Effect of Nitrate, Ammonium and Urea on Growth and Pinnatoxin G Production of Vulcanodinium rugosum
Abstract
:1. Introduction

2. Results and Discussion
2.1. Cell Growth
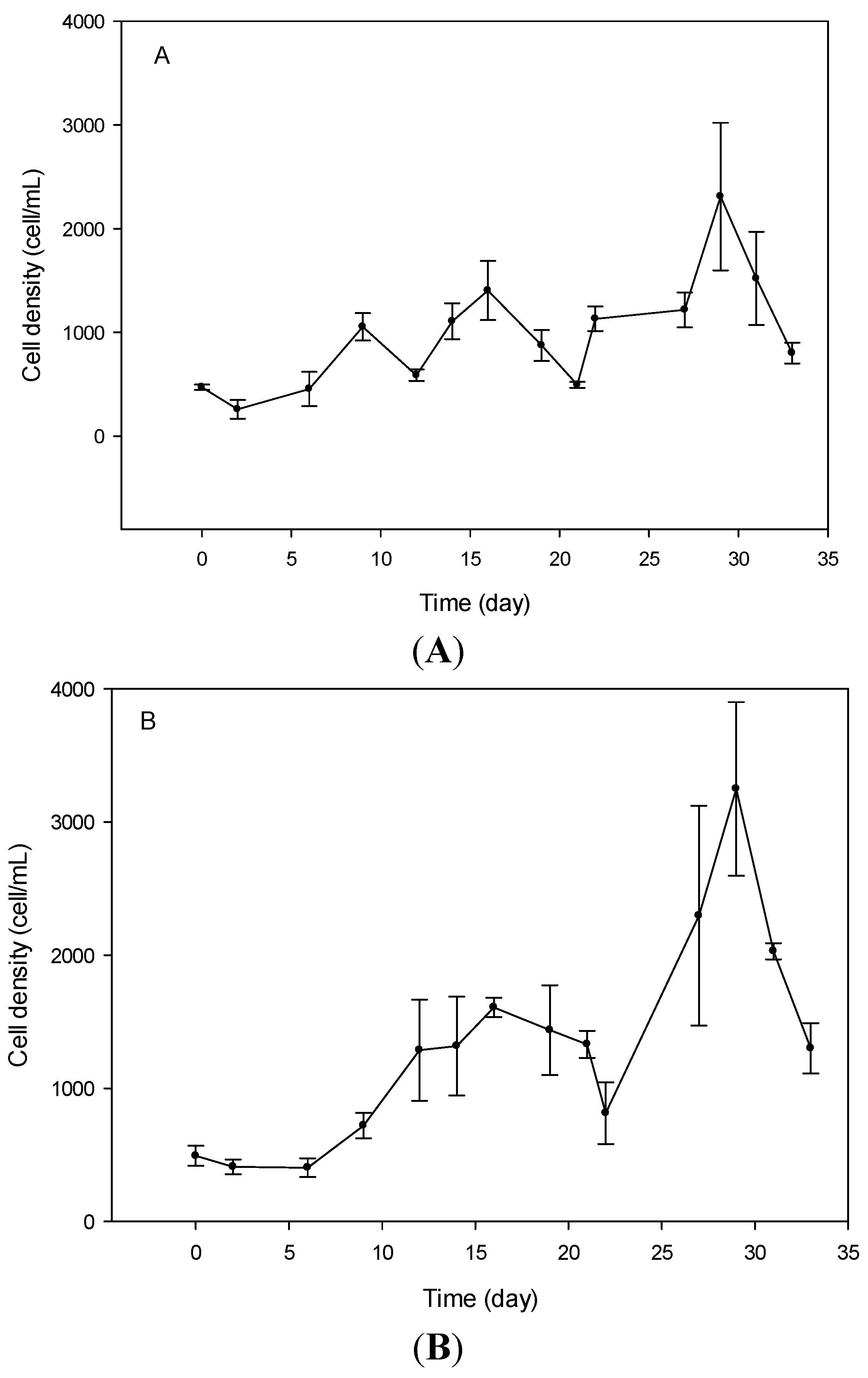
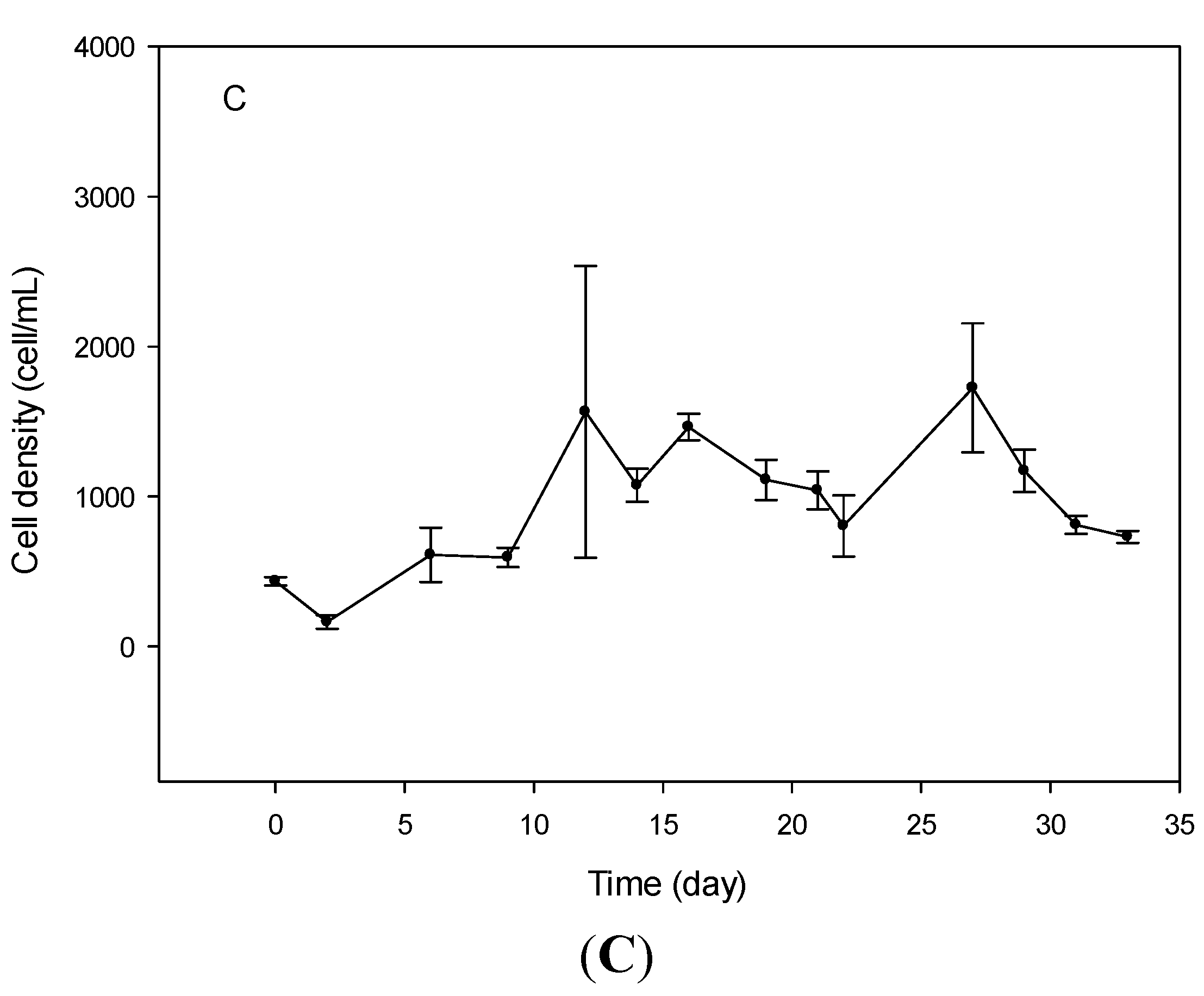
2.2. Cell Morphometry

2.3. Variation of Nitrogen Concentration in Cultures
2.4. Toxin Concentration Variations
2.5. Chlorophyll a Measurements
3. Experimental Section
3.1. Cultures of Vulcanodinium rugosum
3.2. Experimental Design
3.3. Growth Rate Calculation
3.4. Cell Diameter Measurement
3.5. Nitrogen Content in the Cultures
3.6. Toxin Extraction and Quantification
3.7. Chlorophyll a Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smayda, T.J. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Zingone, A.; Oksfeldt Enevoldsen, H. The diversity of harmful algal blooms: A challenge for science and management. Ocean Coast. Manag. 2000, 43, 725–748. [Google Scholar] [CrossRef]
- Glibert, P.M.; Anderson, D.M.; Gentien, P.; Granéeli, E.; Sellner, K.G. Global complex phenomena of harmful algal blooms. Oceanography 2005, 18, 136–147. [Google Scholar] [CrossRef]
- Shumway, S.E.; Allen, S.M.; Dee Boersma, P. Marine birds and harmful algal blooms: Sporadic victims or under-reported events? Harmful Algae 2003, 2, 1–17. [Google Scholar] [CrossRef]
- Bolch, C.J.S.; de Salas, M.F. A review of the molecular evidence for ballast water introduction of the toxic dinoflagellates Gymnodinium catenatum and the Alexandrium tamarensis complex to Australasia. Harmful Algae 2007, 6, 465–485. [Google Scholar] [CrossRef]
- Laabir, M.; Gentien, P. Survival of toxic dinoflagellates after gut passage in the pacific oyster Crassostrea gigas thunburg. J. Shellfish Res. 1999, 18, 217–222. [Google Scholar]
- Burkholder, J.M.; Glibert, P.M.; Skelton, H.M. Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae 2008, 8, 77–93. [Google Scholar] [CrossRef]
- Takada, N.; Umemura, N.; Suenaga, K.; Chou, T.; Nagatsu, A.; Haino, T.; Yamada, K.; Uemura, D. Pinnatoxins B and C, the most toxic components in the pinnatoxin series from the Okinawan bivalve Pinna muricata. Tetrahedron Lett. 2001, 42, 3491–3494. [Google Scholar] [CrossRef]
- Selwood, A.I.; Miles, C.O.; Wilkins, A.L.; van Ginkel, R.; Munday, R.; Rise, F.; McNabb, P. Isolation, structural determination and acute toxicity of pinnatoxins E, F and G. J. Agric. Food Chem. 2010, 58, 6532–6542. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; Smith, K.; Selwood, A.; McNabb, P.; van Ginkel, R.; Holland, P.; Munday, R. Production of pinnatoxins by a peridinoid dinoflagellate isolated from Northland, New Zealand. Harmful Algae 2010, 9, 384–389. [Google Scholar] [CrossRef]
- Nezan, E.; Chomerat, N. Vulcanodinium. rugosum gen. et sp. Nov. (dinophyceae): A new marine dinoflagellate from the French mediterranean coast. Cryptogam. Algologie 2011, 32, 3–18. [Google Scholar] [CrossRef]
- Hess, P.; Abadie, E.; Herve, F.; Berteaux, T.; Sechet, V.; Araoz, R.; Molgo, J.; Zakarian, A.; Sibat, M.; Rundberget, T.; et al. Pinnatoxin G is responsible for atypical toxicity in mussels (Mytilus. galloprovincialis) and clams (Venerupis. decussata) from Ingril, a French mediterranean lagoon. Toxicon 2013, 75, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; Smith, K.; Selwood, A.; McNabb, P.; Molenaar, S.; Munday, R.; Wilkinson, C.; Hallegraeff, G. Production of pinnatoxins E, F and G by scrippsielloid dinoflagellates isolated from Franklin Harbour, South Australia. N.Z. J. Mar. Freshw. Res. 2011, 45, 703–709. [Google Scholar] [CrossRef]
- Garrett, M.J.; Puchulutegui, C.; Selwood, A.I.; Wolny, J. Identification of the harmful dinoflagellate Vulcanodinium. rugosum recovered from a ballast tank of a globally traveled ship in Port Tampa Bay, Florida, USA. Harmful Algae 2014, 39, 202–209. [Google Scholar] [CrossRef]
- Smith, K.F.; Rhodes, L.L.; Suda, S.; Selwood, A.I. A dinoflagellate producer of pinnatoxin G, isolated from sub-tropical Japanese waters. Harmful Algae 2011, 10, 702–705. [Google Scholar] [CrossRef]
- Hernandez-Becerril, D.U.; Rodriguez-Palacio, M.C.; Lozano-Ramirez, C. Morphology and life stages of the potentially pinnatoxin-producing thecate dinoflagellate Vulcanodinium. rugosum from the tropical Mexican Pacific. Bot. Mar. 2013, 56, 535–540. [Google Scholar] [CrossRef]
- Selwood, A.I.; Wilkins, A.L.; Munday, R.; Gu, H.F.; Smith, K.F.; Rhodes, L.L.; Rise, F. Pinnatoxin H: A new pinnatoxin analogue from a south China sea Vulcanodinium. rugosum isolate. Tetrahedron Lett. 2014, 55, 5508–5510. [Google Scholar] [CrossRef]
- Rhodes, L.; Smith, K.; Selwood, A.; McNabb, P.; Munday, R.; Suda, S.; Molenaar, S.; Hallegraeff, G. Dinoflagellate Vulcanodinium rugosum identified as the causative organism of pinnatoxins in Australia, New Zealand and Japan. Phycologia 2011, 50, 624–628. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Leong, S.C.Y.; Murata, A.; Nagashima, Y.; Taguchi, S. Variability in toxicity of the dinoflagellate Alexandrium tamarense in response to different nitrogen sources and concentrations. Toxicon 2004, 43, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.H.; Kwok, O.T.; Ho, K.C.; Lee, F.W.F. Effects of different nitrate and phosphate concentrations on the growth and toxin production of an Alexandrium tamarense strain collected from drake passage. Mar. Envir. Res. 2012, 81, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.T.; Leaw, C.P.; Kobiyama, A.; Ogata, T. Growth and toxin production of tropical Alexandrium minutum halim (dinophyceae) under various nitrogen to phosphorus ratios. J. Appl. Phycol. 2010, 22, 203–210. [Google Scholar] [CrossRef]
- Xu, J.; Ho, A.Y.T.; He, L.; Yin, K.; Hung, C.; Choi, N.; Lam, P.K.S.; Wu, R.S.S.; Anderson, D.M.; Harrison, P.J. Effects of inorganic and organic nitrogen and phosphorus on the growth and toxicity of two Alexandrium species from Hong Kong. Harmful Algae 2012, 16, 89–97. [Google Scholar] [CrossRef]
- Jones, R.I. Mixotrophy in planktonic protists as a spectrum of nutritional strategies. Mar. Microb. Food Webs 1994, 8, 87–96. [Google Scholar]
- Jones, R.I. Mixotrophy in planktonic protists: An overview. Freshw. Biol. 2000, 45, 219–226. [Google Scholar] [CrossRef]
- Nygaard, K.; Tobiesen, A. Bacterivory in algae—A survival strategy during nutrient limitation. Limnol. Oceanogr. 1993, 38, 273–279. [Google Scholar] [CrossRef]
- Jeong, H.J.; Yoo, Y.D.; Kim, J.S.; Kim, T.H.; Kim, J.H.; Kang, N.S.; Yih, W. Mixotrophy in the phototrophic harmful alga Cochlodinium polykrikoides (dinophycean): Prey species, the effects of prey concentration, and grazing impact. J. Eukaryot. Microbiol. 2004, 51, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Kudela, R.M.; Ryan, J.P.; Blakely, M.D.; Lane, J.Q.; Peterson, T.D. Linking the physiology and ecology of Cochlodinium to better understand harmful algal bloom events: A comparative approach. Harmful Algae 2008, 7, 278–292. [Google Scholar] [CrossRef]
- Collos, Y.; Vaquer, A.; Laabir, M.; Abadie, E.; Laugier, T.; Pastoureaud, A.; Souchu, P. Contribution of several nitrogen sources to growth of Alexandrium catenella during blooms in Thau lagoon, Southern France. Harmful Algae 2007, 6, 781–789. [Google Scholar] [CrossRef]
- Mitamura, O.; Saijo, Y. Decomposition of urea associated with photosynthesis of phytoplankton in coastal waters. Mar. Biol. 1975, 30, 67–72. [Google Scholar] [CrossRef]
- Collos, Y.; Bec, B.; Jauzein, C.; Abadie, E.; Laugier, T.; Lautier, J.; Pastoureaud, A.; Souchu, P.; Vaquer, A. Oligotrophication and emergence of picocyanobacteria and a toxic dinoflagellate in Thau lagoon, Southern France. J. Sea Res. 2009, 61, 68–75. [Google Scholar] [CrossRef]
- Laabir, M.; Jauzein, C.; Genovesi, B.; Masseret, E.; Grzebyk, D.; Cecchi, P.; Vaquer, A.; Perrin, Y.; Collos, Y. Influence of temperature, salinity and irradiance on the growth and cell yield of the harmful red tide dinoflagellate Alexandrium catenella colonizing mediterranean waters. J. Plankton Res. 2011, 33, 1550–1563. [Google Scholar] [CrossRef]
- Laabir, M.; Barre, N.; Franco, J.; Brunet, C.; Masseret, E.; Collos, Y. Morphological, biochemical and growth characteristics of Alexandrium catenella (Whedon & Kofoid) Balech, a toxic dinoflagellate expanding in Mediterranean waters. Cah. Biol. Mar. 2012, 53, 365–372. [Google Scholar]
- Pistocchi, R.; Pezzolesi, L.; Guerrini, F.; Vanucci, S.; Dell’Aversano, C.; Fattorusso, E. A review on the effects of environmental conditions on growth and toxin production of Ostreopsis. ovata. Toxicon 2011, 57, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, F.; Pezzolesi, L.; Feller, A.; Riccardi, M.; Ciminiello, P.; Dell’Aversano, C.; Tartaglione, L.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; et al. Comparative growth and toxin profile of cultured Ostreopsis. ovata from the Tyrrhenian and Adriatic Seas. Toxicon 2010, 55, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.L.; Vershinin, A.; Smith, L.L.; Leighfield, T.A.; Pankov, S.; Quilliam, M.A. Seasonality of Dinophysis spp. and Prorocentrum lima in Black Sea phytoplankton and associated shellfish toxicity. Harmful Algae 2009, 8, 629–636. [Google Scholar] [CrossRef]
- Vale, P.; Veloso, V.; Amorim, A. Toxin composition of a Prorocentrum lima strain isolated from the Portuguese coast. Toxicon 2009, 54, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Abadie, E.; Ifremer, Sète, France. Unpublished work. 2015.
- Collos, Y.; Gagne, C.; Laabir, M.; Vaquer, A.; Cecchi, P.; Souchu, P. Nitrogenous nutrition of Alexandrium catenella (dinophyceae) in cultures and in Thau lagoon, Southern France. J. Phycol. 2004, 40, 96–103. [Google Scholar] [CrossRef]
- Collos, Y.; Lespilette, M.; Vaquer, A.; Laabir, M.; Pastoureaud, A. Uptake and accumulation of ammonium by Alexandrium catenella during nutrient pulses. Afr. J. Mar. Sci. 2006, 28, 313–318. [Google Scholar] [CrossRef]
- Twomey, L.J.; Piehler, M.F.; Paerl, H.W. Phytoplankton uptake of ammonium, nitrate and urea in the Neuse River Estuary, NC, USA. Hydrobiologia 2005, 533, 123–134. [Google Scholar] [CrossRef]
- Jauzein, C.; Collos, Y.; Garces, E.; Vila, M.; Maso, M. Short-term temporal variability of ammonium and urea uptake by Alexandrium catenella (Dinophyta) in cultures. J. Phycol. 2008, 44, 1136–1145. [Google Scholar] [CrossRef]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed]
- John, E.H.; Flynn, K.J. Growth dynamics and toxicity of Alexandrium fundyense (Dinophyceae): The effect of changing N:P supply ratios on internal toxin and nutrient levels. Eur. J. Phycol. 2000, 35, 11–23. [Google Scholar] [CrossRef]
- Hadjadji, I.; Masseret, E.; Plisson, B.; Laabir, M.; Cecchi, P.; Collos, Y. Clonal variation in physiological parameters of Alexandrium tamarense: Implications for biological invasions and maintenance. Cah. Biol. Mar. 2012, 53, 357–363. [Google Scholar]
- Harrison, P.J.; Waters, R.E.; Taylor, F.J.R. A broad-spectrum artificial seawater medium for coastal and open ocean phytoplankton. J. Phycol. 1980, 16, 28–35. [Google Scholar] [CrossRef]
- Larsen, J.; Kuosa, H.; Ikavalko, J.; Kivi, K.; Hallfors, S. A redescription of Scrippsiella. hangoei (Schiller) comb-nov—A red tide dinoflagellate from northern Baltic. Phycologia 1995, 34, 135–144. [Google Scholar] [CrossRef]
- Kremp, A.; Parrow, M.W. Evidence for asexual resting cysts in the life cycle of the marine peridinoid dinoflagellate, Scrippsiella hangoei. J. Phycol. 2006, 42, 400–409. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Division rates. In Handbook of Phycological Methods: Culture Methods and Growth Measurements; Cambridge University Press: Cambridge, UK, 1973; pp. 289–311. [Google Scholar]
- Alvarez, E.; Lopez-Urrutia, A.; Nogueira, E.; Fraga, S. How to effectively sample the plankton size spectrum? A case study using FlowCAM. J. Plankton Res. 2011, 33, 1119–1133. [Google Scholar] [CrossRef]
- Collos, Y.; Mornet, F.; Sciandra, A.; Waser, N.; Larson, A.; Harrison, P.J. An optical method for the rapid measurement of micromolar concentrations of nitrate in marine phytoplankton cultures. J. Appl. Phycol. 1999, 11, 179–184. [Google Scholar] [CrossRef]
- Koroleff, F. Determination of ammonium. In Method of Sea Water Analysis; Grasshoff, K., Kremling, K., Ehrhardt, M., Eds.; Wiley-VCH: Weinheim, Germany, 1983; pp. 117–182. [Google Scholar]
- Aminot, A.; Kerouel, R. Automatic-determination of urea in sea-water—A sensible method using diacetylmonoxime. Can. J. Fish. Aquat. Sci. 1982, 39, 174–183. [Google Scholar] [CrossRef]
- Neveux, J.; Lantoine, F. Spectrofluorometric assay of chlorophylls and phaeopigments using the least squares approximation technique. Deep Sea Res. Part I Oceanogr. Res. Pap. 1993, 40, 1747–1765. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abadie, E.; Kaci, L.; Berteaux, T.; Hess, P.; Sechet, V.; Masseret, E.; Rolland, J.L.; Laabir, M. Effect of Nitrate, Ammonium and Urea on Growth and Pinnatoxin G Production of Vulcanodinium rugosum. Mar. Drugs 2015, 13, 5642-5656. https://doi.org/10.3390/md13095642
Abadie E, Kaci L, Berteaux T, Hess P, Sechet V, Masseret E, Rolland JL, Laabir M. Effect of Nitrate, Ammonium and Urea on Growth and Pinnatoxin G Production of Vulcanodinium rugosum. Marine Drugs. 2015; 13(9):5642-5656. https://doi.org/10.3390/md13095642
Chicago/Turabian StyleAbadie, Eric, Lamia Kaci, Tom Berteaux, Philipp Hess, Véronique Sechet, Estelle Masseret, Jean Luc Rolland, and Mohamed Laabir. 2015. "Effect of Nitrate, Ammonium and Urea on Growth and Pinnatoxin G Production of Vulcanodinium rugosum" Marine Drugs 13, no. 9: 5642-5656. https://doi.org/10.3390/md13095642
APA StyleAbadie, E., Kaci, L., Berteaux, T., Hess, P., Sechet, V., Masseret, E., Rolland, J. L., & Laabir, M. (2015). Effect of Nitrate, Ammonium and Urea on Growth and Pinnatoxin G Production of Vulcanodinium rugosum. Marine Drugs, 13(9), 5642-5656. https://doi.org/10.3390/md13095642






