scholar]
- Tubaro, A.; Sosa, S.; Altinier, G.; Soranzo, M.R.; Satake, M.; Della Loggia, R.; Yasumoto, T. Short-term oral toxicity of homoyessotoxins, yessotoxin and okadaic acid in mice. Toxicon 2004, 43, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Kumagai, M.; Yasumoto, T. Toxicological evaluation of yessotoxin. Nat. Toxins 1997, 5, 255–259. [Google Scholar] [CrossRef]
- De la Rosa, L.A.; Alfonso, A.; Vilariño, N.; Vieytes, M.R.; Botana, L.M. Modulation of cytosolic calcium levels of human lymphocytes by yessotoxin, a novel marine phycotoxin. Biochem. Pharmacol. 2001, 61, 827–833. [Google Scholar] [CrossRef]
- Tobío, A.; Fernández-Araujo, A.; Alfonso, A.; Botana, L.M. Role of yessotoxin in calcium and cAMP-crosstalks in primary and K-562 human lymphocytes: The effect is mediated by Anchor kinase a mitochondrial proteins. J. Cell. Biochem. 2012, 113, 3752–3761. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Qu, P.; Gao, C.L.; Tang, X.; Wang, Z.L. Effect of yessotoxin on cytosolic calcium levels in human hepatocellular carcinoma cells. Biomed. Rep. 2014, 2, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Qu, P.; Gao, C.L.; Wang, Z.L. Yessotoxin induces apoptosis in HL7702 human liver cells. Mol. Med. Rep. 2012, 5, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Wang, Z.L.; Gao, C.L.; Qu, P.; Li, H.D. Characterization of apoptotic changes induced by yessotoxin in the Bel7402 human hepatoma cell line. Mol. Med. Rep. 2011, 4, 547–552. [Google Scholar] [PubMed]
- Perez-Gomez, A.; Ferrero-Gutierrez, A.; Novelli, A.; Franco, J.; Paz, B.; Fernandez-Sanchez, M.T. Potent neurotoxic action of the shellfish biotoxin yessotoxin on cultured cerebellar neurons. Toxicol. Sci. 2006, 90, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Dell’Ovo, V.; Bandi, E.; Coslovich, T.; Florio, C.; Sciancalepore, M.; Decorti, G.; Sosa, S.; Lorenzon, P.; Yasumoto, T.; Tubaro, A. In vitro effects of yessotoxin on a primary culture of rat cardiomyocytes. Toxicol. Sci. 2008, 106, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Fato, R.; Angelin, A.; Trombetti, F.; Ventrella, V.; Borgatti, A.R.; Fattorusso, E.; Ciminiello, P.; Bernardi, P.; Lenaz, G.; et al. Yessotoxin, a shellfish biotoxin, is a potent inducer of the permeability transition in isolated mitochondria and intact cells. Biochim. Biophys. Acta 2004, 1656, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Leira, F.; Alvarez, C.; Vieites, J.M.; Vieytes, M.R.; Botana, L.M. Characterization of distinct apoptotic changes induced by okadaic acid and yessotoxin in the BE(2)-M17 neuroblastoma cell line. Toxicol. Vitro 2002, 16, 23–31. [Google Scholar] [CrossRef]
- Korsnes, M.S.; Hetland, D.L.; Espenes, A.; Tranulis, M.A.; Aune, T. Apoptotic events induced by yessotoxin in myoblast cell lines from rat and mouse. Toxicol. Vitro 2006, 20, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, A.; de la Rosa, L.A.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Yessotoxin a novel phycotoxin, activates phosphodiesterase activity. Effect of yessotoxin on cAMP levels in human lymphocytes. Biochem. Pharmacol. 2003, 65, 193–208. [Google Scholar] [CrossRef]
- Alfonso, A.; Alfonso, C. Pharmacology and mechanism of action: Biological detection. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection, 2nd ed.; Botana, L.M., Ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2008; pp. 315–327. [Google Scholar]
- Alfonso, A.; Vale, C.; Vilariño, N.; Rubiolo, J.; Louzao, C.; Vieytes, M.R.; Botana, L.M. Recent developments on the mechanism of action of marine phycotoxins. In Proceedings of 17th Meeting on Toxinology, Paris, France, 2–3 December 2009; pp. 51–56.
- Pazos, M.; Alfonso, A.; Vieytes, M.; Yasumoto, T.; Botana, L. Resonant mirror biosensor detection method based on yessotoxin-phosphodiesterase interactions. Anal. Biochem. 2004, 335, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Alfonso, A.; Vieytes, M.; Yasumoto, T.; Botana, L. Study of the interaction between different phosphodiesterases and yessotoxin using a resonant mirror biosensor. Chem. Res. Toxicol. 2006, 19, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.J.; Alfonso, A.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Kinetic analysis of the interaction between yessotoxin and analogs and immobilized phosphodiesterases using a resonant mirror optical biosensor. Chem. Res. Toxicol. 2005, 18, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Fonfria, E.S.; Vilarino, N.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Feasibility of using a surface plasmon resonance-based biosensor to detect and quantify yessotoxin. Anal. Chim. Acta 2008, 617, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, C.; Alfonso, A.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Quantification of yessotoxin using the fluorescence polarization technique, and study of the adequate extraccion procedure. Anal. Biochem. 2005, 344, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Livigni, A.; Scorziello, A.; Agnese, S.; Adornetto, A.; Carlucci, A.; Garbi, C.; Castaldo, I.; Annunziato, L.; Avvedimento, E.V.; Feliciello, A. Mitochondrial AKAP121 links cAMP and src signaling to oxidative metabolism. Mol. Biol. Cell 2006, 17, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Kotera, J. Overview of PDEs and their regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Feliciello, A.; Gottesman, M.E.; Avvedimento, E.V. The biological functions of A-Kinase anchor proteins. J. Mol. Biol. 2001, 308, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Araujo, A.; Tobio, A.; Alfonso, A.; Botana, L.M. Role of AKAP 149-PKA-PDE4A complex in cell survival and cell differentiation processes. Int. J. Biochem. Cell Biol. 2014, in press. [Google Scholar]
- Alonso, E.; Vale, C.; Vieytes, M.R.; Botana, L.M. Translocation of PKC by yessotoxin in an in vitro model of Alzheimer’s disease with improvement of tau and beta-amyloid pathology. ACS Chem. Neurosci. 2013, 4, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Martín-López, A.; Gallardo-Rodríguez, J.; Sánchez-Mirón, A.; García-Camacho, F.; Molina-Grima, E. Immunoregulatory potential of marine algal toxins yessotoxin and okadaic acid in mouse T lymphocyte cell line EL-4. Toxicol. Lett. 2011, 207, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Araujo, A.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Yessotoxin activates cell death pathways independent of Protein Kinase C in K-562 human leukemic cell line. Toxicol. Vitro 2015, 29, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, C.; Ciminiello, P.; Fattorusso, E.; Rossini, G.P. Caspase activation and death induced by yessotoxin in HeLa cells. Toxicol. Vitro 2002, 16, 357–363. [Google Scholar] [CrossRef]
- Korsnes, M.S.; Hetland, D.L.; Espenes, A.; Aune, T. Induction of apoptosis by YTX in myoblast cell lines via mitochondrial signalling transduction pathway. Toxicol. Vitro 2006, 20, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, D.; Marchesini, E.; Ottaviani, E. Lysosomes as the target of yessotoxin in invertebrate and vertebrate cell lines. Toxicol. Lett. 2006, 167, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Callegari, F.; Rossini, G.P. Yessotoxin inhibits the complete degradation of E-cadherin. Toxicology 2008, 244, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Ronzitti, G.; Rossini, G.P. Yessotoxin induces the accumulation of altered E-cadherin dimers that are not part of adhesive structures in intact cells. Toxicology 2008, 244, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Truman, P.; Boucher, M.; Keyzers, R.; Northcote, P.; Jordan, W.T. The algal metabolite yessotoxin affects heterogeneus nuclear ribonucleoproteins in HepG2 cells. Proteomics 2009, 9, 2529–2542. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Araujo, A.; Sanchez, J.A.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Different toxic effects of YTX in tumor K-562 and lymphoblastoid cell lines. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Korsnes, M.S.; Espenes, A. Yessotoxin as an apoptotic inducer. Toxicon 2011, 57, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Araujo, A.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Key role of phosphodiesterase 4A (PDE4A) in autophagy triggered by yessotoxin. Toxicology 2015, 329, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Rubiolo, J.A.; Lopez-Alonso, H.; Martinez, P.; Millan, A.; Cagide, E.; Vieytes, M.R.; Vega, F.V.; Botana, L.M. Yessotoxin induces ER-stress followed by autophagic cell death in glioma cells mediated by mTOR and BNIP3. Cell Signal. 2014, 26, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Korsnes, M.S.; Roed, S.S.; Tranulis, M.A.; Espenes, A.; Christophersen, B. Yessotoxin triggers ribotoxic stress. Toxicol. Vitro 2014, 28, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Korsnes, M.S.; Espenes, A.; Hetland, D.L.; Hermansen, L.C. Paraptosis-like cell death induced by yessotoxin. Toxicol. Vitro 2011, 25, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Korsnes, M.S.; Espenes, A.; Hermansen, L.C.; Loader, J.I.; Miles, C.O. Cytotoxic responses in BC3H1 myoblast cell lines exposed to 1-desulfoyessotoxin. Toxicol. Vitro 2013, 27, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Korsnes, M.S. Yessotoxin as a tool to study induction of multiple cell death pathways. Toxins 2012, 4, 568–579. [Google Scholar] [PubMed]
- Botana, L.M.; Alfonso, A.; Vale, C.; Vilariño, N.; Rubiolo, R.; Alonso, E.; Cagide, E. The mechanistic complexities of phycotoxins: Toxicology of azaspiracids and yessotoxins. In Advances in Molecular Toxicology; Fishbein, J.C., Heilman, J., Eds.; Elsevier: Philadelphia, PA, USA, 2014; Volume 8, pp. 1–26. [Google Scholar]
- Ares, I.R.; Louzao, M.C.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Actin cytoskeleton of rabbit intestinal cells is a target for potent marine phycotoxins. J. Exp. Biol. 2005, 208 Pt 22, 4345–4354. [Google Scholar] [CrossRef] [PubMed]
- Botana, L.M.; Alfonso, A.; Vieytes, M.R.; Loza, M.I. Therapeutic Use of Yessotoxin as Human Tumor Cell Growth Inhibitor. European Patent EP1875906 A2, 9 January 2008. [Google Scholar]
- Korsnes, M.S.; Korsnes, R. Lifetime distributions from tracking individual BC3H1 cells subjected to Yessotoxin. Front. Bioeng. Biotechnol. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Küntziger, T.; Rogne, M.; Folstad, R.L.; Collas, P. Association of PP1 with its regulatory subunit AKAP149 is regulated by serine phosphorylation flanking the RVXF motif of AKAP149. Biochemistry 2006, 45, 5868–5877. [Google Scholar] [CrossRef] [PubMed]
- Steen, R.L.; Beullens, M.; Landsverk, H.B.; Bollen, M.; Collas, P. AKAP149 is a novel PP1 specifier required to maintain nuclear envelope integrity in G1 phase. J. Cell Sci. 2003, 116, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Canton, D.A.; Scott, J.D. Anchoring proteins encounter mitotic kinases. Cell Cycle 2013, 12, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chaerkady, R.; Wu, J.; Hwang, H.J.; Papadopoulos, N.; Kopelovich, L.; Maitra, A.; Matthaei, H.; Eshleman, J.R.; Hruban, R.H.; et al. Mutant proteins as cancer-specific biomarkers. Proc. Natl. Acad. Sci. USA 2011, 108, 2444–2449. [Google Scholar] [CrossRef] [PubMed]
- Korsnes, M.S.; Hetland, D.L.; Espenes, A.; Aune, T. Cleavage of tensin during cytoskeleton disruption in YTX-induced apoptosis. Toxicol. Vitro 2007, 21, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Martín-López, A.; Gallardo-Rodríguez, J.; Sánchez-Mirón, A.; García-Camacho, F.; Molina-Grima, E. Cytotoxicity of yessotoxin and okadaic acid in mouse T lymphocyte cell line EL-4. Toxicon 2012, 60, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Leira, F.; Alvarez, C.; Cabado, A.G.; Vieites, J.M.; Vieytes, M.R.; Botana, L.M. Development of a F actin-based live-cell fluorimetric microplate assay for diarrhetic shellfish toxins. Anal. Biochem. 2003, 317, 129–135. [Google Scholar] [CrossRef]
- Rios-Doria, J.; Day, M.L. Truncated E-cadherin potentiates cell death in prostate epithelial cells. Prostate 2005, 63, 259–258. [Google Scholar] [CrossRef]
- Pierotti, S.; Albano, C.; Milandri, A.; Callegari, F.; Poletti, R.; Rossini, G.P. A slot blot procedure for the measurement of yessotoxins by a functional assay. Toxicon 2007, 49, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Ronzitti, G.; Callegari, F.; Malaguti, C.; Rossini, G.P. Selective disruption of the E-cadherin-catenin system by an algal toxin. Br. J. Cancer 2004, 90, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Ciminiello, P.; Dell’Aversano, C.; Forino, M.; Malaguti, C.; Tubaro, A.; Poletti, R.; Yasumoto, T.; Fattorusso, E.; Rossini, G.P. Structure-activity relationships of yessotoxins in cultured cells. Chem. Res. Toxicol. 2004, 17, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Callegari, F.; Sosa, S.; Ferrari, S.; Soranzo, M.R.; Pierotti, S.; Yasumoto, T.; Tubaro, A.; Rossini, G.P. Oral administration of yessotoxin stabilizes E-cadherin in mouse colon. Toxicology 2006, 227, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Orsi, C.F.; Colombari, B.; Callegari, F.; Todaro, A.M.; Ardizzoni, A.; Rossini, G.P.; Blasi, E.; Peppoloni, S. Yessotoxin inhibits phagocytic activity of macrophages. Toxicon 2010, 55, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, D.; Casarini, L.; Sacchi, S.; Ottaviani, E. Stress and immune response in the mussel Mytilus galloprovincialis. Fish Shellfish Immunol. 2007, 23, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, D.; Ottaviani, E. Yessotoxin affects fMLP-induced cell shape changes inMytilus galloprovincialis immunocytes. Cell Biol. Int. 2004, 28, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, A.; Cabado, A.G.; Vieytes, M.R.; Botana, L.M. Functional compartments in rat mast cells for cAMP and calcium on histamine release. Cell Signal. 2000, 12, 343–350. [Google Scholar] [CrossRef]
- Botana, L.M.; Alfonso, A.; Vieytes, M.R.; Loza, M.I. Use of Yessotoxin in the Treatment of Allergic and Asthmatic Processes. European Patent EP1875907 A2, 9 January 2008. [Google Scholar]
- Cole, G.; Dobkins, K.R.; Hansen, L.A.; Terry, R.D.; Saitoh, T. Decreased levels of protein kinase C in Alzheimer brain. Brain Res. 1988, 452, 165–174. [Google Scholar] [CrossRef]
- Botana, L.M.; Alonso, E.; Vale, C. Use of Yessotoxin and Analogs and Derivatives thereof for Treating and/or Preventing Neurodegenerative Diseases Linked to Tau and Beta Amyloid. European Patent 2011739430, 19 December 2012. U.S. Patent 13577537, 7 February 2013. [Google Scholar]
- Botana, L.M.; Lopez-Alonso, H.; Rubiolo, J. Use of Yessotoxins and the Derivatives thereof for the Treatment and/or Prevention of Metabolic Diseases. Patent WO2012140298 A1, 18 October 2012. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfonso, A.; Vieytes, M.R.; Botana, L.M. Yessotoxin, a Promising Therapeutic Tool. Mar. Drugs 2016, 14, 30. https://doi.org/10.3390/md14020030
Alfonso A, Vieytes MR, Botana LM. Yessotoxin, a Promising Therapeutic Tool. Marine Drugs. 2016; 14(2):30. https://doi.org/10.3390/md14020030
Chicago/Turabian StyleAlfonso, Amparo, Mercedes R. Vieytes, and Luis M. Botana. 2016. "Yessotoxin, a Promising Therapeutic Tool" Marine Drugs 14, no. 2: 30. https://doi.org/10.3390/md14020030
APA StyleAlfonso, A., Vieytes, M. R., & Botana, L. M. (2016). Yessotoxin, a Promising Therapeutic Tool. Marine Drugs, 14(2), 30. https://doi.org/10.3390/md14020030