Abstract
Four new chromone derivatives, phomopsichins A–D (1–4), along with a known compound, phomoxanthone A (5), were isolated from the fermentation products of mangrove endophytic fungus Phomopsis sp. 33#. Their structures were elucidated based on comprehensive spectroscopic analysis coupled with single-crystal X-ray diffraction or theoretical calculations of electronic circular dichroism (ECD). They feature a tricyclic framework, in which a dihydropyran ring is fused with the chromone ring. Compounds 1–5 showed weak inhibitory activities on acetylcholinesterase as well as α-glucosidase, weak radical scavenging effects on 1,1-diphenyl-2-picrylhydrazyl (DPPH) as well as OH, and weak antimicrobial activities. Compounds 1–4 showed no cytotoxic activity against MDA-MB-435 breast cancer cells. Their other bioactivities are worthy of further study, considering their unique molecular structures.
1. Introduction
The chromone family of natural products exhibit a range of biological activities including anticancer, anti-inflammatory, antibacterial, antiviral, atypical antipsychotic, and anti-platelet properties [1,2,3,4,5,6,7,8,9]. In our continuous investigation of new bioactive secondary metabolites from the mangrove endophytic fungi in the South China Sea, four new chromone derivatives, phomopsichin A–D (1–4), along with a known compound, phomoxanthone A (5), were isolated from the metabolic products of endophytic fungus Phomopsis sp. 33# from the bark of the mangrove plant Rhizophora stylosa. Compounds 1–3 (Figure 1) featured a tricyclic framework in which a dihydropyran ring is fused at C-3 and C-4 of the chromone ring. To our knowledge, the compounds with this type skeleton number approximately 10, which were reported to exhibit the activity attenuating resistin-induced adhesion of HCT-116 colorectal cancer cells to endothelial cells [10], the activity interrupting the dimer formation of Aβ17–42 peptide associated to Alzheimer’s disease [11], inhibitory activity against metallo-β-lactamases [12], moderate antibacterial activity and weak cytotoxic activity [13,14,15]. In this study, we report the isolation, structural elucidation, and exploration on the biological activities of compounds 1–5.
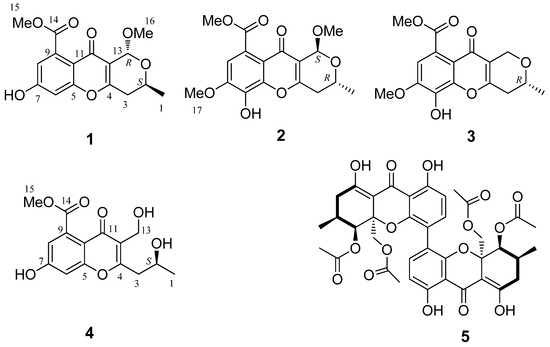
Figure 1.
The chemical structures of compounds 1–5.
2. Results
2.1. Structure Elucidation
Phomopsichin A (1, Figure 1) was obtained as a white solid and had a molecular formula of C16H16O7 as determined by its datum of high resolution electrospray ionization mass spectroscopy (HRESIMS) (observed m/z 319.08184 M−, calculated 319.08233), requiring nine degrees of unsaturation. The 13C-NMR and distortionless enhancement by polarization transfer (DEPT) spectra (Table 1) indicated the presence of two carbonyl groups (δc 169.5 and 173.2), eight olefinic carbons, two sp3 CH groups, one sp3 CH2 group, two methoxy groups, and one methyl group. The 1H-NMR and 1H-1H correlation spectroscopy (COSY) (Table 1 and Figure 2) showed the signals of two m-hydrogens of phenol (δH 6.93 d J = 2.4 Hz; 6.85 d J = 2.4 Hz), two methoxy groups (δH 3.85/3.42), and one 2-oxo-propyl group (δH 1.34 d J = 6.0 Hz; 4.34 m; 2.67 dd J = 18.0, 4.0 Hz; 2.58 dd J = 18.0, 4.0 Hz). The remaining two degrees of unsaturation supported a tricyclic carbon framework of dihydropyrano[4,3-b]chromen-10(1H)-one in 1, which was confirmed by the correlations between H-13 and C-2/C-11 in heteronuclear multiple-bond correlation (HMBC) spectroscopy. In the HMBC spectrum (Figure 2), rich correlation data allowed us to unambiguously establish the locations of substituents on the carbon skeleton. The HMBC correlation between H-8 and C-14 revealed that the carbonyl group was located at the C-9 position; the correlation between H3-1 and C-3 demonstrated that the CH3-1 was located at the C-2 position; and the correlations between H3-15 and C-14 as well as between H3-16 and C-13 indicated that the two methoxy groups were located at the C-14 and C-13 positions, respectively. One hydroxyl group was identified at the C-7 position based on the lower field chemical shift (δc 162.6, C-7).

Table 1.
1H-NMR and 13C-NMR data of compounds 1–4 (400/100 MHz, J in Hz).
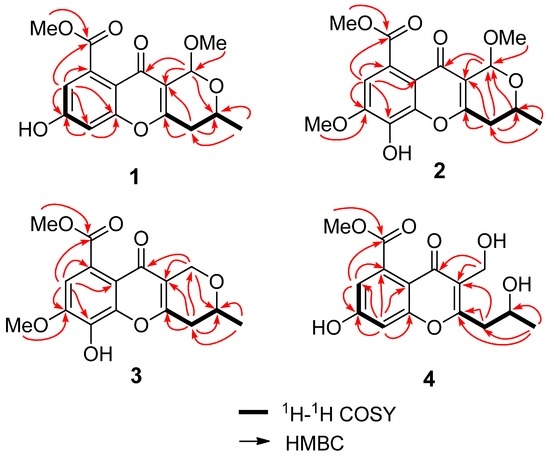
Figure 2.
The key 1H-1H COSY and HMBC correlations of compounds 1–4.
The relative stereochemistry of 1 was established by its nuclear Overhauser effect spectroscopy (NOESY). The NOE correlation between H-2 and H3-16 indicated the relative stereochemistry of 1 as shown in Figure 3.
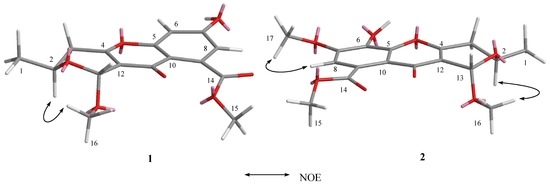
Figure 3.
The key correlations of compounds 1 and 2 in NOESY.
The complete structure and stereochemistry of 1 were further confirmed by X-ray diffraction analysis (Figure 4). The final refinement of the Cu Kα data resulted in a small Flack parameter of 0.02(3), allowing an unambiguous assignment of the absolute configuration of 1 as 2S, 13R (Figure 1).
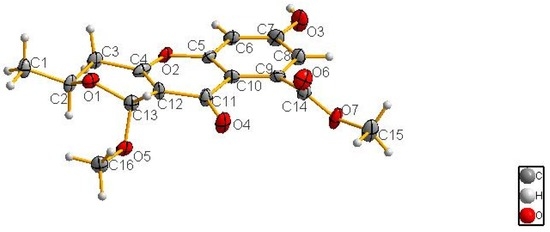
Figure 4.
The X-ray single-crystal structure of 1.
Phomopsichin B (2, Figure 1) was obtained as a white solid and had a molecular formula of C17H18O8 based on HRESIMS data (observed m/z 349.09241 M−, calculated 349.09289), with one more CH3O group than compound 1. The 1H-NMR, 13C-NMR, and HMBC spectra of 2 were very similar to those of 1 (Table 1), except for the absence of the H-6 signal, and an added CH3O-17 signals (δH/C 3.98/56.8). The added CH3O-17 was located at the C-7 position based on the NOE correlation between H-17 and H-8. One hydroxyl group was identified at the C-6 position based on the chemical shift of C-6 (δc 134.7) as well as the HMBC correlation between H-8 and C-6.
The relative stereochemistry of 2 was established by its NOESY. The NOE correlation between H-2 and H3-16, similar to those of 1, indicated the relative stereochemistry of 2 as shown in Figure 3.
Compounds 2 and 1 have identical chiral spheres, just opposite in the signs of their specific rotation data; their ECD spectra were symmetric (Figure 5). The ECD spectrum of 2 showed negative Cotton effect at 318 (Δε −0.48) nm as well as positive one at 291 (Δε +0.53) nm. Meanwhile, the ECD spectrum of 1 displayed opposite Cotton effects at the same wavelengths. For the above reasons, the absolute configuration of 2 was suggested as 2R, 13S.
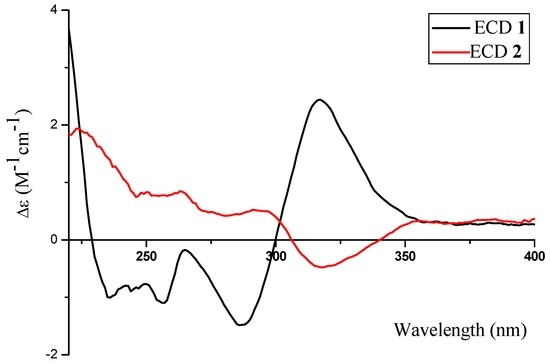
Figure 5.
The ECD spectra of 1 and 2.
Phomopsichin C (3, Figure 1) was obtained as a white solid and had a molecular formula of C16 H16O7 based on HRESIMS data (observed m/z 319.08200 M−, calculated 319.08233). The 1H-NMR, 13C-NMR, 1H-1H COSY, and HMBC spectra of 3 were very similar to those of compound 2 (Table 1, Figure 2), except for the changes of CH-13 signals (δH/C 5.57/94.5) in 2 to CH2-13 signals (δH/C 4.82 d; 4.48 d/62.5) in 3. These results suggested that compound 3 is lacking a methoxy group at the C-13 position. The absolute configuration of compound 3 was determined as 2R by the result that the experimental ECD and calculated ECD spectrum for 2R isomer matched exactly (Figure 6).
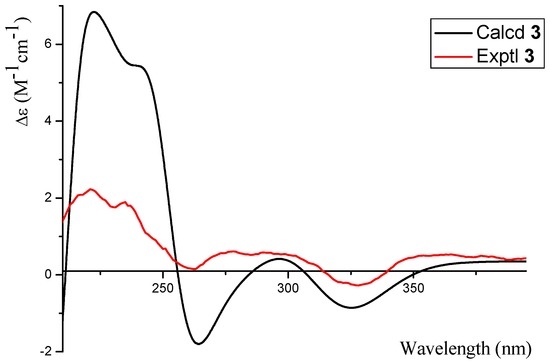
Figure 6.
The calculated and experimental ECD spectra of 3.
Phomopsichin D (4, Figure 1) had a molecular formula of C15H16O7 based on HRESIMS data (observed m/z 307.08194 M−, calculated 307.08233), requiring eight degrees of unsaturation. The 1H-NMR, 13C-NMR, 1H-1H COSY, and HMBC spectra of 4 were very similar to those of 1 (Table 1 and Figure 2), except for the change of CH-13 signals (δH/C 5.40/95.2) in 1 to CH2OH-13 signals (δH/C 4.55/55.0) and the absence of a methoxy group signal in 4. A dicyclic 4H-chromen-4-one segment of 4 was decided based on its eight degrees of unsaturation, which was supported by the absence of HMBC correlation between H-13 and C-2. The hydroxymethyl group was located at C-12 based on the HMBC correlation between H-13 and C-11. A 2-hydroxypropyl group was located at C-4 based on the HMBC correlations between H-1 and C-4, as well as, between H-3 and C-12.
The absolute configuration of 4 was confirmed as 2S based on the result that the experimental data and calculated ECD spectrum for the 2S isomer matched exactly (Figure 7).
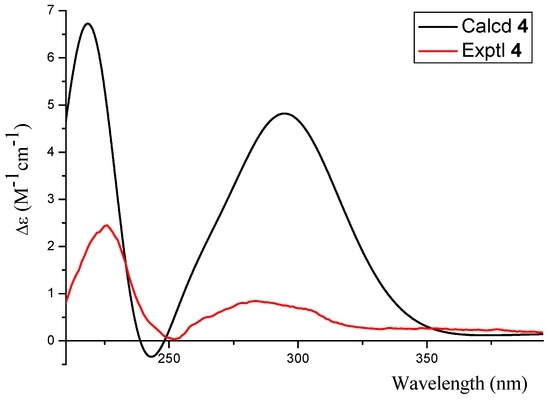
Figure 7.
The calculated and experimental ECD spectra of 4.
Compound 5 was identified as phomoxanthone A (5, Figure 1) by comparison of its spectral data with that of the literature [16,17]; both compound 5 and phomoxanthone A had the same NMR, MS, ECD data (Figure S27, in Supplementary Materials) and specific rotation data.
2.2. Biological Evaluation
The various bioactivities of compounds 1–5 were evaluated in vitro. The five compounds displayed low inhibitory activities on acetylcholinesterase (AchE) as well as α-glucosidase, weak radical scavenging effects on DPPH as well as OH, and low antimicrobial activity against 13 pathogenic bacteria strains (Tables S8 and S9, in Supplementary Materials). Compounds 1–4 showed no cytotoxic activity against MDA-MB-435 breast cancer cells. It was reported that phomoxanthone A (5) has strong pro-apoptotic activity and immunostimulatory activity [17], so we did not consider its cytotoxicity assays in the study.
3. Materials and Methods
3.1. General Experimental Procedures
Acetylcholinesterase (AchE), S-acetylthiocholine iodide, 5,5′-dithio-bis-(2-nitrobenzoic acid), huperzine A, α-glucosidase and p-nitrophenyl-α-d-glucopyranoside were purchased from Sigma (St. Louis, MO, USA); 1,1-diphenyl-2-picrylhydrazyl (DPPH), H2O2, 1,10-phenanthroline, FeSO4, and other reagents were of analytical grade and commercially available; methanol was of chromatographic grade; potato dextrose agar (PDA) medium was purchased from Beijing L and Bridge Technology Co. Ltd. (Beijing, China).
Optical rotation measurements were carried out using a Bellingham-Stanley 37–440 polarimeter (Bellingham Stanley Ltd., Kent, UK). UV spectra were determined using a UV-240 spectrophotometer (Shimadzu, Tokyo, Japan). ECD spectra were measured using a Chirascan Circular Dichroism Spectrometer (Applied Photophysics, London, UK). IR spectra were measured on a TENSOR37 spectrometer (Bruker Optics, Ettlingen, Germany). The 1H-NMR and 13C-NMR data were acquired using a Bruker Avance 400 spectrometer at 400 MHz for 1H nuclei and 100 MHz for 13C nuclei (Bruker Biospin, Rheinstetten, Germany). Tetramethylsilane (TMS) was used as an internal standard, and the chemical shifts (δ) were expressed in ppm. The HRESIMS were obtained using a LTQ-Orbitrap LC-MS (Thermo Fisher, Frankfurt, Germany). Single-crystal data were carried out on an Agilent Technologies Gemini A Ultra system (Agilent Tech, Santa Clara, CA, USA). HPLC was performed using a 515 pump with a UV 2487 detector (Waters, Milford, CT, USA) and an Ultimate XB-C-18 column (250 mm × 10 mm, 5 μm; Welch, Maryland, USA). Normal pressure preparative column chromatography was carried out on RP-18 gel (25–40 μm, Daiso Inc., Osaka, Japan), silica gel (200–400 mesh, Qingdao Marine Chemical Inc., Qingdao, China), or a Sephadex-LH-20 (GE Healthcare, Stockholm, Sweden) for reverse and direct phase elution modes, respectively. The thin-layer chromatography was performed over F254 glass plates (Qingdao Marine Chemical Inc.) and analyzed under UV light (254 and 366 nm).
3.2. Fungal Material
Endophytic fungus Phomopsis sp. 33# was isolated with PDA medium from the bark of the mangrove plant Rhizophora stylosa, collected in the intertidal region of Zhanjiang, in Guangdong Province, China, and identified according to its morphological characteristics and internal transcribed spacer (ITS) region [18]. A voucher specimen is deposited in our laboratory at −20 °C.
3.3. Fermentation, Extraction, and Isolation
Small agar slices bearing mycelia were placed in 1000 mL Erlenmeyer flasks containing rice medium (composed of 60 g rice, 80 mL distilled water, and 0.24 g sea salt) and incubated for 30 days at 28 °C. In total, 140 flasks of culture were obtained. Cultures were extracted with EtOAc. In total, 250 g crude extract was obtained by evaporation of EtOAc. The crude extract was suspended in H2O (3 L) and partitioned with n-hexane (5 L × 2) and EtOAc (5 L × 2) to give n-hexane (90 g) and EtOAc (110 g) extracts, respectively.
The EtOAc extract was subjected to a silica gel column, eluted with a n-hexane-EtOAc gradient (from 100:0 to 0:100) to obtain six fractions (Fractions 1–6). Fraction 2 (15 g) was extracted with 300 mL of chloroform to give dark yellow liquid phases and solid. The chloroform-soluble fraction was evaporated to dryness and washed with methanol (50 mL × 6) to give compound 5 (a light yellow solid, 5.2 g). Fraction 4 (15 g) was chromatographed over a column of RP-18 gel (2.5 cm × 30 cm, MeOH-H2O gradient from: 100:0 to 40:60) to obtain five fractions (Fractions 4.1–4.5). Fraction 4.1 and 4.3–4.5 were separated by HPLC (MeOH-H2O, 20:80, 2 mL/min, 254 nm), respectively, and then were purified separately using a Sephadex LH-20 column (MeOH) until pure compound 1 (17 mg), compound 2 (17mg), compound 3 (10 mg), and compound 4 (22 mg) were obtained.
3.4. Spectral Data
Phomopsichin A (1): white solid; [a] +12.5 (c 0.48, MeOH); UV (MeOH) λmax (logε) 290 (4.0), 218 (4.4) nm; ECD (MeOH) λmax (Δε) 312 (+2.4), 281 (−1.5), 231 (−1.0) nm; IR (KBr) νmax 3410, 2925, 1655 cm−1; for 1H-NMR and 13C-NMR data, see Table 1; ESIMS m/z 319.0 [M−]; HRESIMS m/z 319.08184 [M−] (calculated for C16H15O7, 319.08233).
Crystal structure determination of phomopsichin A (1): crystal data for C16H16O7 (M = 320.29 g/mol): monoclinic, space group P21 (no. 4), a = 7.6234 (9) Å, b = 15.5426 (17) Å, c = 12.4889 (13) Å, β = 96.241 (10)°, V = 1471.0 (3) Å3, Z = 4, T = 150 (2) K, μ (CuKα) = 0.973 mm−1, Dcalc = 1.446 g/cm3, 15,309 reflections measured (7.12° ≤ 2Θ ≤ 133.92°), 5018 unique (Rint = 0.0877, Rsigma = N/A) which were used in all calculations. The final R1 was 0.0849 (>2sigma(I)) and wR2 was 0.2334 (all data). The crystallographic data of 1 have been deposited at the Cambridge Crystallographic Data Centre (CCDC), CCDC Depository Request CRM: 0001000638303.
Phomopsichin B (2): white solid; [a] −8.0 (c 0.89, MeOH); UV (MeOH) λmax (logε) 299 (3.8), 238 (4.4) nm; ECD (MeOH) λmax (Δε) 318 (−0.48), 291 (+0.53) nm; IR (KBr) νmax 3284, 2922, 1649 cm−1; for 1H-NMR and 13C-NMR data, see Table 1; ESIMS m/z 349.0 [M−]; HRESIMS m/z 349.09241 [M−] (calculated for C17H17O8, 349.09289).
Phomopsichin C (3): white solid; [a] −72 (c 0.05, MeOH); UV (MeOH) λmax (logε) 303 (3.9), 239 (4.5) nm; ECD (MeOH) λmax (Δε) 328 (−0.27), 278 (+0.61), 221 (+2.2) nm; IR (KBr) νmax 3325, 2922, 1665 cm−1; for 1H-NMR and 13C-NMR data, see Table 1; ESIMS m/z 319.1 [M−], 206, 175; HRESIMS m/z 319.08200 [M−] (calculated for C16H15O7, 319.08233).
Phomopsichin D (4): white solid; [a] +102 (c 0.05, MeOH); UV (MeOH) λmax (logε) 293 (3.8), 221 (4.2) nm; ECD (MeOH) λmax (Δε) 284 (+0.83), 226 (+2.4) nm; IR (KBr) νmax 3265, 2912, 1707 cm−1; for 1H-NMR and 13C-NMR data, see Table 1; ESIMS m/z 307.0 [M−]; HRESIMS m/z 307.08194 [M−] (calculated for C15H15O7, 307.08233).
3.5. Computational Analyses
All of the theoretical methods and the basis set used for optimization and spectrum calculation were recommended in previous studies [19,20]. All of the theoretical calculations, including geometry optimization, frequency analysis, and ECD spectrum prediction, were carried out with the density functional theory (DFT) and time-dependent density functional theory (TDDFT) methods in the Gaussian 09 software package (Gaussian Inc., Wallingford, CT, USA) [21]. The geometry optimizations were performed at the B3LYP/6-31+G (d) level in the gas phase. Based on the final optimized structure, the ECD spectra were calculated at the PBE1PBE-SCRF/6-311++g (d, p) level using the Polarized Continuum Model (PCM) with methanol as a solvent. The theoretical predicted ECD spectra were fitted in the SpecDis 1.6 software package (University of Würzburg, Würzburg, Germany) [22].
3.6. X-ray Crystallographic Analysis of Compound 1
Single crystals of compound 1 were obtained from CH3OH-EtOAc. A suitable crystal was selected and all crystallographic data were collected at 150 K with Cu/Kα radiation (λ = 1.54178 Å). Using Olex2 (OlexSys Ltd., Durham University, Durham, UK), the structure was solved with the SIR2004 structure solution program using direct methods and refined with the XL refinement package using least squares minimization [23,24,25].
3.7. AchE Inhibitory Assay
The inhibitory activities against AchE of compounds 1–5 were investigated in vitro using the modified Ellman method [26]. The substrates were S-acetylthiocholine iodide and 5,5′-dithio-bis-(2-nitrobenzoic acid). Huperzine A was used as a positive control.
3.8. DPPH Radical Scavenging Assay
The radical scavenging effect on DPPH of compounds 1–5 were determined according to previously reported methods [27,28], and 2,6-ditertbutyl-4-methylphenol was used as a positive control.
3.9. OH-Radical-Scavenging Assay
Radical scavenging effect on OH of compounds 1–5 were carried out according to previously reported methods [29,30]. The indicator used was 1,10-phenanthroline-Fe2+; vitamin C was used as a positive control.
3.10. α-Glucosidase Inhibitory Assay
The inhibitory activities against α-glucosidase of compounds 1–5 were investigated in vitro using the modified method described by Moradi-Afrapoli et al. [31]; p-nitrophenyl-α-d-glucopyranoside was used as the substrates, and trans-resveratrol was used as a positive control.
3.11. Antibacterial Experiment
The antibacterial activity of compounds 1–5 were investigated in vitro using the modified 96 well microtiter-based method described by Pierce et al. [32].
4. Conclusions
Mangrove endophytic fungi from the South China Sea provide rich fungal diversity, and are promising sources of structurally-unprecedented bioactive natural products [33,34,35,36,37]. Five chromone derivatives were isolated from the mangrove endophytic fungus Phomopsis sp. 33#, four of them are new compounds (1–4). Compounds 1–5 showed weak inhibitory activity of AchE as well as α-glucosidase, radical scavenging effects on DPPH as well as OH, and low antimicrobial activity. The compounds (1–4) showed no cytotoxic activity against MDA-MB-435 breast cancer cells. Their other bioactivities are worthy of further study, considering their unique tricyclic molecular structures, in which a dihydropyran ring is fused with the chromone ring.
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/14/11/215/s1. Figure S1: 1H-NMR for phomopsichin A (1). Figure S2: 13C-NMR for phomopsichin A (1). Figure S3: 1H-1H COSY for phomopsichin A (1). Figure S4: heteronuclear single quantum coherence (HSQC) spectroscopy for phomopsichin A (1). Figure S5: HMBC for phomopsichin A (1). Figure S6: NOESY for phomopsichin A (1). Figure S7: HR mass spectrometry for phomopsichin A (1). Figure S8: 1H-NMR for phomopsichin B (2). Figure S9: 13C-NMR for phomopsichin B (2). Figure S10: 1H-1H COSY for phomopsichin B (2). Figure S11: HSQC for phomopsichin B (2). Figure S12: HMBC for phomopsichin B (2). Figure S13: NOESY for phomopsichin B (2). Figure S14: HR mass spectrometry for phomopsichin B (2). Figure S15: 1H-NMR for phomopsichin C (3). Figure S16: 13C-NMR for phomopsichin C (3). Figure S17: 1H-1H COSY for phomopsichin C (3). Figure S18: HSQC for phomopsichin C (3). Figure S19: HMBC for phomopsichin C (3). Figure S20: HR mass spectrometry for phomopsichin C (3). Figure S21: 1H-NMR for phomopsichin D (4). Figure S22: 13C-NMR for phomopsichin D (4). Figure S23: 1H-1H COSY for phomopsichin D (4). Figure S24: HSQC for phomopsichin D (4). Figure S25: HMBC for phomopsichin D (4). Figure S26: HR mass spectrometry for phomopsichin D (4). Figure S27: ECD spectrometry for phomoxanthone A (5). Table S1: Crystal data and structure refinement for phomopsichin A (1). Table S2: Fractional atomic coordinates (× 104) and equivalent isotropic displacement parameters (Å2 × 103) for phomopsichin A (1). Table S3: Anisotropic displacement parameters (Å2 × 103) for phomopsichin A (1). Table S4: Bond lengths for phomopsichin A (1). Table S5: Bond angles for phomopsichin A (1). Table S6: Torsion angles for phomopsichin A (1). Table S7: Hydrogen atom coordinates (Å × 104) and isotropic displacement parameters (Å2 × 103) for phomopsichin A (1). Table S8: Inhibitory activities against AchE as well as α-glucosidase, and the radical scavenging effects on DPPH as well as OH of compounds 1–5. Table S9: Antimicrobial activity of compounds 1–5.
Acknowledgments
We are grateful for the financial support from the National Natural Science Foundation of China (21272286), China’s Marine Commonweal Research Project (201305017), Department of Science and Technology of Guangdong Province (No. 2014A020221006 and No. 2015A030313119), Science and Technology Planning Project of Guangdong Province (No. 2013B021100009), innovative development of marine economic demonstration project, GD2012-D01-001 and Traditional Chinese Medicine Bureau of Guangdong Province (20141052).
Author Contributions
Jun Wang and Lan Liu took charge throughout the research, structural elucidation and writing. Meixiang Huang mainly took part in the extraction and isolation. Jing Li mainly took part in the computational analyses. Sheng Yin mainly took part in biological activity assay. Yongcheng Lin mainly checked the error about structures elucidation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tang, Y.; Ling, J.; Zhang, P.; Zhang, X.; Zhang, N.; Wang, W.; Li, J.; Li, N. Potential therapeutic agents for circulatory diseases from Bauhinia glauca Benth.subsp. pernervosa (Da Ye Guan Men). Bioorg. Med. Chem. Lett. 2015, 25, 3217–3220. [Google Scholar] [CrossRef] [PubMed]
- Hutter, J.A.; Salman, M.; Stavinoha, W.B.; Satsangi, N.; Williams, R.F.; Streeper, R.T.; Weintraub, S.T. Antiinflammatory C-glucosyl chromone from Aloe barbadensis. J. Nat. Prod. 1996, 59, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Speranza, G.; Morelli, C.F.; Tubaro, A.; Altinier, G.; Durì, L.; Manitto, P. Aloeresin I. An anti-inflammatory 5-methylchromone from cape aloe. Planta Med. 2005, 71, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Nastasă, C.M.; Duma, M.; Pîrnău, A.; Vlase, L.; Tiperciuc, B.; Oniga, O. Development of new 5-(chromene-3-yl)methylene-2,4-thiazolidinediones as antimicrobial agents. Clujul Med. 2016, 89, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.S.; Xu, S.Q.; Cheng, M.; Chen, Y.; Xia, P.; Qian, K.; Xia, Y.; Yang, Z.Y.; Chen, C.H.; Morris-Natschke, S.L.; et al. Anti-AIDS agents 87. New bio-isosteric dicamphanoyl-dihydropyranochromone (DCP) and dicamphanoyl-khellactone (DCK) analogues with potent anti-HIV activity. Bioorg. Med. Chem. Lett. 2011, 21, 5831–5834. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Shi, Q.; Chen, C.H.; Zhu, H.; Huang, L.; Ho, P.; Lee, K.H. Anti-AIDS agents 79. Design, synthesis, molecular modeling and structure-activity relationships of novel dicamphanoyl-2′,2′-dimethyldihydropyranochromone (DCP) analogs as potent anti-HIV agents. Bioorg. Med. Chem. 2010, 18, 6678–6689. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.D.; Lecerf-Schmidt, F.; Guragossian, N.; Pazinato, J.; Gozzi, G.J.; Winter, E.; Valdameri, G.; Veale, A.; Boumendjel, A.; Di Pietro, A.; et al. New, highly potent and non-toxic, chromone inhibitors of the human breast cancer resistance protein ABCG2. Eur. J. Med. Chem. 2016, 122, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Guru, S.K.; Jain, S.K.; Joshi, P.; Gandhi, S.G.; Bharate, S.B.; Bhushan, S.; Bharate, S.S.; Vishwakarma, R.A. A chromatography-free isolation of rohitukine from leaves of Dysoxylum binectariferum: Evaluation for in vitro cytotoxicity, CDK inhibition and physicochemical properties. Bioorg. Med. Chem. Lett. 2016, 26, 3457–3463. [Google Scholar] [CrossRef] [PubMed]
- Bolós, J.; Anglada, L.; Gubert, S.; Planas, J.M.; Agut, J.; Príncep, M.; De la Fuente, N.; Sacristán, A.; Ortiz, J.A. 7-[3-(1-piperidinyl)propoxy]chromenones as potential atypical antipsychotics. 2. Pharmacological profile of 7-[3-[4-(6-fluoro-1, 2-benzisoxazol-3-yl)-piperidin-1-yl]propoxy]-3-(hydroxymeth yl)chromen-4-one (abaperidone. FI-8602). J. Med. Chem. 1998, 41, 5402–5409. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.S.; Yang, J.T.; Lu, C.C.; Chang, S.F.; Chen, C.N.; Su, Y.P.; Lee, K.C. Fulvic acid attenuates resistin-induced adhesion of HCT-116 colorectal cancer cells to endothelial cells. Int. J. Mol. Sci. 2015, 16, 29370–29382. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, A.; Mishra, A. The effect of fulvic acid on pre- and postaggregation state of Aβ(17-42): Molecular dynamics simulation studies. Biochim. Biophys. Acta 2013, 1834, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Payne, D.J.; Hueso-Rodríguez, J.A.; Boyd, H.; Concha, N.O.; Janson, C.A.; Gilpin, M.; Bateson, J.H.; Cheever, C.; Niconovich, N.L.; Pearson, S.; et al. Identification of a series of tricyclic natural products as potent broad-spectrum inhibitors of metallo-beta-lactamases. Antimicrob. Agents Chemother. 2002, 46, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Zhang, Y.; Li, L.; Wang, X.; Ding, G. Chaetochromones A and B, two new polyketides from the fungus Chaetomium indicum (CBS.860.68). Molecules 2013, 18, 10944–10952. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, H.Y.; Wu, C.S.; Jiao, Y.; Li, M.; Wang, Y.Y.; Wang, S.Q.; Zhao, Z.T.; Lou, H.X. Austdiol. Fulvic acid and citromycetin derivatives from an endolichenic fungus, Myxotrichum sp. Phytochem. Lett. 2013, 6, 662–666. [Google Scholar]
- Lösgen, S.; Schlörke, O.; Meindl, K.; Herbst-Irmer, R.; Zeeck, A. Structure and biosynthesis of chaetocyclinones, new polyketides produced by an endosymbiotic Fungus. Eur. J. Org. Chem. 2007, 2191–2196. [Google Scholar] [CrossRef]
- Elsässer, B.; Krohn, K.; Flörke, U.; Root, N.; Aust, H.-J.; Draeger, S.; Schulz, B.; Antus, S.; Kurtán, T. X-ray structure determination, absolute configuration and biological activity of phomoxanthone A. Eur. J. Org. Chem. 2005, 21, 4563–4570. [Google Scholar] [CrossRef]
- Rönsberg, D.; Debbab, A.; Mándi, A.; Vasylyeva, V.; Böhler, P.; Stork, B.; Engelke, L.; Hamacher, A.; Sawadogo, R.; Diederich, M.; et al. Pro-apoptotic and immunostimulatory tetrahydroxanthone dimmers from the endophytic fungus Phomopsislongicolla. J. Org. Chem. 2013, 78, 12409–12425. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Qiu, Z.; You, J.; Tan, H.; Zhou, S. Isolation and characterization of endophytic streptomycete antagonists of fusarium wilt pathogen from surface-sterilized banana roots. FEMS Microbiol. Lett. 2005, 247, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; revision a.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bruhn, T.; Schaumloffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Harada, N. ECD cotton effect approximated by the Gaussian curve and other methods. Chirality 2010, 22, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Van Zandt, M.C.; Jones, M.L.; Gunn, D.E.; Geraci, L.S.; Jones, J.H.; Sawicki, D.R.; Sredy, J.; Jacot, J.L.; Dicioccio, A.T.; Petrova, T.; et al. Discovery of 3-[(4,5,7-trifluorobenzothiazol-2-yl)methyl]indole-N-acetic acid (lidorestat) and congeners as highly potent and selective inhibitors of aldose reductase for treatment of chronic diabetic complications. J. Med. Chem. 2005, 48, 3141–3152. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Cryst. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, J.V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–92. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Wu, W.H.; Wang, J.; Lan, M.B. Antioxidant properties of polysaccharide from the brown seaweed Sargassum graminifolium (Turn.), and its effects on calcium oxalate crystallization. Mar. Drugs 2012, 10, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, L.; Fang, Z.; Hu, Y.; Chen, S.; Sugawara, T.; Ye, X. The Effect of the Molecular Architecture on the Antioxidant Properties of Chitosan Gallate. Mar. Drugs 2016, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Harasit, K.M.; Tapas, M.; Ambikesh, M. Kinetics of the basic hydrolysis of tris (1,10-phenenthroline) Fe (II): Influence of polymer-surfactant interactions. Colloid Surf. A-Physicochem. Eng. Asp. 2011, 380, 300–307. [Google Scholar]
- Moradi-Afrapoli, F.; Asghari, B.; Saeidnia, S.; Ajani, Y.; Mirjani, M.; Malmir, M.; Dolatabadi Bazaz, R.; Hadjiakhoondi, A.; Salehi, P.; Hamburger, M.; et al. In vitro α-glucosidase inhibitory activity of phenolic constituents from aerial parts of Polygonum hyrcanicum. Daru 2012, 20, 37. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Debbab, A.; Aly, A.H.; Proksch, P. Endophytes and associated marine derived fungi-ecological and chemical perspectives. Fungal Divers. 2012, 57, 45–83. [Google Scholar] [CrossRef]
- Imhoff, J.F. Natural Products from Marine Fungi-Still an Underrepresented Resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Cai, X.; Xu, F.; She, Z.; Chan, W.L.; Vrijmoed, L.L.; Jones, E.B.; Lin, Y. Three metabolites from the mangrove endophytic fungus Sporothrix sp. (#4335) from the South China Sea. J. Org. Chem. 2009, 74, 1093–1098. [Google Scholar] [PubMed]
- Ma, Y.H.; Li, J.; Huang, M.X.; Liu, L.; Wang, J.; Lin, Y.C. Six New Polyketide Decalin Compounds from Mangrove Endophytic Fungus Penicillium aurantiogriseum 328#. Mar. Drugs 2015, 13, 6306–6318. [Google Scholar] [PubMed]
- Li, J.; Xue, Y.Y.; Yuan, J.; Lu, Y.J.; Zhu, X.; Lin, Y.C.; Liu, L. Lasiodiplodins from Mangrove Endophytic Fungus Lasiodiplodia sp. 318#. Nat. Prod. Res. 2015, 30, 1–6. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).