Hunt for Palytoxins in a Wide Variety of Marine Organisms Harvested in 2010 on the French Mediterranean Coast
Abstract
:1. Introduction
2. Results and Discussion
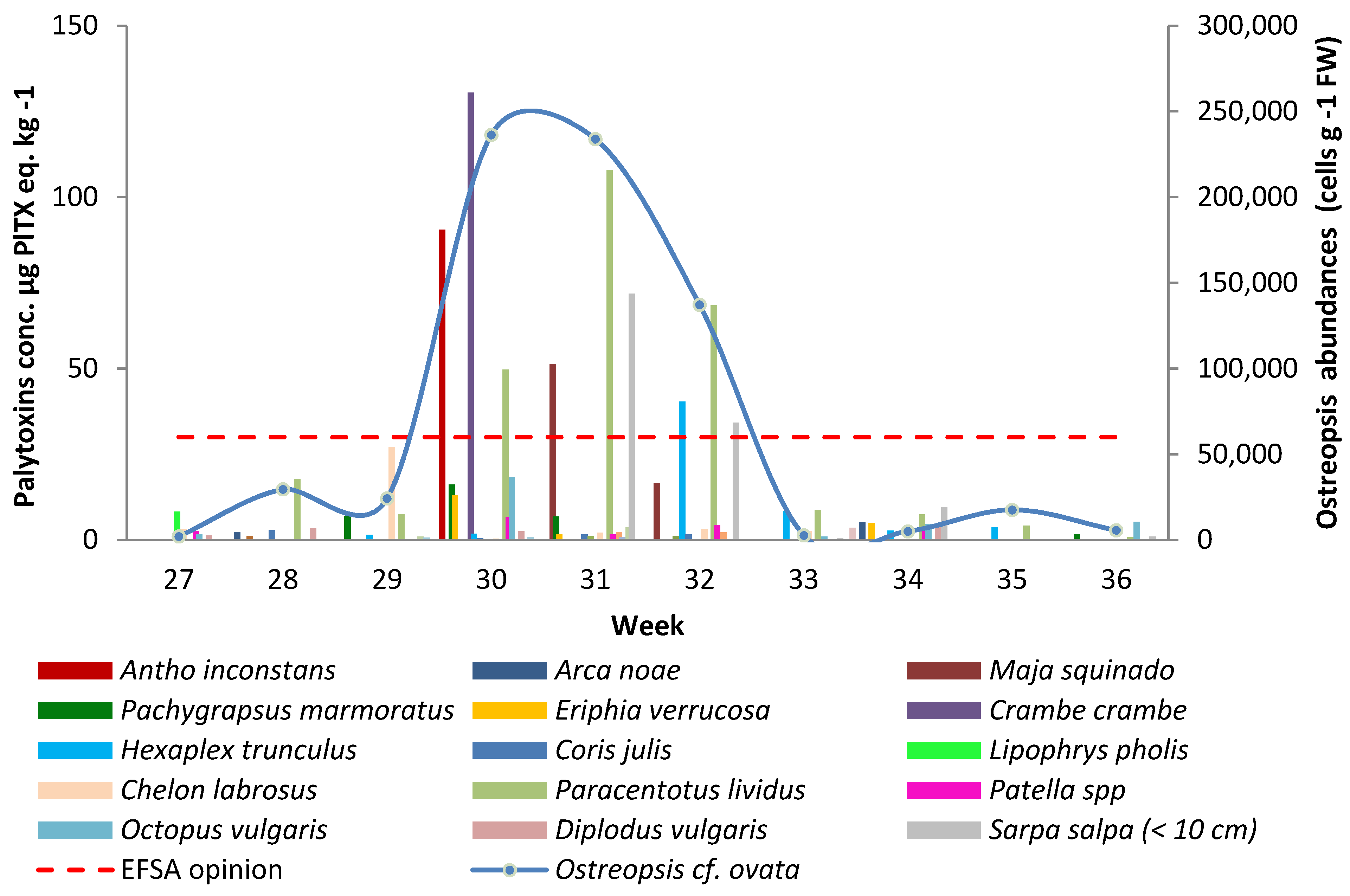
2.1. Evolution of the O. cf. ovata Abundances and Toxin Concentrations in Marine Organisms
| Group | Common Name | Species | Authorities | Tissue Analysed 1 | Diet 2 | Major Food Source |
|---|---|---|---|---|---|---|
| Fishes | Combtooth blenny | Lipophrys pholis | Linnaeus, 1758 | DT, RT | O | Crustaceans, other invertebrates and plants |
| Common two-banded seabream | Diplodus vulgaris | Geoffroy Saint-Hilaire, 1817 | WF | C | Benthic invertebrates, crustaceans, worms and molluscs | |
| Corkwing wrasse | Symphodus melops | Linnaeus, 1758 | WF | C | Shellfish and crustaceans | |
| East Atlantic peacock wrasse | Symphodus tinca | Linnaeus, 1758 | DT, RT | C | Sea urchins, brittle stars, bivalves, shrimps and crabs | |
| Five-spotted wrasse | Symphodus roissali | Risso, 1810 | WF | C | Small invertebrates like crustaceans, molluscs and sea urchins | |
| Gobbi | Gobiidae | WF | C | Most Gobiidae are carnivorous, but some feed on phytoplankton | ||
| Lizard fish | Saurida undosquamis | Richardson, 1848 | DT, RT | C | Fish, crustaceans, and other invertebrates | |
| Mediterranean moray | Muraena helena | Linnaeus, 1758 | DT, RT | C | Fish, crayfish and cephalopods, but also dead animals | |
| Mediterranean rainbow wrasse | Coris julis | Linnaeus, 1758 | WF | C | Amphipods, isopods, sea urchins, polychaetes, shrimp, and small gastropods | |
| Ornate wrasse | Thalassoma pavo | Linnaeus, 1758 | WF | C | Small invertebrates including crustaceans, molluscs and worms | |
| Oscellated wrasse | Symphodus ocellatus | Linnaeus, 1758 | WF | C | Benthic invertebrates including crustaceans, worms, molluscs and other small prey | |
| Painted comber | Serranus scriba | Linnaeus, 1758 | WF | C | Crustaceans, molluscs and fish | |
| Pointed-snout wrasse | Symphodus rostratus | Bloch, 1791 | WF | C | Small prey, mainly crustaceans | |
| Red mullet | Mullus surmuletus | Linnaeus, 1758 | WF | C | Worms and crustaceans found while excavating soft substrates | |
| Saddled seabream | Oblada melanura | Linnaeus, 1758 | DT, RT | O | Algae, zooplankton, small animals (crustaceans, larvae) and fish and invertebrate eggs. The proportion of planktonic prey decreases with growth in favour of benthic prey | |
| Salema porgy (seabream < 10 cm) | Sarpa salpa | Linnaeus, 1758 | DT, RT | H | Feeding behaviour depending on age. Adults feed on macroalgae and Posidonia leaves | |
| Salema porgy (seabream > 20 cm) | Sarpa salpa | Linnaeus, 1758 | DT, RT | H | ||
| Scorpion fish | Scorpaena porcus | Linnaeus, 1758 | DT, RT | C | Crabs, shrimps and fish | |
| Thicklip grey mulet | Chelon labrosus | Risso, 1827 | DT, RT | O | Plants, benthic microorganisms, small invertebrates and fingerlings | |
| White seabream | Diplodus sargus | Linnaeus, 1758 | DT, RT | C | Crustaceans, molluscs and echinoderms |
| Group | Common Name | Species | Authorities | Tissue Analysed 1 | Diet 2 | Major Food Source |
|---|---|---|---|---|---|---|
| Crustaceans | Marbled crab | Pachygrapsus marmoratus | Fabricius, 1787 | WF | O | Algae and animals, particularly mussels, limpets and members of its own species |
| Spinous spider crab | Maja squinado | Herbst, 1788 | WF | C | Molluscs and small crustaceans | |
| Warty/yellow crab | Eriphia verrucosa | Forskâl, 1775 | WF | C | Opportunist, feeds on dead or live prey | |
| Xantho crab | Xantho poressa | Olivi, 1792 | WF | C | Opportunist, feeds on dead or live prey | |
| Bivalve molluscs | Noah’s ark | Arca noae | Linnaeus, 1758 | WF | O | Plankton and fine organic particles filtered from the water column (via gills) |
| Gastropods | Banded dye-murex | Hexaplex trunculus | Linnaeus, 1758 | WF | C | Feeds on various organisms (bivalves, gastropods, hermit crabs, barnacles, tunicates, worms, etc.) and also scavenges (mainly dead fish) |
| Patella | Patella spp. | Linnaeus, 1758 | WF | H | Microalgae and cyanobacteria found on the substrate they live on | |
| Echinoderms | Sea urchin | Paracentotus lividus | Lamarck, 1816 | DT, roe | H | Macroalgae |
| Cephalopods | Octopus | Octopus vulgaris | Cuvier, 1797 | DT, RT | C | Crustaceans and molluscs |
| Sponges | Elephant’s hide sponge | Pachymatisma johnstonia | Bowerbank in Johnston, 1842 | WF | O | Bacteria, organic debris and unicellular algae |
| Orange-red encrusting sponge | Crambe crambe | Schmidt, 1862 | WF | O | ||
| Red encrusting sponge | Antho inconstans | Topsent, 1925 | WF | O |

2.2. Mean Toxin Concentrations in Marine Organisms
2.2.1. Marine Organisms Consumed as Whole Flesh
| Species | Common Name | Tissue Analysed | N | %LC | Mean | Min | Max | >30 µg·kg−1 | |
|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB/UB | LB/UB | % | |||||
| Fish | Lipophrys pholis | WF | 1 | 0% | 8.3 | 9.3 | 8.3/9.3 | 8.3/9.3 | 0% |
| Diplodus vulgaris | WF | 4 | 0% | 2.9 | 3.2 | 1.3/1.3 | 4.2/4.8 | 0% | |
| Symphodus melops | WF | 1 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Symphodus tinca | WF | 6 | 83% | 0.2 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Symphodus roissali | WF | 6 | 83% | 0.2 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Gobiidae | WF | 1 | 0% | 1.1 | 1.1 | 1.1/1.1 | 1.1/1.1 | 0% | |
| Saurida undosquamis | WF | 1 | 0% | 0.2 | 1.2 | 0.2/1.2 | 0.2/1.2 | 0% | |
| Murena helena | WF | 4 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Coris julis | WF | 7 | 43% | 0.9 | 1.6 | 0.0/1.2 | 2.8/2.8 | 0% | |
| Thalassoma pavo | WF | 2 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Symphodus ocellatus | WF | 1 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Serranus scriba | WF | 3 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Symphodus rostratus | WF | 2 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Mullus surmuletus | WF | 3 | 67% | 0.3 | 1.3 | 0.0/1.2 | 0.9/1.5 | 0% | |
| Oblada melanura | WF | 3 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Sarpa salpa (juveniles < 10 cm) | WF | 6 | 33% | 19.4 | 20.1 | 0.0/1.2 | 71.8/72.3 | 33% | |
| Sarpa salpa (adults > 20 cm) | WF | 3 | 33% | 0.6 | 1.6 | 0.1/1.2 | 0.9/1.9 | 0% | |
| Scorpaena porcus | WF | 7 | 71% | 0.6 | 1.5 | 0.0/1.2 | 2.3/2.3 | 0% | |
| Chelon labrosus | WF | 7 | 14% | 5.5 | 7.2 | 0.0/1.2 | 27.1/27.1 | 0% | |
| Diplodus sargus | WF | 7 | 86% | 0.5 | 1.6 | 0.0/1.2 | 3.7/3.7 | 0% | |
| Total group mean | WF | 75 | 61% | 2.0 | 3.0 | 0.0/1.2 | 71.8/72.3 | 3% | |
| Crustaceans | Pachygrapsus marmoratus | WF | 7 | 43% | 4.5 | 5.0 | 0.0/1.2 | 16.2/16.2 | 0% |
| Maja squinado | WF | 3 | 33% | 22.6 | 23.0 | 0.0/1.2 | 51.3/51.3 | 33% | |
| Eriphia verrucosa | WF | 5 | 40% | 3.9 | 4.4 | 0.0/1.2 | 13.0/13.0 | 0% | |
| Xantho poressa | WF | 1 | 0% | 3.6 | 3.6 | 3.6/3.6 | 3.6/3.6 | 0% | |
| Total group mean | WF | 16 | 38% | 8.7 | 9.0 | 0.0/1.2 | 51.3/51.3 | 6% | |
| Bivalve molluscs | Arka noae | WF | 2 | 0% | 3.8 | 3.8 | 2.3/2.3 | 5.2/5.2 | 0% |
| Gastropods | Hexaplex trunculus | WF | 8 | 25% | 7.3 | 7.7 | 0.0/1.2 | 40.4/40.4 | 13% |
| Patella spp. | WF | 8 | 38% | 2.4 | 2.8 | 0.0/1.2 | 6.6/6.6 | 0% | |
| Total group mean | WF | 16 | 31% | 4.9 | 5.3 | 0.0/1.2 | 40.4/40.4 | 6% | |
| Echinoderms | Paracentrotus lividus | WF | 9 | 0% | 30.3 | 30.7 | 0.8/1.3 | 107.6/108.0 | 33% |
| Cephalopods | Octopus vulgaris | WF | 5 | 0% | 6.1 | 6.3 | 0.5/1.6 | 18.3/18.3 | 0% |
| Overall group mean | WF | 123 | 46% | 4.3 | 5.1 | 0.0/1.2 | 107.6/108.0 | <1% | |
| Species | Common Name | Tissue Analysed | N | %LC | Mean | Min | Max | >30 µg·kg−1 | |
|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB/UB | LB/UB | % | |||||
| Fish | Lipophrys pholis | RT | 1 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% |
| Symphodus tinca | RT | 2 | 50% | 1.0 | 1.6 | 0.0/1.2 | 2.0/2.0 | 0% | |
| Saurida undosquamis | RT | 1 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Muraena helena | RT | 4 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Oblada melanura | RT | 2 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Sarpa salpa (juvenile < 10 cm) | RT | 4 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Sarpa salpa (adult > 20 cm) | RT | 3 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Scoprpaena porcus | RT | 6 | 83% | 0.2 | 1.2 | 0.0/1.2 | 1.4/1.4 | 0% | |
| Chelon labrosus | RT | 3 | 67% | 0.7 | 1.5 | 0.0/1.2 | 2.2/2.2 | 0% | |
| Diplodus sargus | RT | 2 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% | |
| Total group mean | RT | 28 | 89% | 0.2 | 1.3 | 0.0/1.2 | 2.2/2.2 | 0% | |
| Echinoderms | Paracentrotus lividus | Roe | 9 | 100% | 0.0 | 1.2 | 0.0/1.2 | 0.0/1.2 | 0% |
| Cephalopods | Octopus vulgaris | RT | 5 | 20% | 6.5 | 6.7 | 0.0/1.2 | 19.9/19.9 | 0% |
| Overall group mean | RT/Roe | 42 | 83% | 0.7 | 1.7 | 0.0/1.2 | 19.9/19.9 | 0% | |
2.2.2. Marine Organisms Consumed as Remaining Tissues
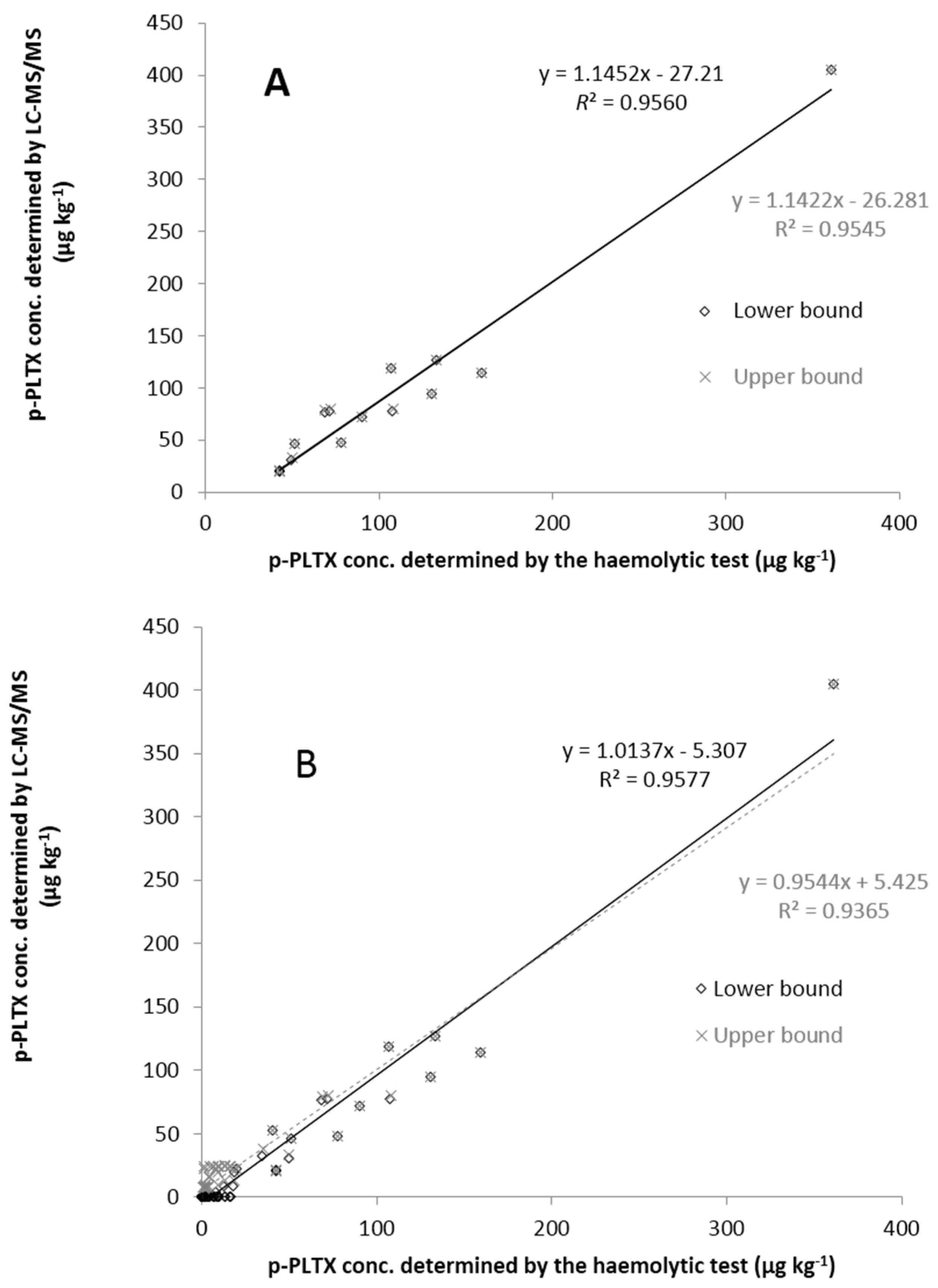
2.3. Comparison of the Toxin Levels Determined by Haemolytic Test and LC-MS/MS
3. Experimental Section
3.1. Sampling Location
3.2. Sampling Periods
3.3. Sampling of Marine Organisms
3.4. Sampling Techniques and Sample Preparation
3.5. Determination of the Toxin Content of the Marine Organisms
3.5.1. Reagents Used
3.5.2. Haemolytic Test
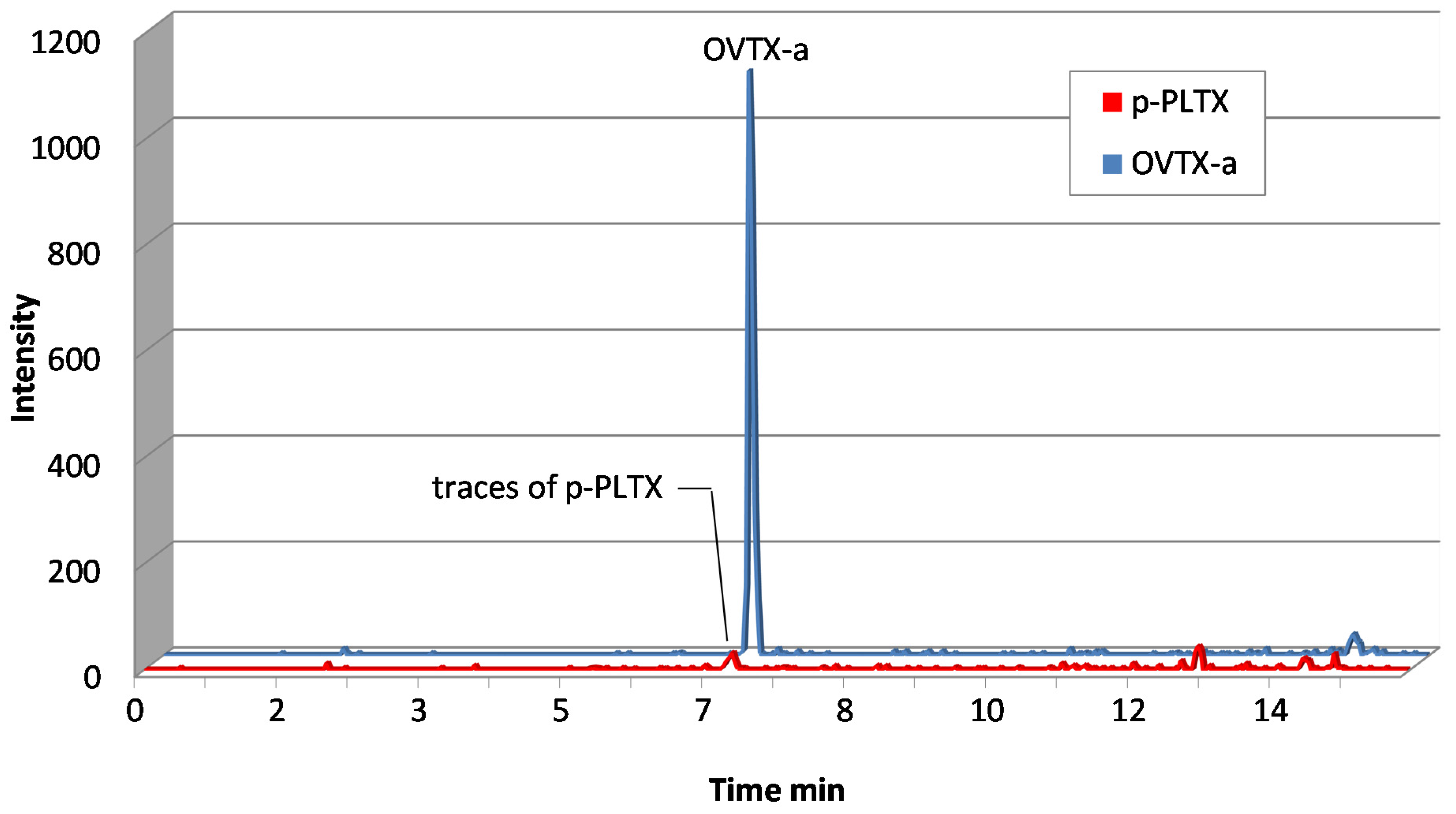
3.5.3. Tandem Mass Spectrometry (LC-MS/MS)
| Toxins | [M + 2H]2+ → [A moiety + H − H2O]+ | [M + 2H − H2O]2+ → [A moiety + H − H2O]+ |
|---|---|---|
| p-PLTX | 1340.3 → 327.3 | 1331.3 → 327.3 |
| OST-D | 1318.3 → 327.3 | 1309.3 → 327.3 |
| OVTX-a | 1324.3 → 327.3 | 1315.3 → 327.3 |
| OVTX-b | 1346.3 → 371.2 | 1337.3 → 371.2 |
| OVTX-c | 1354.3 → 371.2 | 1345.3 → 371.2 |
| OVTX-d | 1332.3 → 327.3 | 1323.3 → 327.3 |
3.5.4. Data Treatment
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moore, R.E.; Scheuer, P.J. Palytoxin: A new marine toxin from a coelenterate. Science 1971, 172, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Gleibs, S.; Mebs, D.; Werding, B. Studies on the origin and distribution of palytoxin in a Caribbean coral reef. Toxicon 1995, 33, 1531–1537. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Florio, C.; Lorenzon, P.; de Bortoli, M.; et al. Stereostructure and biological activity of 42-hydroxy-palytoxin: A new palytoxin analogue from Hawaiian Palythoa subspecies. Chem. Res. Toxicol. 2009, 22, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Brissard, C.; Herrenknecht, C.; Séchet, V.; Hervé, F.; Pisapia, F.; Harcouet, J.; Lémée, R.; Chomérat, N.; Hess, P.; Amzil, Z. Complex toxin profile of French Mediterranean Ostreopsis cf. ovata strains, seafood accumulation and ovatoxins prepurification. Mar. Drugs 2014, 12, 2851–2876. [Google Scholar] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Grillo, C.; Melchiorre, N. Putative palytoxin and its new analogue, ovatoxin-a, in Ostreopsis ovata collected along the Ligurian coasts during the 2006 toxic outbreak. J. Am. Soc. Mass Spectrom. 2008, 19, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Uemura, D.; Hirata, Y.; Iwashita, T.; Naoki, H. Studies on palytoxins. Tetrahedron 1985, 41, 1007–1017. [Google Scholar] [CrossRef]
- Rossi, R.; Castellano, V.; Scalco, E.; Serpe, L.; Zingone, A.; Soprano, V. New palytoxin-like molecules in Mediterranean Ostreopsis cf. ovata (dinoflagellates) and in Palythoa tuberculosa detected by liquid chromatography-electrospray ionization time-of-flight mass spectrometry. Toxicon 2010, 56, 1381–1387. [Google Scholar] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Guerrini, F.; Pezzolesi, L.; Pistocchi, R.; et al. Isolation and structure elucidation of ovatoxin-a, the major toxin produced by Ostreopsis ovata. J. Am. Chem. Soc. 2012, 134, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Guerrini, F.; Pistocchi, R. Complex palytoxin-like profile of Ostreopsis ovata. Identification of four new ovatoxins by high-resolution liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2735–2744. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dellaversano, C.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Battocchi, C.; Crinelli, R.; Carloni, E.; Magnani, M.; et al. Unique toxin profile of a mediterranean Ostreopsis cf. ovata strain: HR LC-MSn characterization of ovatoxin-f, a new palytoxin congener. Chem. Res. Toxicol. 2012, 25, 1243–1252. [Google Scholar]
- Brissard, C.; Hervé, F.; Sibat, M.; Séchet, V.; Hess, P.; Amzil, Z.; Herrenknecht, C. Characterization of ovatoxin-h, a new ovatoxin analog, and evaluation of chromatographic columns for Ovatoxin analysis and purification. J. Chromatogr. A 2015, 1388, 87–101. [Google Scholar] [CrossRef] [PubMed]
- García-Altares, M.; Tartaglione, L.; Dell’Aversano, C.; Carnicer, O.; De La Iglesia, P.; Forino, M.; Diogène, J.; Ciminiello, P. The novel ovatoxin-g and isobaric palytoxin (so far referred to as putative palytoxin) from Ostreopsis cf. ovata (NW Mediterranean Sea): Structural insights by LC-high resolution MSn. Anal. Bioanal. Chem. 2014, 407, 1191–1204. [Google Scholar]
- Suzuki, T.; Watanabe, R.; Uchida, H.; Matsushima, R.; Nagai, H.; Yasumoto, T.; Yoshimatsu, T.; Sato, S.; Adachi, M. LC-MS/MS analysis of novel ovatoxin isomers in several Ostreopsis strains collected in Japan. Harmful Algae 2012, 20, 81–91. [Google Scholar] [CrossRef]
- Usami, N.; Satatake, M.; Ishida, S.; Inoue, A.; Kan, Y.; Yasumoto, T. Palytoxin analogs from the dinoflagellate Ostreopsis siamensis. J. Am. Chem. Soc. 1995, 117, 5389–5390. [Google Scholar] [CrossRef]
- Ukena, T.; Satake, M.; Usami, M.; Oshima, Y.; Naoki, H.; Fujita, T.; Kan, Y.; Yasumoto, T. Structure elucidation of ostreocin D, a palytoxin analog isolated from the dinoflagellate Ostreopsis siamensis. Biosci. Biotechnol. Biochem. 2001, 65, 2585–2588. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, S.; Ten-Hage, L.; Turquet, J.; Quod, J.P.; Hennion, M.C. Characterisation of new analogues of palytoxin isolated from an Ostreopsis mascarenensis bloom in the South-Western Indian Ocean. Afr. J. Mar. Sci. 2006, 28, 389–391. [Google Scholar] [CrossRef]
- Lenoir, S.; Ten-Hage, L.; Turquet, J.; Quod, J.P.; Bernard, C.; Hennion, M.C. First evidence of palytoxin analogues from an Ostreopsis mascarenensis (Dinophyceae) benthic bloom in South-Western Indian Ocean. J. Phycol. 2004, 40, 1042–1051. [Google Scholar] [CrossRef]
- Mangialajo, L.; Ganzin, N.; Accoroni, S.; Asnaghi, V.; Blanfuné, A.; Cabrini, M.; Cattaneo-Vietti, R.; Chavanon, F.; Chiantore, M.; Cohu, S.; et al. Trends in Ostreopsis proliferation along the Northern Mediterranean coasts. Toxicon 2011, 57, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Shears, N.T.; Ross, P.M. Blooms of benthic dinoflagellates of the genus Ostreopsis; an increasing and ecologically important phenomenon on temperate reefs in New Zealand and worldwide. Harmful Algae 2009, 8, 916–925. [Google Scholar] [CrossRef]
- Armengaud, A.; Lasalle, J.L.; De Haro, L.; Grossel, H.; Lemée, R.; Rambaud, L.; Moissonnier, B.; Tichadou, L.; Glaizal, M.; Kantin, R.; et al. Bilan de la surveillance et de la prévention des effets sanitaires d’Ostreopsis sur le littoral méditerranéen en France (2007–2010). Bull. Epidémiol. Hebd. 2011, 29–30, 321–324. [Google Scholar]
- EFSA. Scientific opinion on marine biotoxins in shellfish—Palytoxin group. EFSA J. 2009, 7, 1393–1430. [Google Scholar]
- Amzil, Z.; Sibat, M.; Chomerat, N.; Grossel, H.; Marco-Miralles, F.; Lemee, R.; Nezan, E.; Sechet, V. Ovatoxin-a and palytoxin accumulation in seafood in relation to Ostreopsis cf. ovata blooms on the French Mediterranean coast. Mar. Drugs 2012, 10, 477–496. [Google Scholar] [PubMed]
- Biré, R.; Trotereau, S.; Lemée, R.; Delpont, C.; Chabot, B.; Aumond, Y.; Krys, S. Occurrence of palytoxins in marine organisms from different trophic levels of the French Mediterranean coast harvested in 2009. Harmful Algae 2013, 28, 10–22. [Google Scholar] [CrossRef]
- Cohu, S.; Mangialajo, L.; Thibaut, T.; Blanfuné, A.; Marro, S.; Lemée, R. Proliferation of the toxic dinoflagellate Ostreopsis cf. ovata in relation to depth, biotic substrate and environmental factors in the North West Mediterranean Sea. Harmful Algae 2013, 24, 32–44. [Google Scholar]
- Pistocchi, R.; Pezzolesi, L.; Guerrini, F.; Vanucci, S.; Dell’Aversano, C.; Fattorusso, E. A review on the effects of environmental conditions on growth and toxin production of Ostreopsis ovata. Toxicon 2011, 57, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Cohu, S.; Lemée, R. Vertical distribution of the toxic epibenthic dinoflagellates Ostreopsis cf. ovata, Prorocentrum lima and Coolia monotis in the NW Mediterranean Sea. Cahier Biol. Mar. 2012, 53, 373–380. [Google Scholar]
- Lopes, V.M.; Lopes, A.R.; Costa, P.; Rosa, R. Cephalopods as vectors of harmful algal bloom toxins in marine food webs. Mar. Drugs 2013, 11, 3381–3409. [Google Scholar] [CrossRef] [PubMed]
- Aligizaki, K.; Katikou, P.; Milandri, A.; Diogene, J. Occurrence of palytoxin-group toxins in seafood and future strategies to complement the present state of the art. Toxicon 2011, 57, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Aligizaki, K.; Katikou, P.; Nikolaidis, G.; Panou, A. First episode of shellfish contamination by palytoxin-like compounds from Ostreopsis species (Aegean Sea, Greece). Toxicon 2008, 51, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Yasumoto, T.; Battocchi, C.; Giacobbe, M.; Amorim, A.; et al. Investigation of toxin profile of Mediterranean and Atlantic strains of Ostreopsis cf. siamensis (Dinophyceae) by liquid chromatography-high resolution mass spectrometry. Harmful Algae 2013, 23, 19–27. [Google Scholar]
- FAO/WHO. Principles and methods for the risk assessment of chemicals in food. In Environmental Health Criteria 240; WHO Press: Geneva, Switzerland, 2009; pp. 1–98. [Google Scholar]
- European Food Safety Authority. Management of left-censored data in dietary exposure assessment of chemical substances. EFSA J. 2010, 8, 96. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biré, R.; Trotereau, S.; Lemée, R.; Oregioni, D.; Delpont, C.; Krys, S.; Guérin, T. Hunt for Palytoxins in a Wide Variety of Marine Organisms Harvested in 2010 on the French Mediterranean Coast. Mar. Drugs 2015, 13, 5425-5446. https://doi.org/10.3390/md13085425
Biré R, Trotereau S, Lemée R, Oregioni D, Delpont C, Krys S, Guérin T. Hunt for Palytoxins in a Wide Variety of Marine Organisms Harvested in 2010 on the French Mediterranean Coast. Marine Drugs. 2015; 13(8):5425-5446. https://doi.org/10.3390/md13085425
Chicago/Turabian StyleBiré, Ronel, Sophie Trotereau, Rodolphe Lemée, Davide Oregioni, Christine Delpont, Sophie Krys, and Thierry Guérin. 2015. "Hunt for Palytoxins in a Wide Variety of Marine Organisms Harvested in 2010 on the French Mediterranean Coast" Marine Drugs 13, no. 8: 5425-5446. https://doi.org/10.3390/md13085425
APA StyleBiré, R., Trotereau, S., Lemée, R., Oregioni, D., Delpont, C., Krys, S., & Guérin, T. (2015). Hunt for Palytoxins in a Wide Variety of Marine Organisms Harvested in 2010 on the French Mediterranean Coast. Marine Drugs, 13(8), 5425-5446. https://doi.org/10.3390/md13085425





