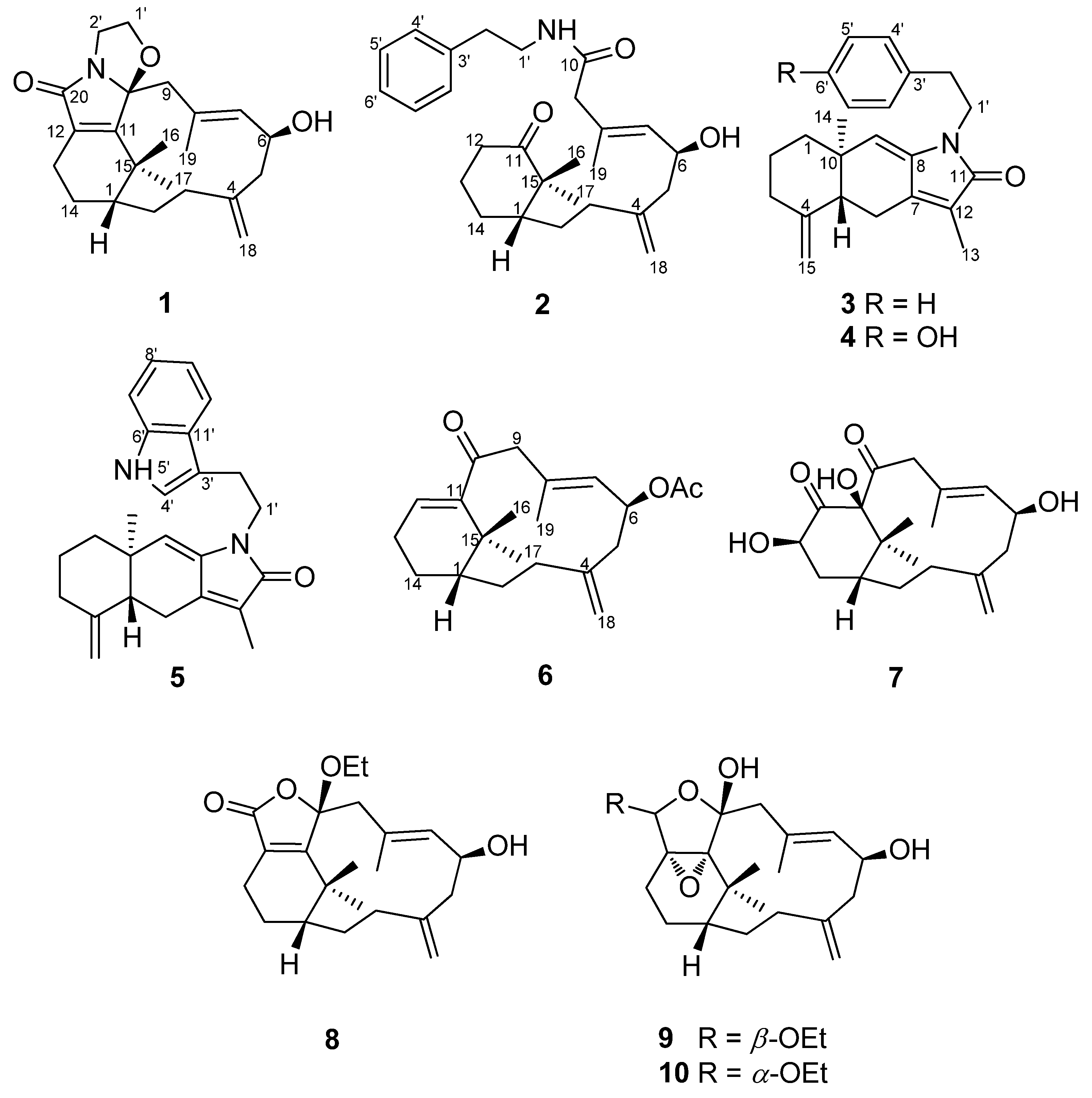
2. Results and Discussion
The EtOAc-MeOH (1:1) extract of C. taeniata was partitioned between H2O and EtOAc to give an EtOAc-soluble fraction. Extensive column chromatography and HPLC purification allowed the separation of ten new compounds (1–10).
Cespilamide A (
1),
−118.2 (CH
2Cl
2), had a molecular formula of C
22H
31O
3N as deduced from the NMR and HRESIMS (
m/
z 358.2380 [M + Na]
+, calcd. 358.2382) data, indicating eight indices of hydrogen deficiency. The IR spectrum revealed the presence of hydroxy (3421 cm
−1) and conjugated amide (1695 cm
−1) moieties. The
1H and
13C-NMR data (
Table 1 and
Table 2) showed the presence of an amidocarbonyl (δ
C 177.1), a trisubstituted olefinic unit [δ
C 134.8 (s), 132.3 (d); δ
H 5.50, d,
J = 8.0 Hz], a tetrasubstituted olefinic moity (δ
C 166.2, 133.5), and an exomethylene group [δ
C 146.2 (s), 114.2 (t); δ
H 4.87, 4.83, each brs]. The DEPT NMR spectrum indicated an oxygenated quarternary carbon (δ
C 102.8), an oxygenated methine carbon (δ
C 68.5 d), eight methylene carbons (δ
C 17.9, 24.4, 32.3, 33.0, 42.5, 43.8, 46.6, 68.8), and three methyl groups (δ
H 1.47, 1.19, 1.59, each 3H and s; δ
C 17.2, 34.3, 35.2). The
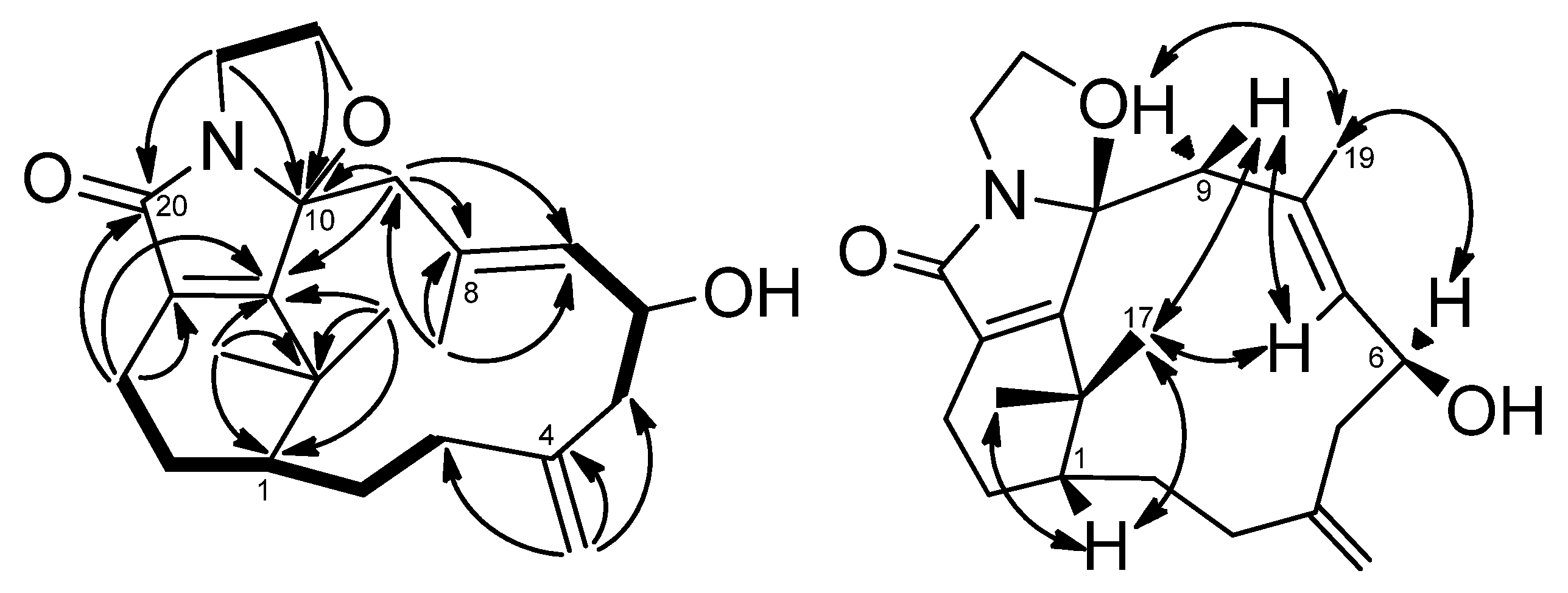
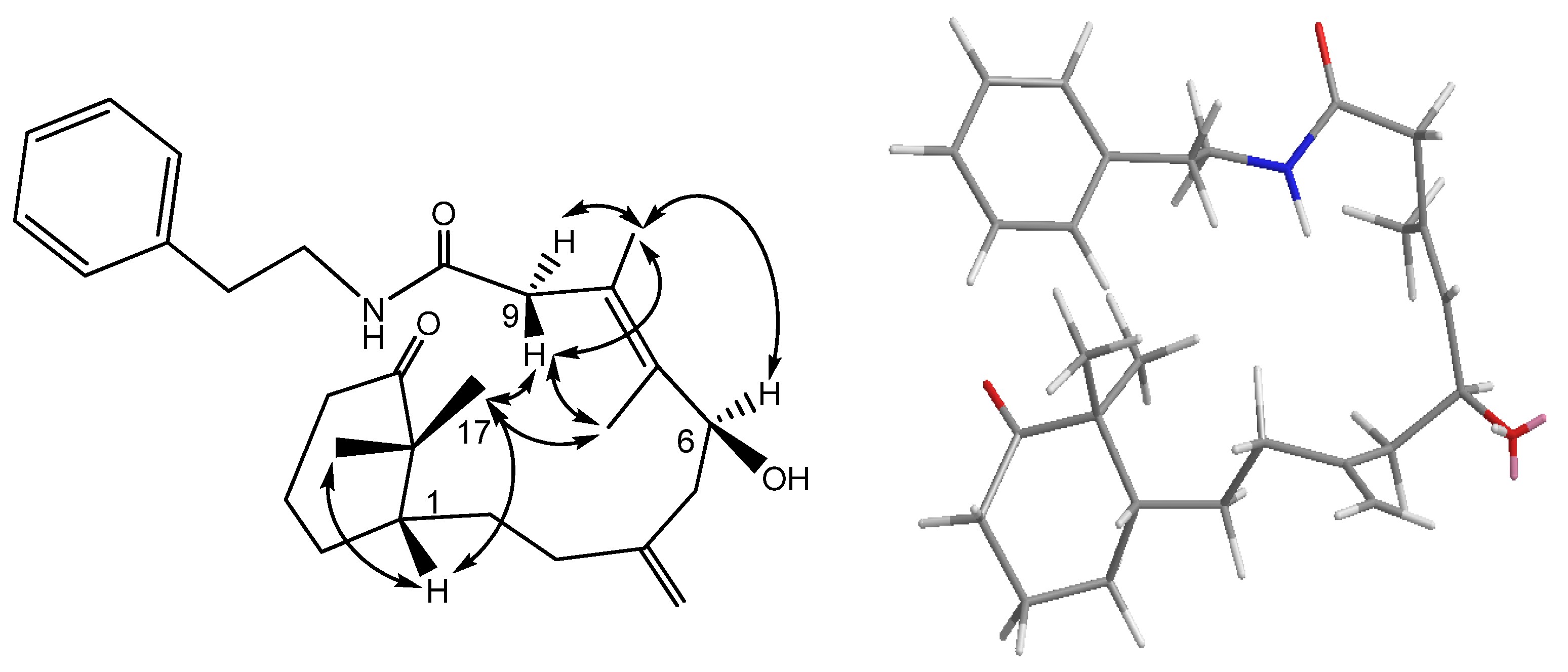
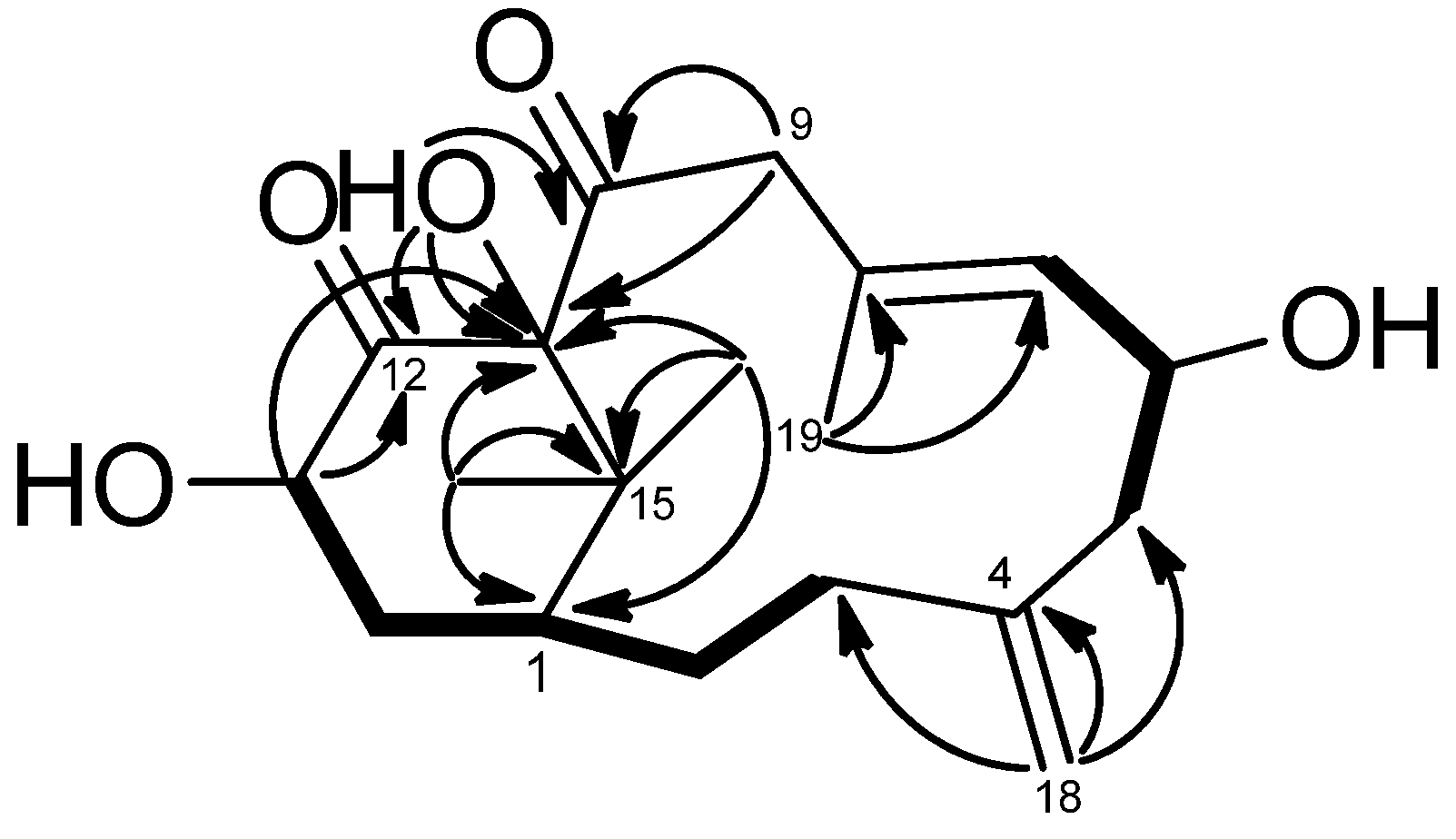
1H–
1H COSY experiment (
Figure 2) showed three sets of correlations, H-1′′/H-2′′, H-7/H-6/H-5 and H-3/H-2/H-1/H-14/H-13, and the latter two sets of proton sequences were further connected by the HMBC correlations (
Figure 2) of H-18/C-3 (δ
C 33.0), C-4 (δ
C 146.2), and C-5 (δ
C 43.8). Furthermore, the HMBC correlations of CH
3-16, CH
3-17/C-15 (δ
C 37.9), C-1 (δ
C 43.5), C-11 (δ
C 166.2) and H-13/C-12 (δ
C 133.5), C-20 (δ
C 177.1), C-11 indicated that compound
1 possesses a 2′,2′-dimethylcyclohexene moiety. The HMBC correlations of H-9/C-7 (δ
C 134.8), C-8 (δ
C 132.3), C-10 (δ
C 102.8), C-11 and CH
3-19/C-7, C-8, C-9 (δ
C 46.6) were used to establish the planar structure of compound
1, except for the C1′-C2′ moiety. Comparison of the
1H- and
13C-NMR data of
1 with those of cespitulactam D revealed that they have similar verticillene skeletons [
12].
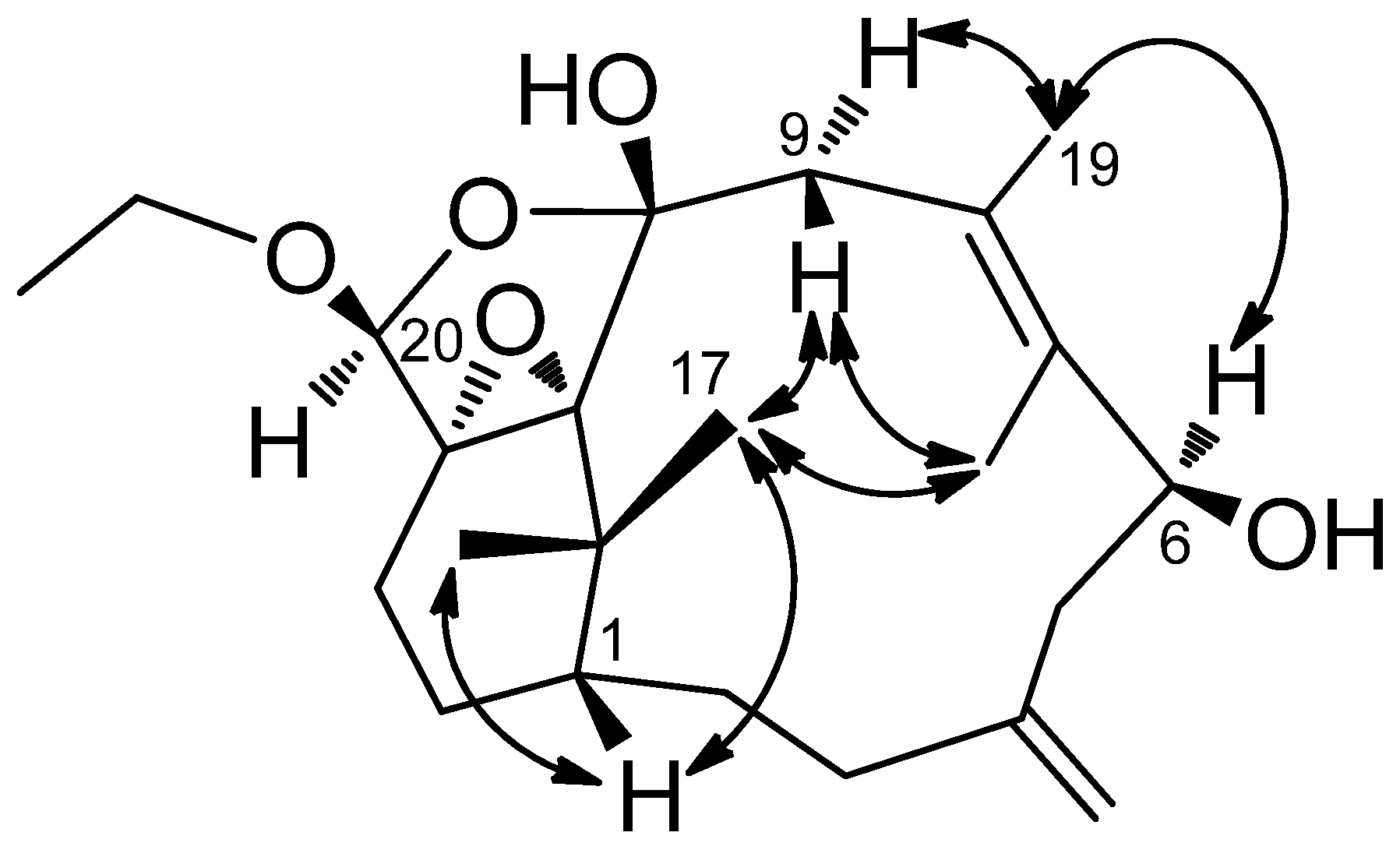
1H–
1H COSY correlations of H-1′ (δ
H 3.84, m; 4.11, m)/H-2′ (δ
H 3.27, m; 3.90, m) and HMBC correlations of H-2′/C-10, C-20 and H-1′/C-10, C-11 suggested that there is an ethylene moiety between the C-10 oxygen function and the nitrogen of the amide moiety. The configuration of compound
1 was determined by NOESY correlations and the Mosher’s ester method. It was assumed that compound
1 has the same absolute configuration at C-1 as naturally-occurring verticillene diterpenoids, such as cespitulactams, cespitularines, and toxoids [
10,
12,
13]. NOESY (
Figure 2) correlations of H-1/Me-16, Me-17 and H-7/ Me-17 indicated the β-orientation of Me-16 and Me-17. Moreover, NOESY correlations of H-6/Me-19/H-9α (δ
H 2.83) and H-7/H-9β (δ
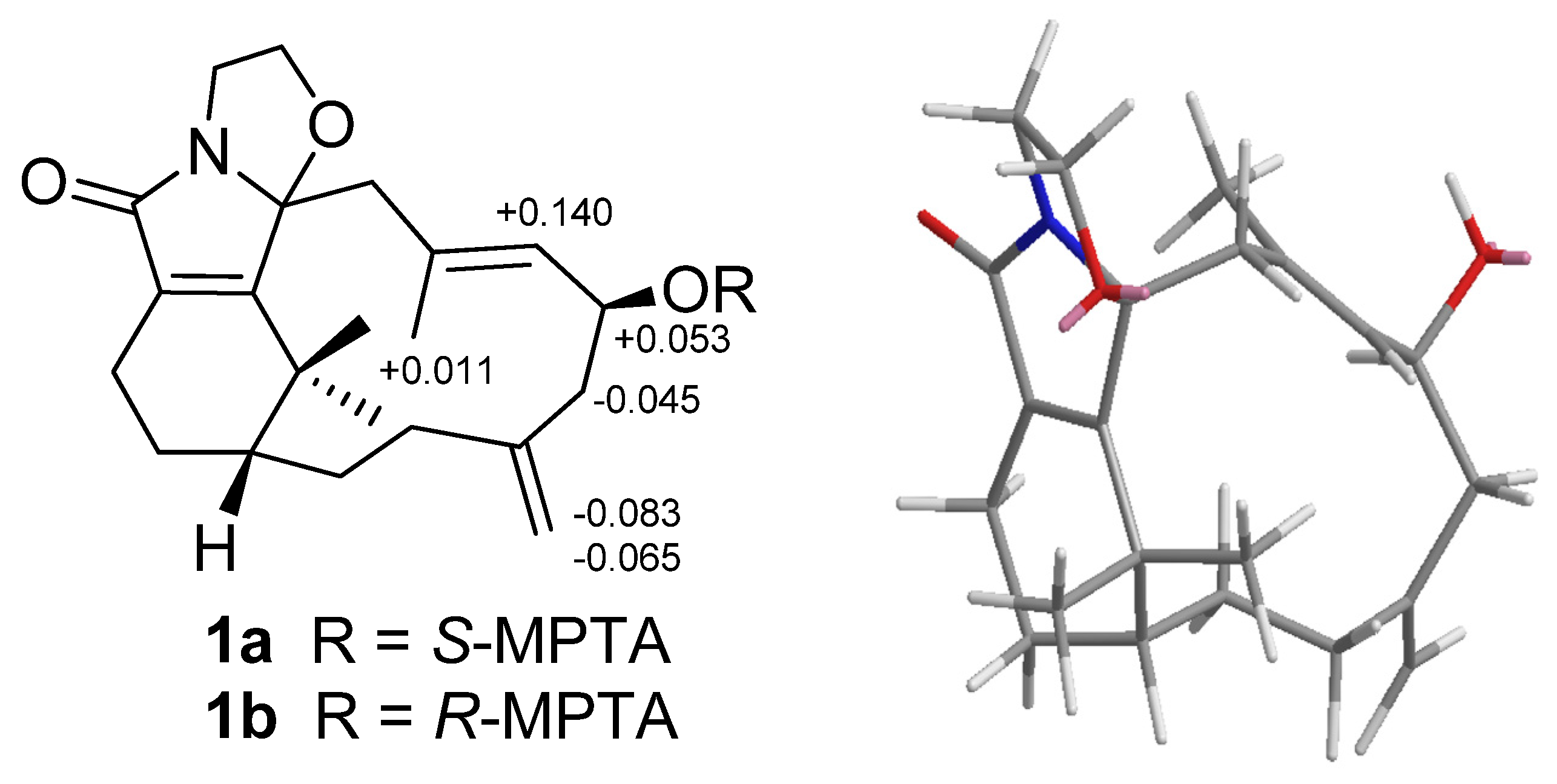
H 2.58) suggested that H-6 is α-oriented. The configuration of the hydroxy group at C-6 was further determined by Mosher’s reactions to yield products
1a and
1b. The results, illustrated in
Figure 3, suggested that C-6 has the
S configuration. A computer-generated MM2 structure for compound
1 calculated for the lowest energy is illustrated in
Figure 3. The result also agreed with a
S configuration at C-6. Due to lack of NOE interaction between H-7 and Me-19, the geometry of the 7,8-double bond in
1 was deduced to be
E.
Table 1.
1H-NMR data for compounds 1–10 a.
Table 1.
1H-NMR data for compounds 1–10 a.
| Position | 1
b | 2
c | 3
b | 4
b | 5
b | 6
b | 7
b | 8
b | 9
c | 10
b |
|---|
| 1 | 1.59, m | 1.44, m | 1.39, m | 1.59, m | 1.32, m | 1.66, m | 2.18, m | 1.60, m | 1.46, m | 1.43, m |
| | | | 1.46, m | | 1.43, m | | | | | |
| 2 | 1.54, m | 1.25, m | 1.56, m | 1.66, m | 1.61, m | 1.50, m | 1.12, m | 2.24, m | 2.30, m | 2.27, m |
| | | 1.62, m | | | | 1.98, m | | | | |
| 3 | 2.11, m | 1.97, m | 2.01, m | 2.36, m | 2.00, m | 2.68, m | 1.93, m | 2.15, m | 2.08, m | 2.13, m |
| | 2.30, m | 2.13, m | 2.33, m | | 2.31, m | | 2.25, m | | 2.18, m | |
| 5 | 2.38, m | 2.19, m | 2.18, m | 2.18, m | 2.11, m | 2.28, m | 2.73, dd (3.9, 12.6) | 2.40, m | 2.23, m | 2.20, m |
| | | | | | | 2.50, m | | | 2.65, m | 2.60, m |
| 6 | 4.37, m | 4.44, dt (5.5, 8.5) | 2.42, m | 2.63, m | 2.36, m | 5.38, dt (8.4, 2.4) | 4.55, dt (3.9, 9.6) | 4.36, dt (3.9, 7.8) | 4.50, dt (3.0, 8.5) | 4.40, dt (3.0, 8.7) |
| | | | 2.60, m | | 2.57, m | | | | | |
| 7 | 5.50, d (8.0) | 5.28, d (8.5) | | | | 5.15, d (8.4) | 5.56, d (9.3) | 5.51, d (7.8) | 5.45, d (8.5) | 5.43, d (8.7) |
| 9 | 2.58, d (13.8) | 2.89, s | 5.05, s | 5.14, s | 5.06, s | 3.07, d (15.9) | 2.84, d (13.5) | 2.85, d (14.1) | 2.51, d (14.5) | 2.53, d (14.4) |
| | 2.83, d (13.8) | | | | | 3.40, d (15.9) | 3.89, d (13.5) | 3.02, d (14.1) | 3.02, d (14.5) | 3.01, d (14.4) |
| 12 | | 2.31, m | | | | 6.20, t (3.6) | | | | |
| | | 2.50, m | | | | | | | | |
| 13 | 1.63, m | 1.99, m | 1.87, s | 1.87, s | 1.88, s | 2.31, m | 4.39, t (3.3) | 1.47, m | 1.59, m | 1.63, m |
| | 2.15, m | | | | | | | | 1.69, m | |
| 14 | 2.15, m | 1.88, m | 0.80, s | 0.84, s | 0.73, s | 2.25, m | 2.14, m | 1.66, m | 1.08, m | 1.16, m |
| | 2.35, m | | | | | | | 2.20, m | 1.86, m | 1.92, m |
| 15 | | | 4.61, s | 4.61, s | 4.59, s | | | | | |
| | | | 4.86, s | 4.87, s | 4.85, s | | | | | |
| 16 | 1.47, s | 1.11, s | | | | 1.27, s | 0.77, s | 1.24, s | 0.94, s | 0.97, s |
| 17 | 1.19, s | 1.03, s | | | | 1.20, s | 1.47, s | 1.44, s | 1.32, s | 1.31, s |
| 18 | 4.83, br s | 4.81, s | | | | 4.80, s | 4.92, s | 4.83, s | 4.92, s | 4.92, s |
| | 4.82, br s | 4.86, s | | | | 4.77, s | 4.96, s | 4.84, s | 4.92, s | 4.92, s |
| 19 | 1.59, s | 1.67, s | | | | 1.76, s | 1.89, s | 1.56, s | 1.82, s | 1.84, s |
| 20 | | | | | | | | | 4.46, s | 4.56, s |
| 1′ | 3.84, m | 3.51, m | 3.74, t (7.5) | 3.72, t (7.5) | 3.84, dt (7.2, 14.4) | | | 3.43, m | 3.50, m | 3.56, m |
| | 4.11, m | | | | | | | 3.63, m | 3.86, m | 3.77, m |
| 2′ | 3.27, m | 2.81, t (6.5) | 2.86, t (7.5) | 2.78, t (7.5) | 3.03, t (7.2) | | | 1.20, t (6.9) | 1.24, t (7.0) | 1.15, t (6.9) |
| | 3.90, m | | | | | | | | | |
| 4′ | | 7.18, d (7.0) | 7.17, d (6.6) | 7.01, d (8.4) | 7.02, d (1.5) | | | | | |
| 5′ | | 7.22, t (7.0) | 7.19, t (6.6) | 6.74, d (8.4) | 8.05, (N
H) | | | | | |
| 6′ | | 7.31, t (7.0) | 7.26, t (6.6) | | | | | | | |
| 7′ | | 7.22, t (7.0) | 7.19, t (6.6) | 6.74, d (8.4) | 7.35, d (7.8) | | | | | |
| 8′ | | 7.18, d (7.0) | 7.17, d (6.6) | 7.01, d (8.4) | 7.18, t (7.2) | | | | | |
| 9′ | | | | | 7.10, t (7.2) | | | | | |
| 10′ | | | | | 7.59, d (7.8) | | | | | |
| OAc | | | | | | 2.01 s | | | | |
Table 2.
13C-NMR data for compounds 1–10 a.
Table 2.
13C-NMR data for compounds 1–10 a.
| Position | 1
b | 2
c | 3
b | 4
b | 5
b | 6
b | 7
b | 8
b | 9
c | 10
b |
|---|
| 1 | 43.5 d | 47.1 d | 39.5 t | 39.5 t | 39.2 t | 43.1 d | 46.8 d | 44.0 d | 44.2 d | 44.5 d |
| 2 | 32.3 t | 27.7 t | 23.2 t | 23.2 t | 23.1 t | 30.6 t | 32.9 t | 17.6 t | 26.2 t | 25.4 t |
| 3 | 33.0 t | 34.3 t | 36.3 t | 36.3 t | 36.2 t | 31.3 t | 39.2 t | 33.6 t | 37.8 t | 37.9 t |
| 4 | 146.2 s | 145.7 s | 148.6 s | 148.8 s | 148.8 s | 146.4 s | 144.8 s | 145.9 s | 145.8 s | 147.2 s |
| 5 | 43.8 t | 43.9 t | 48.9 d | 49.0 d | 48.8 d | 41.1 t | 46.8 t | 43.7 t | 45.8 t | 47.1 t |
| 6 | 68.5 d | 65.8 d | 22.3 t | 22.3 t | 22.1 t | 72.3 d | 70.2 d | 68.2 d | 69.2 d | 69.2 d |
| 7 | 134.8 d | 132.5 d | 139.8 s | 139.8 s | 140.0 s | 129.0 d | 132.7 d | 135.6 d | 133.2 d | 134.1 d |
| 8 | 132.3 s | 132.9 s | 137.1 s | 137.3 s | 137.2 s | 133.3 s | 133.2 s | 131.4 s | 132.8 s | 131.1 s |
| 9 | 46.6 t | 47.5 d | 119.1 d | 119.6 d | 119.1 d | 50.7 t | 49.4 t | 47.0 t | 41.0 t | 41.3 t |
| 10 | 102.8 s | 170.2 s | 38.7 s | 37.9 s | 37.5 s | 202.1 s | 208.1 s | 110.9 s | 94.2 s | 93.0 s |
| 11 | 166.2 s | 216.0 s | 170.0 s | 171.1 s | 170.2 s | 148.0 s | 92.2 s | 166.6 s | 72.8 s | 72.4 s |
| 12 | 133.5 s | 37.8 t | 123.9 s | 124.2 s | 124.1 s | 135.4 d | 214.5 s | 129.5 s | 78.0 s | 79.1 s |
| 13 | 24.4 t | 25.0 t | 8.4 q | 8.4 q | 8.4 q | 23.8 t | 74.8 d | 32.1 t | 31.6 t | 26.0 t |
| 14 | 17.9 t | 25.9 t | 18.6 q | 18.6 q | 18.3 q | 22.8 t | 24.3 t | 24.4 t | 33.9 t | 34.4 t |
| 15 | 37.9 s | 48.9 s | 107.0 t | 107.1 t | 106.8 t | 35.4 s | 46.8 s | 37.4 s | 37.6 s | 37.5 s |
| 16 | 35.2 q | 22.8 q | | | | 32.8 q | 25.8 q | 33.7 q | 25.1 q | 25.0 q |
| 17 | 34.3 q | 19.9 q | | | | 24.8 q | 26.5 q | 24.5 q | 26.0 q | 26.1 q |
| 18 | 114.2 t | 113.0 t | | | | 113.5 t | 115.5 t | 114.5 t | 115.6 t | 114.0 t |
| 19 | 17.2 q | 16.7 q | | | | 19.5 q | 17.6 q | 17.1 q | 17.3 q | 16.5 q |
| 20 | 177.1 s | | | | | | | 170.5 s | 103.5 d | 107.3 d |
| 1′ | 68.8 t | 40.5 t | 41.0 t | 41.1 t | 39.9 t | | | 58.8 t | 65.2 t | 65.4 t |
| 2′ | 42.5 t | 35.2 t | 35.4 t | 34.3 t | 24.8 t | | | 15.1 q | 15.0 q | 14.8 q |
| 3′ | | 138.7 s | 139.2 s | 130.8 s | 113.3 s | | | | | |
| 4′ | | 128.7 d | 128.9 d | 130.0 d | 121.9 d | | | | | |
| 5′ | | 126.5 d | 126.4 d | 115.4 d | | | | | | |
| 6′ | | 128.6 d | 128.5 d | 154.6 s | 124.7 s | | | | | |
| 7′ | | | | | 111.1 d | | | | | |
| 8′ | | | | | 121.9 d | | | | | |
| 9′ | | | | | 119.3 d | | | | | |
| 10′ | | | | | 118.6 d | | | | | |
| 11′ | | | | | 127.6 s | | | | | |
| OAc | | | | | | 170.1 s | | | | |
| | | | | | | 21.3 q | | | | |
Figure 2.
COSY (bold bond), HMBC (arrow) and selected NOESY correlations of 1.
Figure 2.
COSY (bold bond), HMBC (arrow) and selected NOESY correlations of 1.
Figure 3.
Mosher reaction products (1a, 1b), Data are difference values of ΔS-R (ppm); and computer-generated perspective model of 1.
Figure 3.
Mosher reaction products (1a, 1b), Data are difference values of ΔS-R (ppm); and computer-generated perspective model of 1.
Cespilamide B (
2),
−8.0 (CH
2Cl
2), was assigned a molecular formula of C
27H
39O
3N, as deduced from the HRESIMS (
m/
z 448.2825 [M + Na]
+, calcd. 448.2827), indicating nine indices of hydrogen deficiency. The presence of hydroxy, amide, and benzyl functionalities was indicated by IR absorptions at 3371, 1701, and 1647 cm
−1. The
1H and
13C-NMR spectra revealed the presence of a ketocarbonyl (δ
C 216.0), an amide carbonyl (δ
C 170.2), a trisubstituted olefin [δ
C 132.9 (s), 132.5 (d); δ
H 5.28, d,
J = 8.5 Hz], a 1,1-disubstituted olefin (δ
C 145.7) with an exomethylene group (δ
C 113.0; δ
H 4.86, 4.81, each s), an oxygenated methine carbon (δ
C 65.8), and a phenyl group [δ
C 138.7 (s), 128.7 (d, 2C), 126.5 (d, 2C), 128.6 (d); δ
H 7.18, d,
J = 7.0 Hz (2H), δ
H 7.22 t,
J = 7.0 Hz, δ
H 7.31 t,
J = 7.0 Hz (2H)]. Thus, eight degrees of unsaturation were counted, leaving one further ring to be elucidated. The
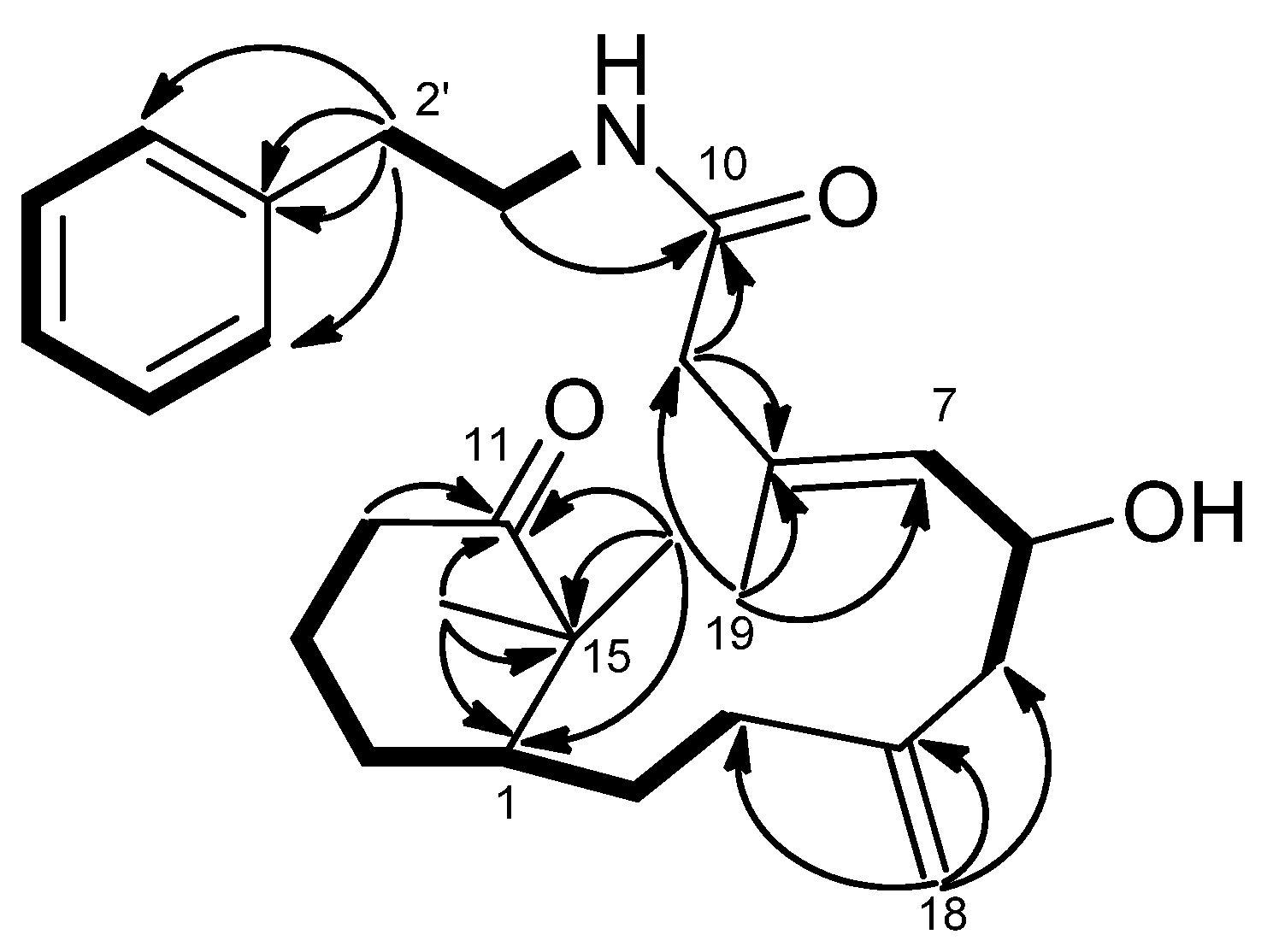
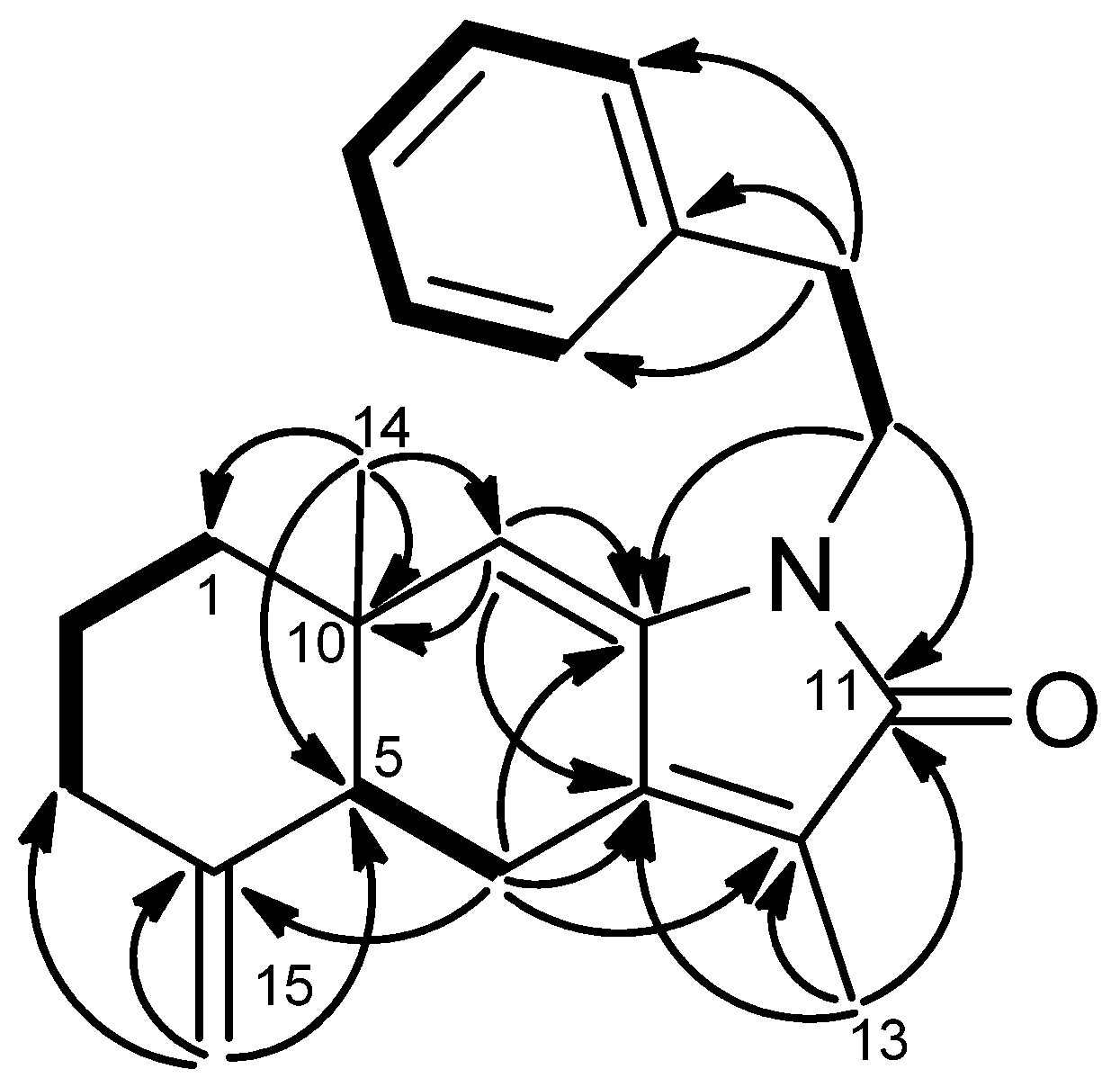
1H–
1H COSY (
Figure 4) correlations of H-7/H-6/H-5, H-3/H-2/H-1/H-14/H-13/H-12, NH (δ
H 5.71, brs)/H-1′/H-2′ and H-4′/H-5′/H-6′/H-7′/H-8′ revealed the sequences of three fragments including H-5 to H-7, H-3 to H-12 and a benzylethyl amine side chain. The HMBC correlations (
Figure 4) of H-9/C-10, C-8, H-12/C-11, Me-16/C-11, Me-17/C-11 and H-1′/C-10 permitted assignment of the two carbonyls at C-10 and C-11. Also, it established the connectivity between C-10 and C-1′. The absence of HMBC correlations between H-9/C-11, and H-12/C-10 indicated that compound
2 represents an unusual C-20 norditerpenoid [
13] with bond cleavage between C-10 and C-11. The relative configuration of compound
2 was determined by NOESY experiments (
Figure 5) and computer-generated perspective models using the MM2 force field calculation. A NOESY correlation between Me-19 and H-6, and the lack of a correlation between Me-19 and H-7 suggested that the 7,8-double bond has an
E geometry, similar to compound
1.
Figure 4.
COSY (bold bond) and HMBC (arrow) correlations of 2.
Figure 4.
COSY (bold bond) and HMBC (arrow) correlations of 2.
Figure 5.
Selected NOESY correlations and computer-generated perspective model of 2.
Figure 5.
Selected NOESY correlations and computer-generated perspective model of 2.
The HRESIMS determined the molecular formula of compound
3 as C
23H
27ON (
m/
z 356.1992 [M + Na]
+, calcd. 356.1990) and indicated eleven degrees of unsaturation. The IR absorption of 1676 cm
−1 suggested the presence of a conjugated amide group. The
1H,
13C (
Table 1 and
Table 2) and DEPT NMR spectroscopic data revealed the presence of an amide carbonyl (δ
C 170.0), a trisubstituted olefin [δ
C 137.1 (s), 119.1 (d); δ
H 5.05, s], an exomethylene group [δ
C 148 (s), 107.0 (t); δ
H 4.86, 4.61, each s], a tetrasubstituted olefin (δ
C 123.9, 139.8), a phenyl group [δ
C 139.2 (s), 128.9 (d, 2C), 126.4 (d, 2C), 128.5 (d); δ
H 7.17, d,
J = 6.6 Hz (2H), δ
H 7.19, t,
J = 6.6 Hz (2H), δ
H 7.26, t,
J = 6.6 Hz], an aliphatic CH group (δ
H 2.18, m; δ
C 48.9), and four aliphatic CH
2 group (δ
C 39.5, 23.2, 36.2, 22.3). The above findings accounted for five of the eight degrees of unsaturation, indicating that compound
3 is a tricyclic sesquiterpene with a phenyl group.
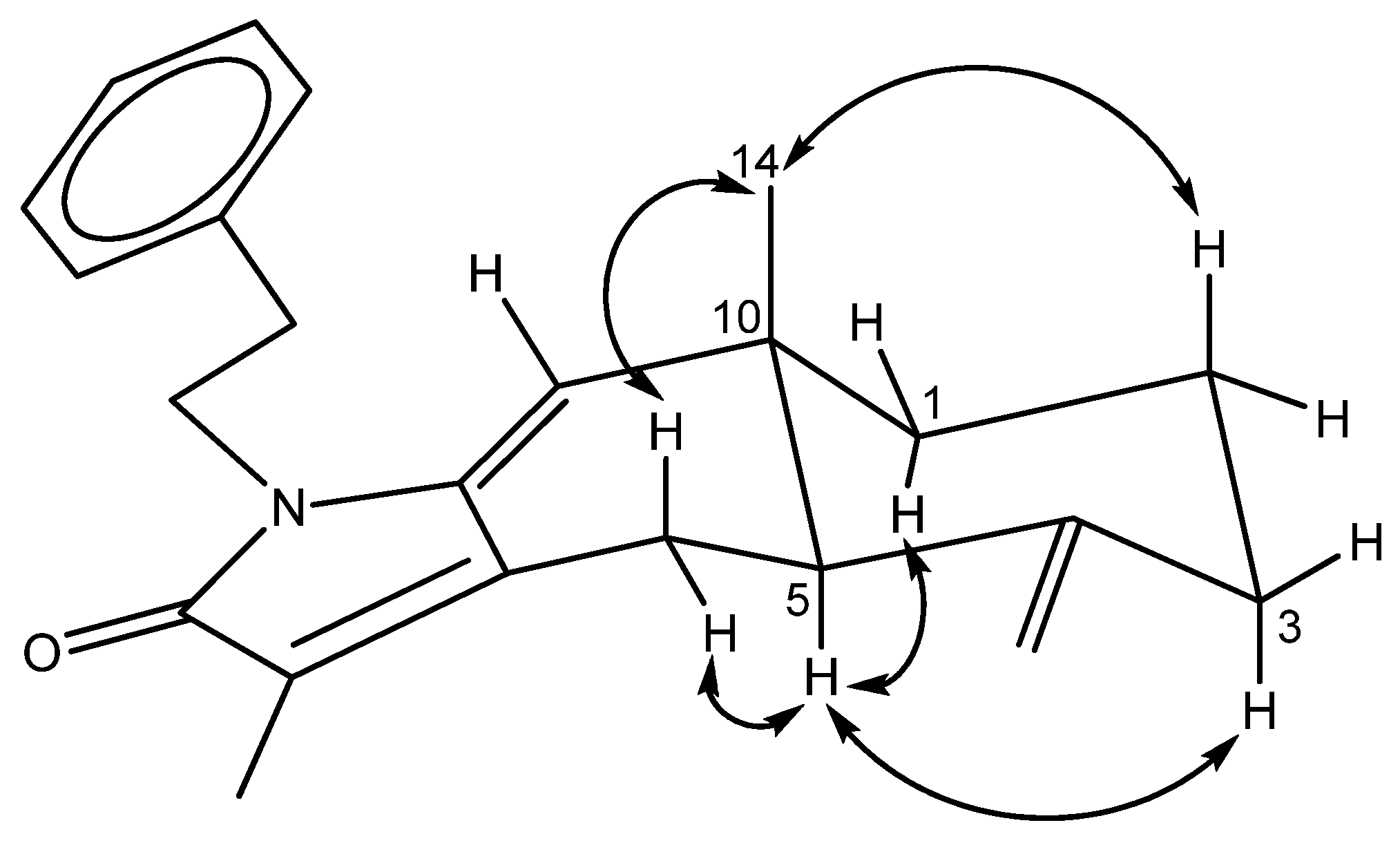
1H–
1H COSY spectrum of
3 showed four sets of correlations, H-1/H-2/H-3, H-5/H-6, H-1′/H-2′, and H-4′/ H-5′/ H-6′/ H-7′/ H-8′. The HMBC correlations (
Figure 6) of H
2-15/C-2, C-4, C-5 confirmed an exocyclic double bond between C-3 and C-5. The HMBC correlations of CH
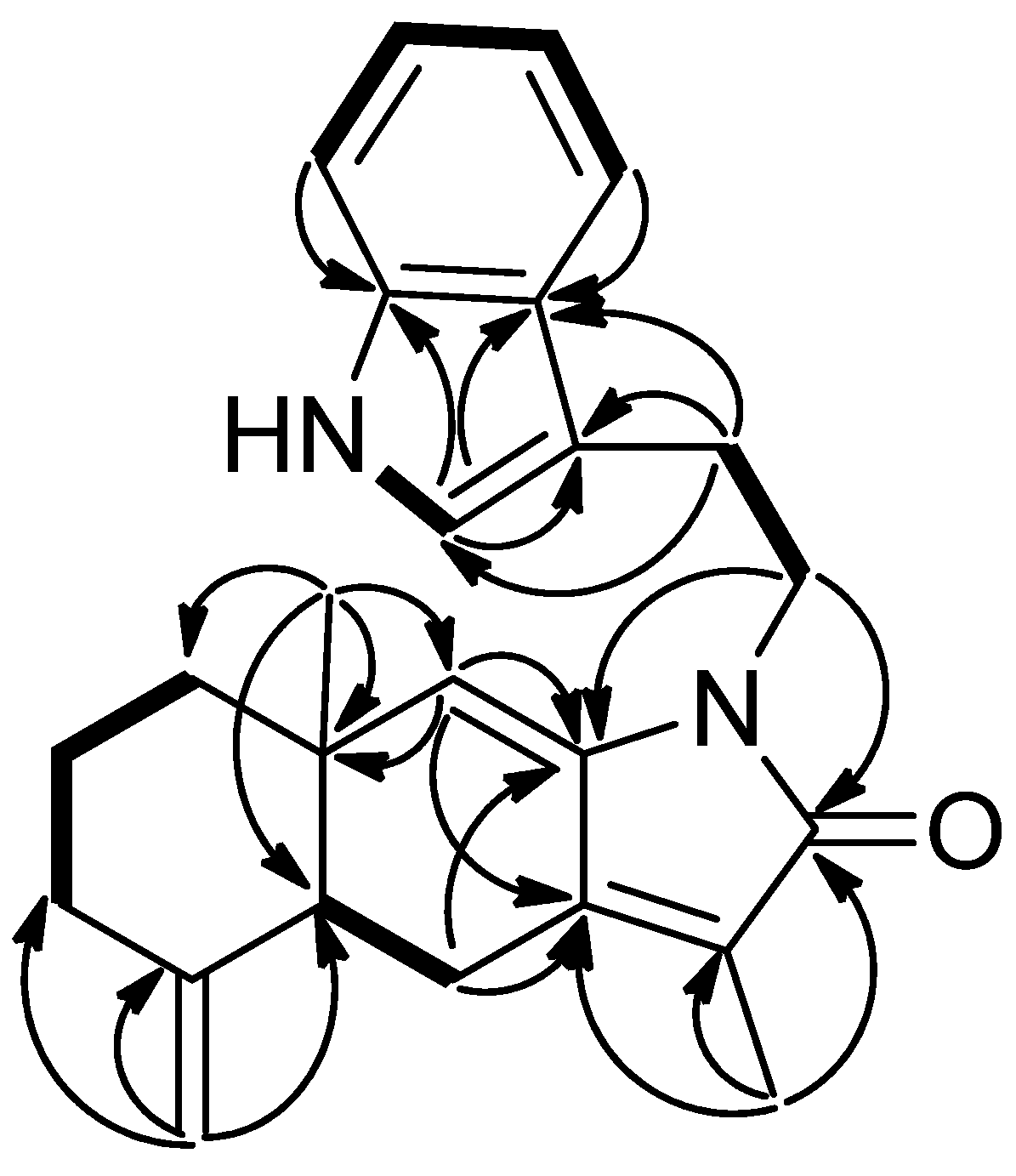
3-13/C-12, C-11, C-7; Me-14/C-10, C-1, C-9, C-5, and H-9/C-10, C-8, C-7 not only suggested the occurrence of double bonds between C-7/C-12 and C-8/C-9 but also assign the methyl group at C-10 and C-12. The presence of an α,β-unsaturated δ-lactam was inferred from the IR and HMBC spectra. Moreover, the HMBC correlations of H-1′/C-11, C-8 and H-2′/C-3′, C-4′, C-8′ indicated an amide carbonyl at C-11 and a phenylethyl side chain attached to a nitrogen atom. The relative configuration of
3 was determined on the basis of NOESY experiment and comparison with the optical rotation and NMR data of recent published compounds, taenialactams A and B, which were isolated from
C. taeniata [
14]. Assuming that H-5 possesses an α-orientation similar to that of taenialactams, the lack of NOESY correlation between H-5 and Me-14, suggested that Me-14 is β-oriented (
Figure 7).
Figure 6.
COSY (bold bond), HMBC (arrow) correlations of 3.
Figure 6.
COSY (bold bond), HMBC (arrow) correlations of 3.
Figure 7.
Selected NOESY correlation of 3.
Figure 7.
Selected NOESY correlation of 3.
The molecular formula of
4 was determined to be C
23H
27O
2N (Δ = 11) by HRESIMS data (
m/
z 372.1937 [M + Na]
+, calcd. 372.1939). The IR spectrum revealed the presence of hydroxy (3421 cm
−1) and α,β-unsaturated γ-lactam (1695 cm
−1) moieties. The
1H and
13C NMR spectra (
Table 1 and
Table 2) of compound
4 were similar to those of
3, suggesting structural similarity with the exception that compound
4 contains a
para-hydroxyphenylethyl side chain [δ
H 7.01, d,
J = 8.4 Hz (2H), 6.74, d,
J = 8.4 Hz (2H); δ
C 154.6 (s), 130.8 (s), 130.0 (d), 115.4 (d), 41.0 (t), 34.3 (t)] on the nitrogen atom, rather than a phenylethyl group as found in compound
3. Interpretation of
1H–
1H COSY and HMBC spectra of compound
4 also indicated the presence of a hydroxy group at C-6′. The relative configuration of compound
4 was determined by comparison with the NMR and the optical rotation of compound
3.
The molecular formula of compound
5 was shown to be C
25H
28ON
2 (Δ = 13), as deduced from HRESIMS at
m/
z 395.2099 ([M + Na]
+, calcd. 395.2099). Spectroscopic data of compound
5 were found to be similar to those of
3 and
4 except for the evidence of an ethylindole moiety. The LRMS of compound
5 exhibited a peak at
m/
z 229 [M + H − C
10H
10N]
+, also consistent with the presence of an ethylindole group. In the
1H and
13C NMR spectra (
Table 1 and
Table 2), signals for a 3-ethylindole group [δ
H 3.84, dt,
J = 14.4, 7.2 Hz, 3.03, t,
J = 7.2 Hz, 7.02, d,
J = 1.5 Hz, 7.35, d,
J = 7.8 Hz, 7.18, t,
J = 7.2 Hz, 7.10, t,
J = 7.2 Hz, 7.59, t,
J = 7.8 Hz, 8.05, s (NH); δ
C 39.9 (t), 24.8 (t), 113.3 (s), 121.9 (d), 124.7 (s), 111.1 (d), 121.9 (d), 119.3 (d), 118.6 (d), and 127.6 (s)] were also observed. The 3-ethylindole group on the tertiary nitrogen in
5 was revealed by detailed analysis of 2D NMR spectra (
Figure 8). The HMBC correlations of H-1′/C-11 (δ
C 170.2), C-8 (δ
C 137.2) as well as correlations of H-2′/C-3′, C-4′ and C-11′ indicated that the phenylethyl side chain at the nitrogen in compound
3 was replaced by the 3-ethylindole group in compound
5. Assignment of the
1H and
13C-NMR spectroscopic data of
5 were accomplished by application of
1H–
1H COSY, HMQC, and HMBC correlations. The relative configuration of compound
5 was assigned the same as those of compounds
3 and
4.
Figure 8.
COSY (bold bond) and HMBC (arrow) correlations of 5.
Figure 8.
COSY (bold bond) and HMBC (arrow) correlations of 5.
Cespitaenin A (
6) was isolated as a colorless, amorphous solid. The molecular formula, C
21H
30O
3, was established by the HRESIMS at
m/
z 353.2096 [M + Na]
+ (calcd. 353.2093). The IR bands at 1720 and 1706 cm
−1 were attributed to an ester and a carbonyl group, which were confirmed by the presence of the acetate (δ
C 170.1) and ketocarbonyl (δ
C 202.1). The
13C-NMR (
Table 2) and DEPT spectra of compound
6 revealed 21 carbons including three methyl carbons (δ
C 19.5, 24.8, and 32.8), six aliphatic methylene carbons (δ
C 30.6, 31.3, 41.1, 50.7, 23.8 and 22.8), a methine carbon (δ
C 43.1), an oxygenated methine carbon (δ
C 72.3), an aliphatic quaternary carbon (δ
C 35.4), two olefinic methine carbons (δ
C 129.0 and 135.4), an olefinic methylene carbon (δ
C 113.5), three olefinic quaternary carbons (δ
C 146.4, 133.3, and 148.0), and two additional carbonyl signals. The
1H–
1H COSY spectrum showed the connectivities of H-7/H-6/H-5 and H-3/H-2/H-1/H-14/H-13/H-12. Resonances at δ
C 133.3 (C-8) and 129.0 (C-7) were correlated in the HMBC spectrum with proton signals at δ
H 5.15 (d,
J = 8.4 Hz, H-7), and with the vinylic methyl protons at δ
H 1.76 (Me-19), and suggested that compound
6 contains an
E-trisubstituted double bond bearing a methyl group [
14]. In addition, a trisubstituted double bond [δ
C 148.0 (s), 135.4 (d), δ
H 6.30, t,
J = 8.4 Hz] and a 1,1-disubstituted olefin (δ
C 144.7) with an exomethylene group (δ
C 115.5; δ
H 4.87, 4.95, each s) were also implied by interpretation of the HMBC data of compound
6. Moreover, HMBC correlations of δ
H 5.38 (dt,
J = 8.4, 2.4 Hz, H-6) with δ
C 170.1 indicated that C-6 (δ
C 72.3) is attached to an acetoxy group (δ
C 21.3). HMBC correlations of H-12/C-11, C-10, C-15, H-9/C-10, C-11, Me-16/C-11, C-15, C-1 and Me-17/C-11, C-15, C-1, H-18/C-3, C-5 established the final structure of
6. The relative configuration of
6 was determined by NOESY analysis and comparison of the coupling constants of
6 with the data reported [
14,
15,
16,
17]. Assuming that H-1 is at the β position, the correlations between H-1/Me-16/Me-17 indicated the β-disposition of Me-16 and Me-17. The spin pattern and coupling constants of H-6, and NOESY correlations of H-6/Me-19/H-9α and H-7/H-9β agreed with a β-orientation of the acetoxy group at C-6.
Cespitaenin B (
7),
−109 (CH
2Cl
2), was isolated as a colorless, amorphous solid. Its molecular formula was determined to be C
19H
28O
5 (Δ= 6) from HRESIMS at
m/
z 359.1837 [M + Na]
+. Its IR bands showed the presence of a hydroxy (3397 cm
−1) and conjugated carbonyl (1697 cm
−1) groups. The
1H and
13C-NMR spectroscopic (
Table 1 and
Table 2) and DEPT data indicated the presence of two ketocarbonyls (δ
C 214.5 and 208.1), a trisubstituted olefin [δ
C 133.4 (s), 132.7 (d); δ
H 5.56, d,
J = 9.3 Hz], and an exocyclic double bond [δ
C 144.8 (s), 115.5 (t); δ
H 4.92, 4.96, each s). In the aliphatic region, a quaternary carbon (δ
C 46.8), two oxygenated methine carbons (δ
C 70.2 and 74.8), an oxygenated tertiary carbon (δ
C 92.2), five methylene carbons (δ
C 32.9, 39.2, 46.8, 49.4, and 24.3), and three methyl groups (δ
C 25.8, 26.5, and 17.6; δ
H 0.77, 1.47, and 1.89, each s) were observed. HMQC correlations of δ
H 4.55 (dt,
J = 9.6, 3.9 Hz, H-6) with δ
C 70.2 (d, C-6) and δ
H 4.39 (t,
J = 3.3 Hz, H-13) with δ
C 74.8 (d, C-13) suggested that C-6 and C-13 are hydroxylated. The
1H–
1H COSY spectrum indicated the connectivities of H-7/H-6/H-5 and H-3/H-2/H-1/H-14/H-13 to be similar with those of compound
6 (
Figure 9). The two ketocarbonyls assigned at C-10 and C-12, and the hydroxyl group assigned at C-11 were deduced from the interpretation of HMBC correlations of H-9/C-10, C-11; H-13/C-12, C-11; Me-16, Me-17/C-1, C-11, C-15; O
H-11 (δ
H 3.13, br s)/C-11, C-10, C-12. The remaining HMBC correlations of Me-16/C-15, C-1, Me-17/C-15, C-1 also indicated that compound
7 has the same 6/12 bicyclic system as compound
6. The NOESY spectrum showed correlations of H-1/Me-16, Me-17, O
H-11/Me-16 indicating that the hydroxy on C-11 is β
-oriented, while H-6 is α
-oriented due to the correlations of H-6/Me-19/H-9α (δ
H 3.89) and H-7/Me-17/H-9β (δ
H 2.84). The lack of correlations of H-13/H-1, Me-16, Me-17 was consistent with an α-orientation of H-13.
Figure 9.
COSY (bold bond) and HMBC (arrow) correlations of 7.
Figure 9.
COSY (bold bond) and HMBC (arrow) correlations of 7.
The molecular formula of cespitaenin C (
8) was determined to be C
22H
32O
4, as derived from a
quasi-molecular ion at
m/
z 361.2378 ([M + Na]
+, calcd. 361.2379), and seven indices of hydrogen deficiency. The IR spectrum displayed absorption bands suggestive of hydroxyl (3385 cm
−1) and ester carbonyl (1738 cm
−1) moieties. The
1H and
13C-NMR spectra (
Table 1 and
Table 2) exhibited an exomethylene double bond [δ
C 145.9 (s), 114.5 (t); δ
H 4.83, 4.84, each s], a trisubstituted double bond [δ
C 131.4 (s), 135.6 (d); δ
H 5.51, d,
J = 7.8 Hz, H-7), a tetrasubstituted double bond (δ
C 166.6, C-11; 129.5, C-12), and an ester carbonyl (δ
C 170.5), accounting for four degrees of unsaturation. These findings implied that
8 is a tricyclic compound. The
1H–
1H COSY correlations of H-7/H-6/H-5, H-3/H-2/H-1/H-14/H-13, and H-1′/H-2′, along with the HMBC correlations of H
2-9/C-10, C-11, H-13/C-12, C-11, C-20; Me-16/C-11, C-12; Me-17/C-11, C-12 clearly indicated that compound
8 contains a common verticillene skeleton. HMBC correlations of H-1′/C-10 suggested the ethoxy group at C-10 and thus a carbonyl at C-20 (δ
C 170.5). The relative configuration of compound
8 was deduced from the NOESY analysis and comparison with chemical shifts and coupling constants of cespihypotin V [
18]. The NOESY correlations of H-1′/Me-16, H-1/Me-17/Me-17 and H-6/Me-19 indicated that Me-16, Me-17, H-1, and the OEt were β-oriented, while H-6 is α-oriented.
The HRESIMS data of cespitaenin D (
9) established the molecular formula of C
22H
34O
5 (
m/
z 401.2306, [M + Na]
+), and indicated six indices of hydrogen deficiency. The IR spectrum displayed an absorption band indicative of hydroxy (3444 cm
−1) group. The
1H and
13C-NMR spectroscopic data (
Table 1 and
Table 2) showed an exomethylene double bond (δ
C 145.8 (s), 115.6 (t); δ
H 4.92, s, 2H), a trisubstituted double bond [δ
C 133.2 (d), 132.8 (s); δ
H 5.45, d,
J = 8.5 Hz, H-7), and a tetrasubstituted double bond, revealing two degrees of unsaturation. This implied that compound
9 possesses a tetracyclic ring system. The similar
1H,
13C-NMR, COSY, and HMBC data suggested that
9 should have the same verticillene skeleton as
8. However, HMBC correlations of H-1′/C-20; H-13/C-12, C-11, C-20; H-20/C-12, C-11; Me-16, Me-17/C-11 indicated an ethoxy group at C-20 (δ
C 103.5) and an epoxy ring at C-11 (δ
C 72.8) and C-12 (δ
C 78.0). The epoxy ring at C-11 and C-12 was tentatively assigned the α-configuration due to the steric hindrance of the two β-faced methyl groups (Me-16 and Me-17). NOESY correlations (
Figure 10) among H-1/Me-16, Me-17, H-6/Me-19/H-9α (δ
H 3.01) and H-7/H-9β (δ
H 2.53), and lack of NOESY correlation between H-20 and Me-17 indicated the β
-orientation of the ethoxy group at C-20 and the α-disposition of H-6.
Figure 10.
Selected NOESY correlation of 9.
Figure 10.
Selected NOESY correlation of 9.
Cespitaenin E (
10) was found to have the same molecular formula, C22H34O5, as
9. It displayed as a sodium adduct ion at
m/
z 401.2305 ([M + Na]+) in the HRESIMS. There were very few differences between the 1H-NMR spectroscopic data (
Table 1) of
9 and
10. Comparison of their 13C-NMR spectra (
Table 2) revealed that the differences occurred in the chemical shifts of C-13 (δC 26.0,
10; 31.6,
9) and C-20 (δC 107.3,
10; 103.5,
9). Furthermore, the COSY and HMBC correlations were closely comparable (Supporting Information). The NOESY correlations of H-20/Me-17 in
10 confirmed the β
-orientation of H-20. The only difference between
9 and
10 is the configuration of the ethoxy group at C-20. The optical rotations of
10 [
0.1 (CH2Cl2)] and
9 [
−20.6 (CH
2Cl
2)] supported the conclusion to be made that compound
10 is the 20-epimer of cespitaenin D.
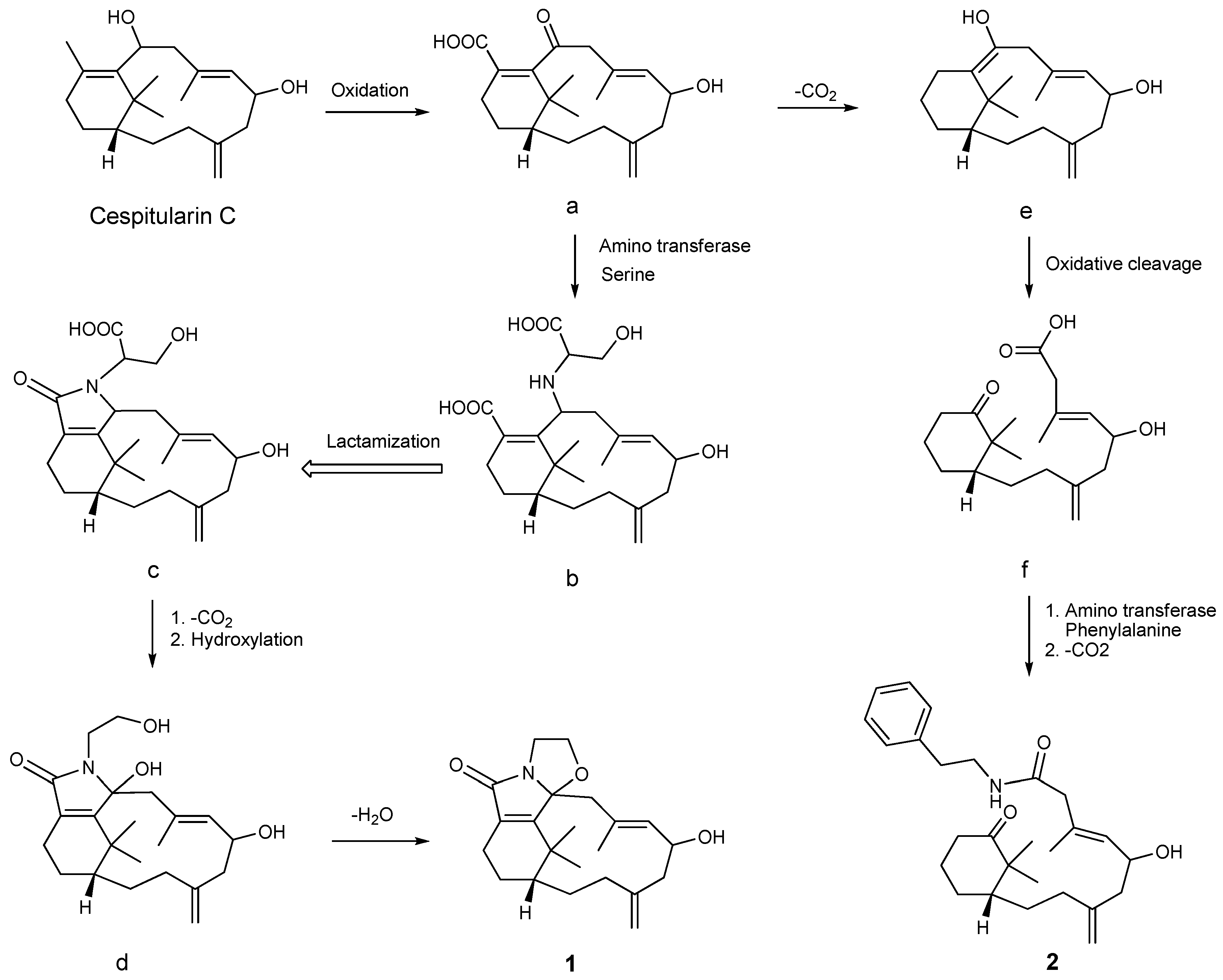
A postulated biosynthetic pathway for compounds
1 and
2 is illustrated in
Scheme 1. Compound
1 is probably produced from cespitularin C [
19] via intermediates
a–
d, involving steps of oxidation, serine transformation, lactamization, decarboxylation, hydroxylation, and dehydration. Compound
2 may be generated from the nor-verticillene
a through intermediates
e and
f. These reactions deal with decarboxylation, cleavage of the double bond between C-10 and C-11 [
19], and phenylalanine transformation leading to an amide formation.
Four human cancer cell lines were chosen to test the
in vitro cytotoxicity of compounds
1–
10 (
Table 3). Compound
5 exhibited cytotoxicity against human breast adenocarcinoma (MCF-7), medulloblastoma (Daoy), and cervical epitheloid carcinoma (Hela) cancer cells with IC
50 of 17.5, 22.3, and 24.7 μM, respectively. Compound
6 showed significant cytotoxicity against human breast adenocarcinoma (MCF-7) cancer cells with the IC
50 at 21.2 μM.
Scheme 1.
A postulated biosynthetic pathway for compounds 1 and 2.
Scheme 1.
A postulated biosynthetic pathway for compounds 1 and 2.
Table 3.
Cytotoxicity of compounds 1–10 against human cancer cells (IC50, μM) a.
Table 3.
Cytotoxicity of compounds 1–10 against human cancer cells (IC50, μM) a.
| Compound | Hela | Daoy | WiDr | MCF-7 |
|---|
| 3 | 30.9 | 34.8 | 49.5 | 30.6 |
| 5 | 24.7 | 22.3 | 34.1 | 17.5 |
| 6 | 28.5 | 31.5 | 36.4 | 21.2 |
| mitomycin C | 0.32 | 0.32 | 0.32 | 0.32 |