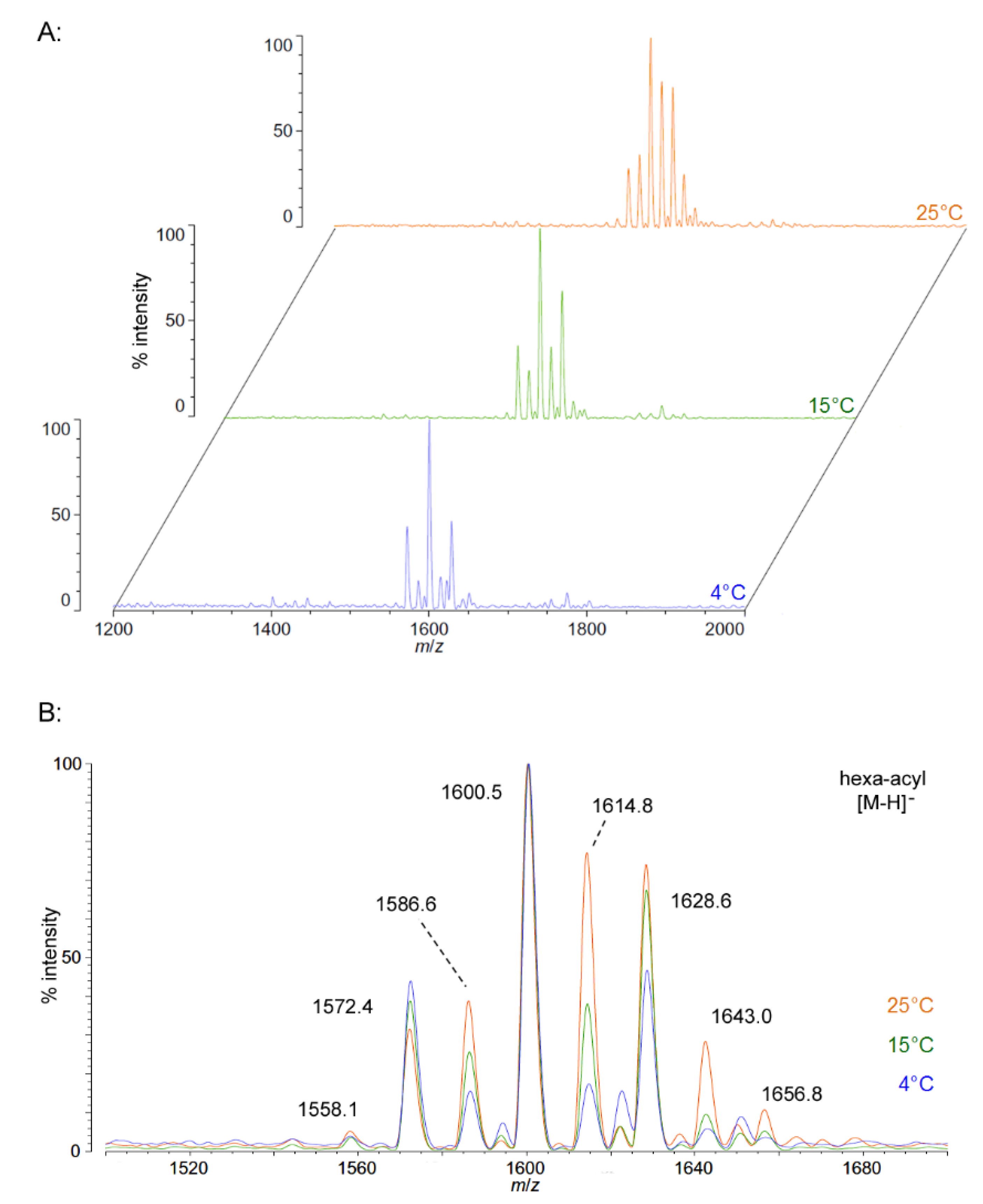
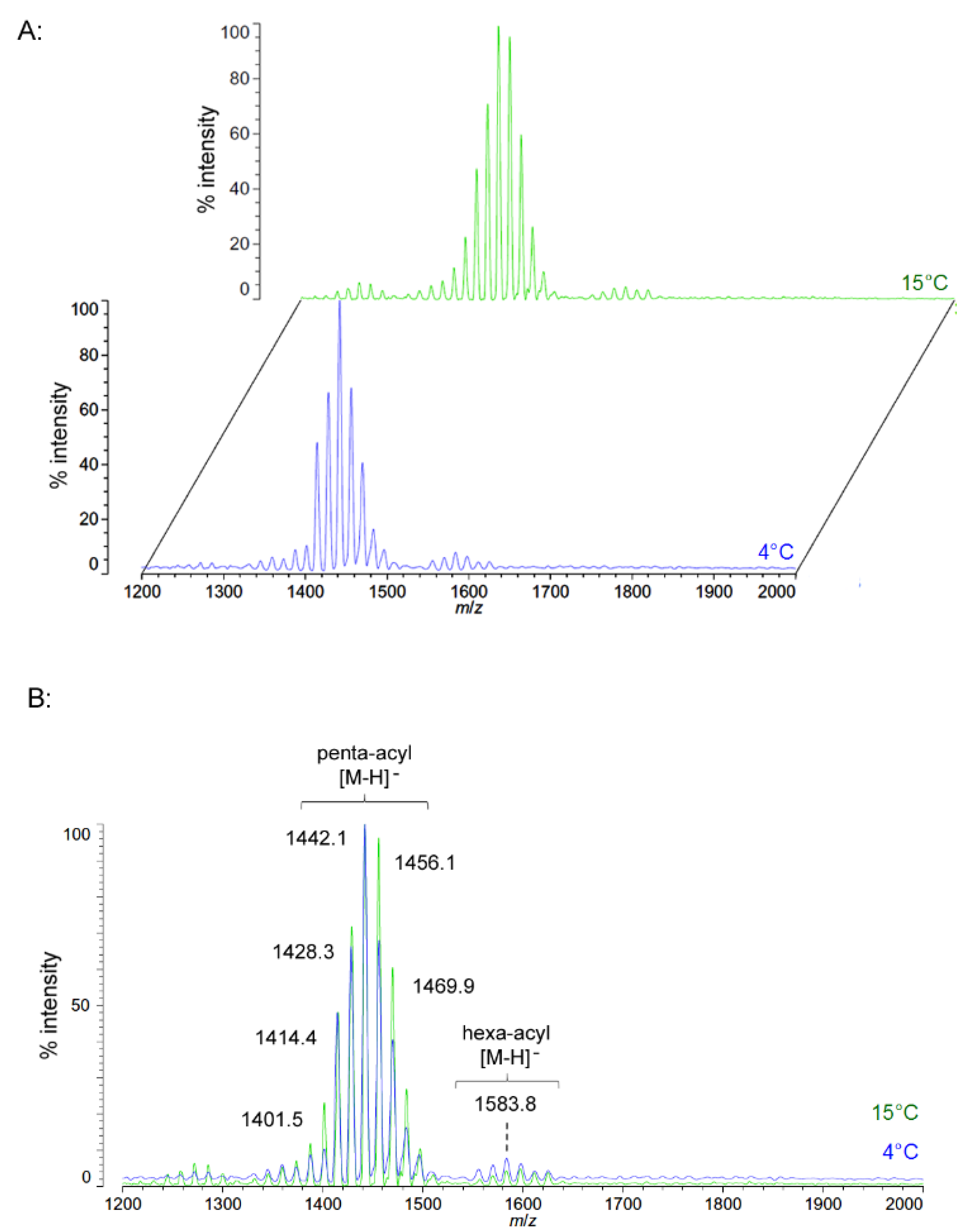
2.1. Structural Characterization of P. cryohalolentis Lipid A at 4 °C, 15 °C and 25 °C
We isolated
P. cryohalolentis lipid A from cultures grown at 4 °C, 15 °C or 25 °C by the modified Bligh-Dyer method [
23] and characterized the structure of this lipid by MALDI-TOF MS and FAME GC-MS.
P. cryohalolentis produces a surprisingly diverse set of lipid A acyl forms at 25 °C, as has been previously reported [
17]. Negative ion MALDI-TOF MS of this lipid A reveals at least six significant acyl variants differing by single methylenes (CH
2) in the dominant penta-acyl set of structures, centered on the most abundant [M − H]
− peak at an
m/
z ratio of 1600.5 (
Figure 1), corresponding to the known molecular mass of the predominant
P. cryohalolentis lipid A of 1601.99 Da (
Table 1). The well-characterized lipid A from
E. coli W3110 was used as a control and a standard for all procedures in this work, and structural examples of the negative and positive ions characteristic of lipid A in MALDI-TOF MS, as well as a summary of lipid A nomenclature can be found in the
E. coli control spectra (
Figure S1).
As growth temperature decreases,
P. cryohalolentis lipid A exhibits consistent alterations in its suite of acyl length variants. Negative ion MALDI-TOF MS at 15 °C shows a significantly lower proportion of the forms containing odd-length acyl chains (the peaks at
m/
z 1586.6 and 1614.8), less of the peak that is +2(CH
2) larger than average (
m/
z 1628.6), more of the peak −2(CH
2) smaller than average (
m/
z 1572.4) and almost complete loss of the peaks +3(CH
2) and +4(CH
2) larger than average (
m/
z 1643.0 and 1656.8), while the minor peak −3(CH
2) lesser than average (
m/
z 1558.1) is not reduced with temperature. Negative ion MALDI-TOF at 4 °C reveals that these shifts show a trend in proportion with temperature; the changes described above at 15 °C are continued and extended in the structure of lipid A from organisms grown at 4 °C, and comparison of the acyl distribution patterns of the highest and lowest temperatures indicates a significant shift to smaller acyl forms as the growth temperature drops. As an illustration of this,
Figure 1B shows that the +1(CH
2) and +2(CH
2) flanking peaks, which are nearly as large as the main peak at 25 °C, are reduced markedly at 4 °C to the point that they are equal to the amounts of the −1(CH
2) and −2(CH
2) peaks) and also shows the nearly complete loss of odd-length acyl chain incorporation into lipid A at cold temperatures.
Table 1.
Summary of MALDI-TOF MS observations and predicted masses of ions.
Table 1.
Summary of MALDI-TOF MS observations and predicted masses of ions.
| Molecule | [M − H]− Predicted Mass (Da) | [M − H]− Observed m/z | B1+ Predicted Mass (Da) | B1+ Observed m/z | B2+ Predicted Mass (Da) | B2+ Observed m/z | [M + Na]+ Predicted Mass (Da) | [M + Na]+ Observed m/z |
|---|
| E. coli hexa-acyl | 1797.36 | 1797.5 | 1087.51 | 1087.6 | 1701.38 | 1701.1 | 1821.35 | 1821.57 |
| P. cryohalolentis −(CH2)3 | 1558.91 | 1558.1 | 905.17 | -- | 1462.93 | -- | 1582.90 | 1582.4 |
| −(CH2)2 | 1572.93 | 1572.4 | 919.19 | 919.1 | 1476.95 | 1476.4 | 1596.93 | 1596.6 |
| −CH2 | 1586.96 | 1586.6 | 933.22 | 933.3 | 1490.98 | 1490.5 | 1610.96 | 1610.8 |
| hexa-acyl | 1600.98 | 1600.5 | 947.25 | 947.2 | 1505.01 | 1504.5 | 1624.98 | 1624.7 |
| +CH2 | 1615.01 | 1614.8 | 961.27 | 961.4 | 1519.03 | 1518.4 | 1639.01 | 1639.5 |
| +(CH2)2 | 1629.04 | 1628.6 | 975.30 | 975.3 | 1533.06 | 1532.6 | 1653.04 | 1652.9 |
| +(CH2)3 | 1643.06 | 1643.0 | 989.33 | -- | 1547.09 | -- | 1667.06 | 1667.7 |
| C. piezophila −(CH2)3 | 1428.76 | 1429.5 | 777.00 | 777.7 | 1332.78 | 1334.3 | 1452.76 | 1454.8 |
| −(CH2)2 | 1442.79 | 1443.0 | 791.02 | 791.7 | 1346.81 | 1348.1 | 1466.79 | 1468.4 |
| −CH2 | 1456.82 | 1456.3 | 805.05 | 805.7 | 1360.84 | 1361.5 | 1480.81 | 1481.4 |
| penta-acyl | 1470.84 | 1470.0 | 819.08 | 819.7 | 1374.86 | 1374.6 | 1494.84 | 1495.1 |
| +CH2 | 1484.87 | 1483.9 | 833.10 | 833.3 | 1388.89 | 1388.4 | 1508.87 | 1509.1 |
| +(CH2)2 | 1498.90 | 1497.5 | 847.13 | 847.0 | 1402.92 | 1402.2 | 1522.89 | 1522.9 |
| +(CH2)3 | 1512.92 | 1511.1 | 861.15 | -- | 1416.94 | * | 1536.92 | 1536.9 |
| C. hornerae−(CH2)3 | 1400.71 | 1401.5 | 748.94 | -- | 1304.73 | 1306.4 | 1424.71 | 1426.7 |
| −(CH2)2 | 1414.74 | 1414.4 | 762.97 | 763.3 | 1318.76 | 1319.3 | 1438.73 | 1439.7 |
| −CH2 | 1428.76 | 1428.3 | 777.00 | 777.4 | 1332.78 | 1333.2 | 1452.76 | 1453.4 |
| penta-acyl | 1442.79 | 1442.1 | 791.02 | 791.0 | 1346.81 | 1346.2 | 1466.79 | 1467.0 |
| +CH2 | 1456.82 | 1456.1 | 805.05 | 805.5 | 1360.84 | * | 1480.81 | 1481.3 |
| +(CH2)2 | 1470.84 | 1469.9 | 819.08 | 816.3 | 1374.86 | * | 1494.84 | 14495.5 |
| +(CH2)3 | 1484.87 | 1483.8 | 833.10 | -- | 1388.89 | * | 1508.87 | 1509.7 |
Figure 1.
Negative ion MALDI-TOF MS of
P. cryohalolentis lipid A at 4 °C, 15 °C and 25 °C. Lipid A was prepared as described in the Materials and Methods. Spectral data were collected by Shimadzu Axima Confidence MS with a power setting of 75–80 and pulsed extraction at 2000 Da as the average of at least 1000 profiles in the linear mode, displayed in 3D offset comparison by temperature (
A) or as a stack of the main peak region (
B). Peak interpretation is shown on the spectra and in
Table 1.
Figure 1.
Negative ion MALDI-TOF MS of
P. cryohalolentis lipid A at 4 °C, 15 °C and 25 °C. Lipid A was prepared as described in the Materials and Methods. Spectral data were collected by Shimadzu Axima Confidence MS with a power setting of 75–80 and pulsed extraction at 2000 Da as the average of at least 1000 profiles in the linear mode, displayed in 3D offset comparison by temperature (
A) or as a stack of the main peak region (
B). Peak interpretation is shown on the spectra and in
Table 1.
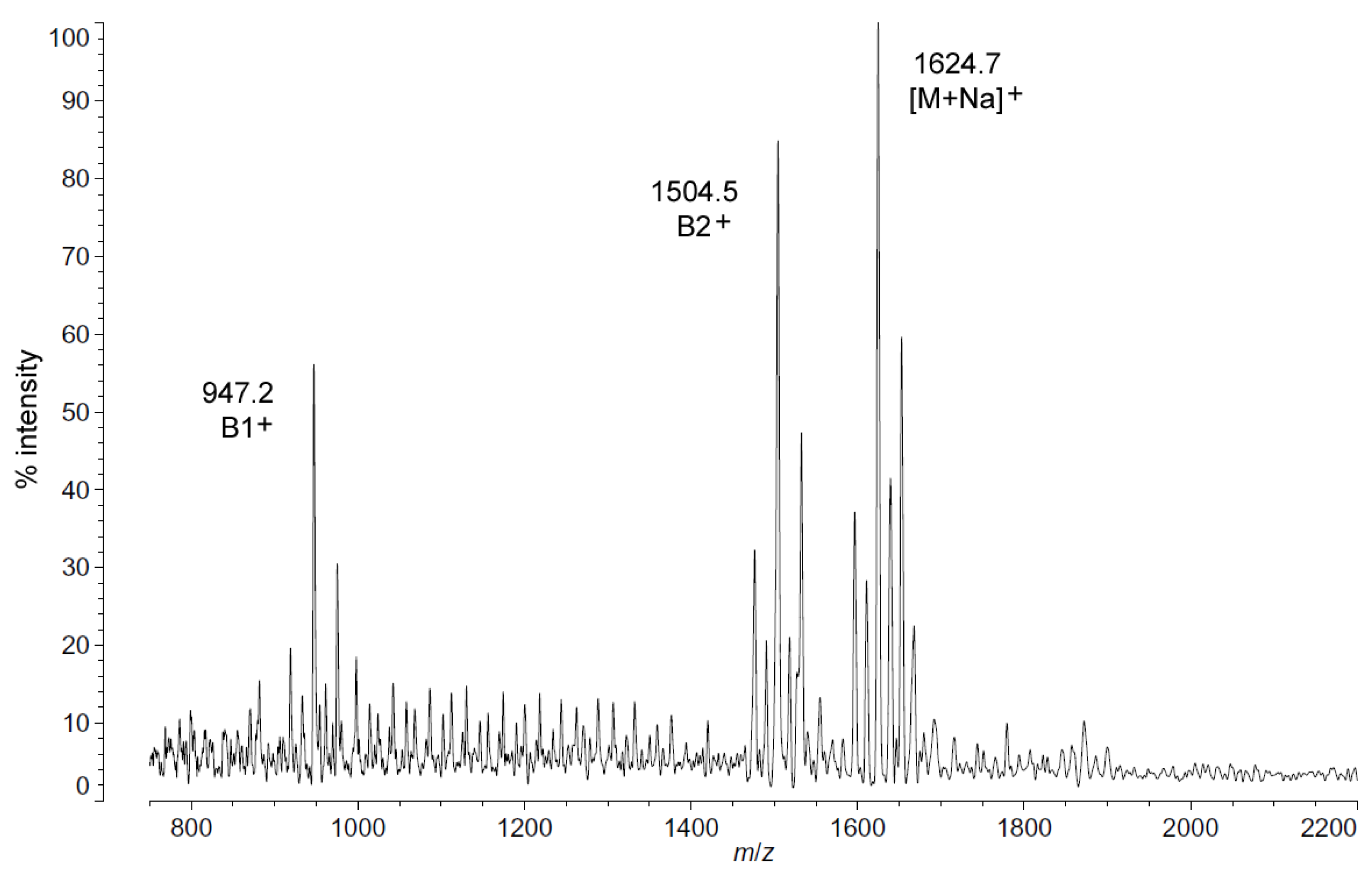
Positive ion MALDI-TOF MS (
Figure 2) also shows this shift in pattern at low temperatures in the structure of the characteristic B1
+, B2
+ and [M + Na]
+ ions. These fragments and adducts are centered on
m/
z of 947.2, 1504.5 and 1624.7, respectively. They correspond to the predominant molecular mass of 1601.99 Da (
Table 1) and aid in establishing the acyl distribution of
P. cryohalolentis, as has been previously shown [
17]. The altered acyl pattern shown in
Figure 1 as characteristic of cold-grown
P. cryohalolentis is clear as well in the B1
+ and B2
+ ions of
Figure 2, though it is a bit obscured in the [M + Na]
+ cluster due to the presence of [M + K]
+ adducts (the difference of ~16 Da between sodium and potassium overlaps with that of the ~14 Da difference between acyl variants).
Figure S2 shows the positive ion spectrum at 25 °C for comparison, in which the large amounts of the +1(CH
2) and +2(CH
2) flanking peaks characteristic of room-temperature
P. cryohalolentis growth are evident.
FAME GC-MS results are consistent with the acyl structural changes in
P. cryohalolentis at the cold temperatures that are described above. At 25 °C, the lipid A-derived FAMEs seen in
P. cryohalolentis include odd-chain 3-OH undecanoyl methyl ester, 3-OH tridecanoyl methyl ester, tridecanoyl methyl ester and pentadecanoyl methyl ester, as well as even-chain FAMEs (
Table 2), while at 4 °C, only the pentadecanoyl methyl ester and the even-chain acyl units remain.
Figure 2.
Positive ion MALDI-TOF mass spectra of
P. cryohalolentis lipid A at 4 °C. Lipid A was prepared as described in the Materials and Methods. Spectral data were collected by Shimadzu Axima Confidence MS with a power setting of 80, pulsed extraction and 1500 profiles in the linear mode. Peak interpretation is shown on the spectra and in
Table 1.
Figure 2.
Positive ion MALDI-TOF mass spectra of
P. cryohalolentis lipid A at 4 °C. Lipid A was prepared as described in the Materials and Methods. Spectral data were collected by Shimadzu Axima Confidence MS with a power setting of 80, pulsed extraction and 1500 profiles in the linear mode. Peak interpretation is shown on the spectra and in
Table 1.
2.2. Structural Characterization of C. piezophila Lipid A at 4 °C and 15 °C
Unlike
P. cryohalolentis lipid A, the obligate psychrophile
C. piezophila does not show any alteration of lipid A acyl size or distribution with respect to temperature. Negative ion MALDI-TOF MS of
C. piezophila lipid A at both 4 °C and its upper growth limit of 15 °C show essentially identical spectra. However, this species’ lipid A is unusual in that it has an even greater variety of single-methylene acyl length variant forms than
P. cryohalolentis. The most abundant negative ion [M − H]
− peak at
m/
z 1470.1 is flanked by many other significant forms, all differing by ~14 mu, indicative of single methylene differences in chain length of the acyl units (
Figure 3 and
Table 1). There are also less abundant groups of single-methylene variants clustered around main peaks with alternative numbers of acyl chains, including a tetra-acyl cluster centered on
m/
z 1285.8 and a hexa-acyl one centered on
m/
z 1611.7. The mass gained (+Δ141.7 mu) or lost (−Δ184.3 mu) between the most abundant peak of the main cluster (a predicted molecular mass of 1471.85 Da) and those of these lesser clusters is useful in determining the details of the chemical structure of this lipid A, as described in the Discussion Section and shown in
Figure 4.
Table 2.
FAME GC-MS observations and structural interpretations.
Table 2.
FAME GC-MS observations and structural interpretations.
| Acyl Residue | Standard Retention Time | E. coli | P. cryohalolentis | C. piezophila | C. hornerae | |
|---|
| at 4 °C | at 25 °C |
|---|
| Lipid A-derived 3-hydroxylated fatty acids: | | | | | | |
| 3-hydroxydecanoate (3-OH C10:0) | 3.7 * | -- | -- | -- | 3.680 | 3.680 |
| 3-hydroxyundecanoate (3-OH C11:0) | 4.2 * | -- | -- | 4.220 | 4.220 | 4.250 |
| 3-hydroxydodecanoate (3-OH C12:0) | 4.717 | -- | 4.727 | 4.727 | 4.727 | 4.727 |
| 3-hydroxytridecanoate (3-OH C13:0) | 5.2 * | -- | -- | 5.187 | 5.187 | 5.191 |
| 3-hydroxytetradecanoate (3-OH C14:0) | 5.600 | 5.600 | -- | -- | -- | -- |
| Lipid A-derived non-hydroxylated fatty acids: | | | | | | |
| decanoate (C10:0) | 2.887 | -- | -- | 2.907 | 2.912 | -- |
| dodecanoate (C12:0) | 3.979 | 3.973 | 3.987 | 3.987 | 3.980 | -- |
| tridecanoate (C13:0) | 4.493 | -- | -- | 4.500 | -- | -- |
| tetradecanoate (C14:0) | 4.971 | 4.967 | 4.967 | 4.973 | 4.970 | 4.973 |
| pentadecanoate (C15:0) | 5.400 | -- | 5.400 | 5.400 | 5.400 | 5.400 |
| Phospholipid-derived fatty acids: | | | | | | |
| hexadecanoate (C16:0) | 5.793 | 5.787 | 5.793 | 5.793 | 5.793 | 5.793 |
| hexadecenoate (C16:1) | 5.727 | -- | 5.720 | 5.720 | 5.722 | 5.720 |
| heptadecanoate (C17.0) | 6.153 | -- | -- | 6.153 | 6.152 | 6.153 |
| heptadecenoate (C17.1) | 6.1 * | -- | -- | 6.080 | -- | -- |
| octadecanoate (C18:0) | 6.493 | 6.487 | 6.493 | 6.493 | 6.493 | 6.493 |
| octadecenoate (C18:1) | 6.413 | 6.413 | 6.420 | 6.420 | 6.420 | 6.420 |
| octadecadienoate (C18:2) | 6.365 | -- | 6.360 | 6.360 | 6.360 | 6.360 |
Figure 3.
Negative ion MALDI-TOF mass spectra of
C. piezophila lipid A at 4 °C and 15 °C. Lipid A was prepared as described in the Materials and Methods. Spectral data were collected by Shimadzu Axima Confidence MS with a power setting of 75 and pulsed extraction at 2000 Da as the average of at least 1000 profiles in the linear mode in offset comparison by temperature (
A) and as a stack of the main peak region (
B). Peak interpretation is shown on the spectra and in
Table 1.
Figure 3.
Negative ion MALDI-TOF mass spectra of
C. piezophila lipid A at 4 °C and 15 °C. Lipid A was prepared as described in the Materials and Methods. Spectral data were collected by Shimadzu Axima Confidence MS with a power setting of 75 and pulsed extraction at 2000 Da as the average of at least 1000 profiles in the linear mode in offset comparison by temperature (
A) and as a stack of the main peak region (
B). Peak interpretation is shown on the spectra and in
Table 1.
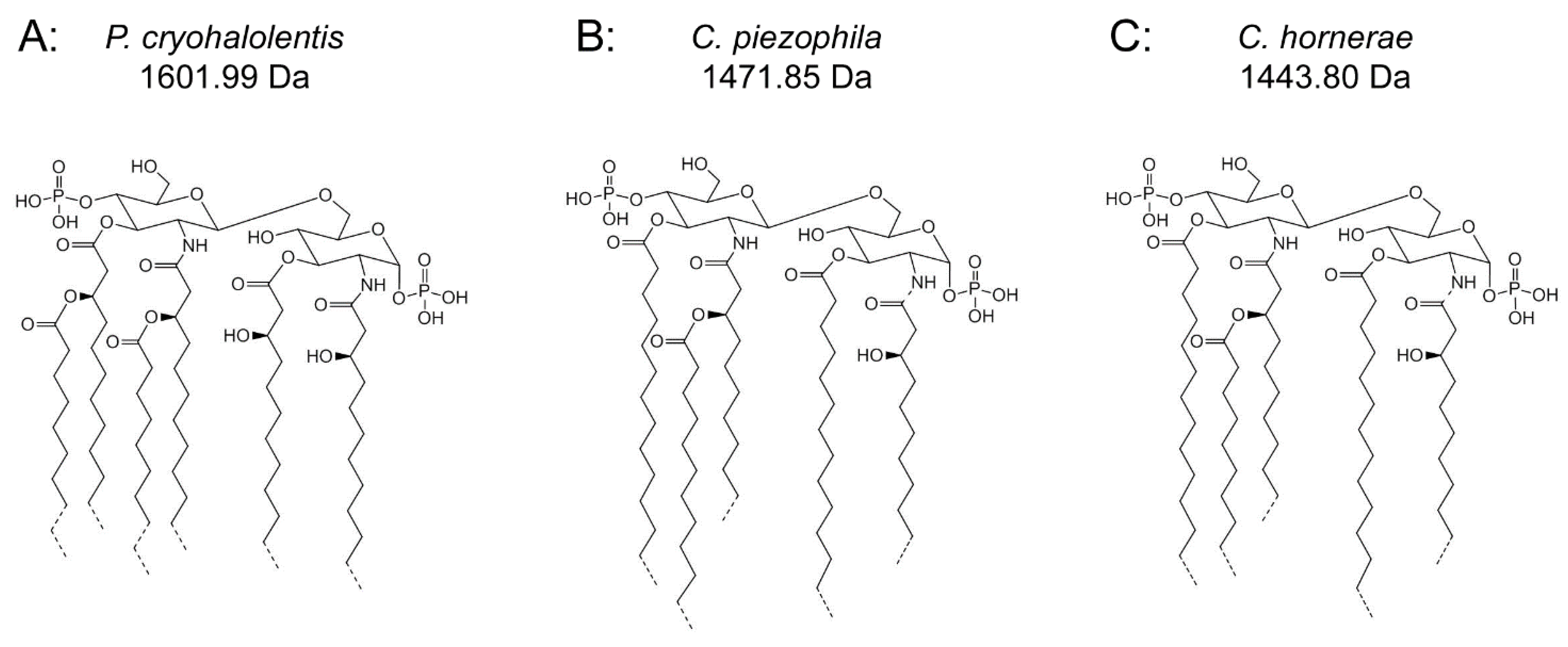
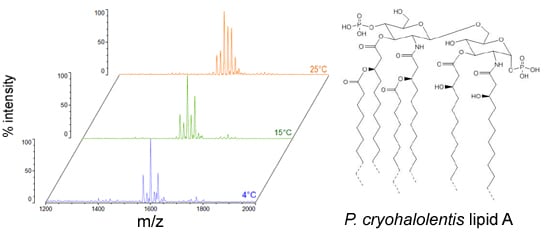
Figure 4.
Proposed psychrophilic lipid A structures. These represent the most likely average structures of the most abundant molecular forms of lipid A in P. cryohalolentis (A), C. piezophila (B) and C. hornerae (C) based on the summation of all structural data. All of these organisms show significant variability in acyl chain length; hashed bonds indicate non-stoichiometric substituents that give rise to the major molecular forms.
Figure 4.
Proposed psychrophilic lipid A structures. These represent the most likely average structures of the most abundant molecular forms of lipid A in P. cryohalolentis (A), C. piezophila (B) and C. hornerae (C) based on the summation of all structural data. All of these organisms show significant variability in acyl chain length; hashed bonds indicate non-stoichiometric substituents that give rise to the major molecular forms.
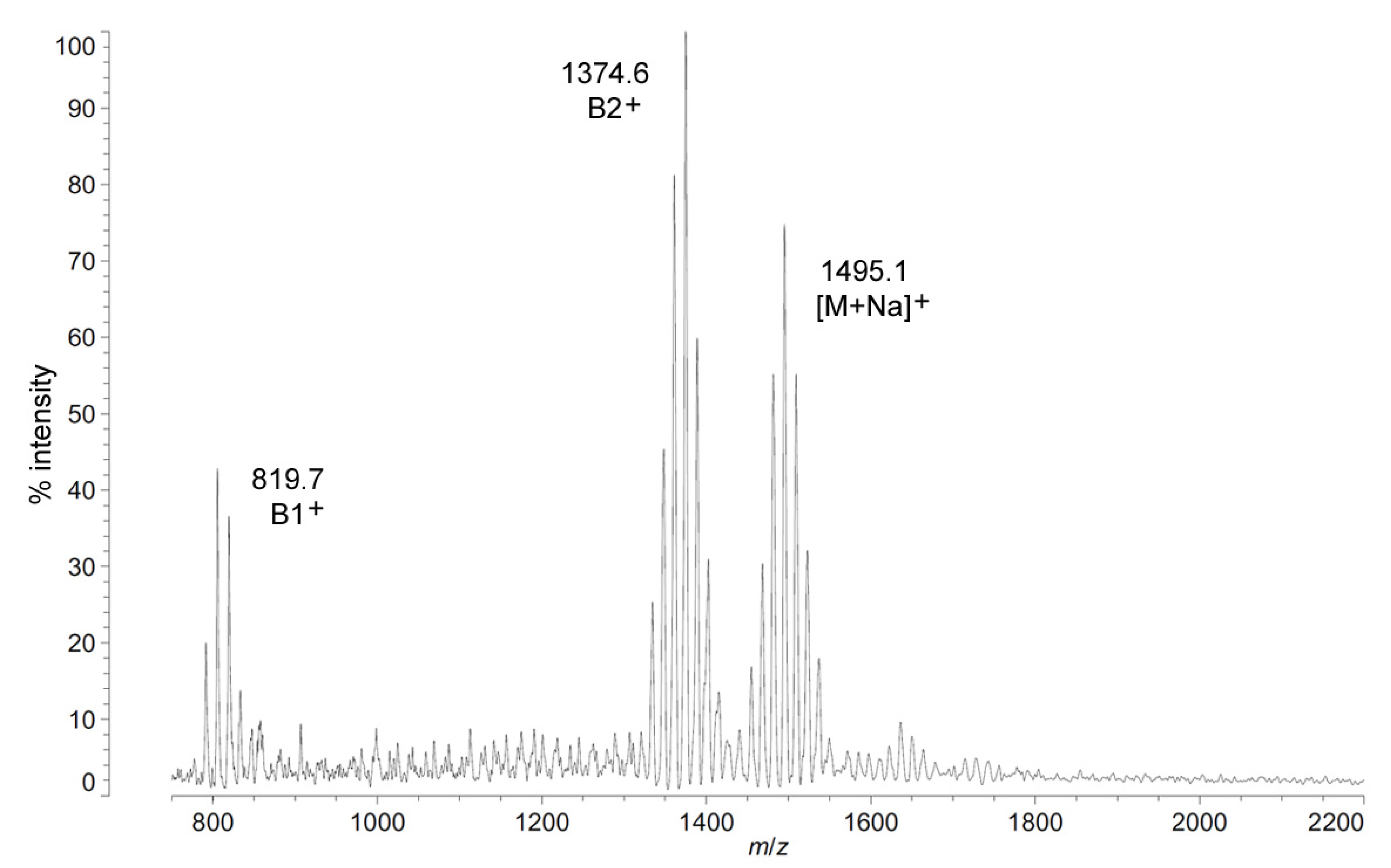
The positive ion MALDI-TOF MS of
C. piezophila lipid A shows the same clustering pattern as the negative ion mode (
Figure 5 and
Table 1). As with the distances between tetra-, penta- and hexa-acyl variants in the negative ion mode, the differences between the B1
+ and B2
+ ions of
C. piezophila inform about the locations and sizes of the acyl units. The B1
+ ion cluster of this spectrum shows at least four single-methylene variants in acyl length, centered on a most abundant peak of
m/
z 819.7. This is 554.9 mu smaller than the most abundant ion in the B2
+ ion cluster, at
m/
z 1374.6. This mass difference indicates that the glucosamine I fragment lost to generate the B1
+ ion is a diacylglucosamine, and the remaining glucosamine II fragment must therefore be triacylated. The third cluster in the spectrum, centered on
m/
z 1495.1, represents the sodium adduct [M + Na]
+ of the dominant penta-acyl molecular structure. B2
+ and [M + Na]
+ clusters are also present for the hexa-acyl form of
C. piezophila lipid A; however, if the B1
+ ion is distinct from that of the penta-acyl species (
i.e., if the sixth fatty acid is on the non-reducing glucosamine II end of the structure), it cannot be observed due to the high background of the lower region of the positive ion spectrum. Similarly, no positive ions can be discerned that would correspond to the tetra-acyl structure seen in the negative mode.
FAME GC-MS analysis of
C. piezophila lipid A determined the acyl composition of this lipid A and displays some differences from the other bacterial species in this study that are critical to understanding the proposed structure shown in
Figure 4B. Most distinctively, the set of hydroxylated FAMEs seen in
C. piezophila are shorter even than those seen in
P. cryohalolentis. While
P. cryohalolentis has only a small amount of 3-OH undecanoyl methyl ester, this length is the most abundant hydroxy-acyl chain in
C. piezophila, along with lesser amounts of 3-OH decanoyl methyl ester and 3-OH dodecanoyl methyl ester and a trace of 3-OH tridecanoyl methyl ester.
Tetradecanoyl methyl ester dominates the lipid A-derived non-hydroxy acyl units, with lesser amounts of pentadecanoyl methyl ester, dodecanoyl methyl ester (but no tridecanoyl methyl ester) and decanoyl methyl ester observed, as well. Combined with the MALDI-TOF data, we therefore propose a plausible average molecular structure in
Figure 4B with 3-OH undecanoyl and tetradecanoyl primary acyl chains and a dodecanoyl acyl-oxyacyl (secondary) unit.
Figure 5.
Positive ion MALDI-TOF mass spectra of
C. piezophila lipid A at 4 °C. Lipid A was prepared as described in the Materials and Methods. Spectral data were collected by Shimadzu Axima Confidence MS with a power setting of 75, pulsed extraction, and 2000 profiles in the linear mode. Peak interpretation is shown on the spectra and in
Table 1.
Figure 5.
Positive ion MALDI-TOF mass spectra of
C. piezophila lipid A at 4 °C. Lipid A was prepared as described in the Materials and Methods. Spectral data were collected by Shimadzu Axima Confidence MS with a power setting of 75, pulsed extraction, and 2000 profiles in the linear mode. Peak interpretation is shown on the spectra and in
Table 1.
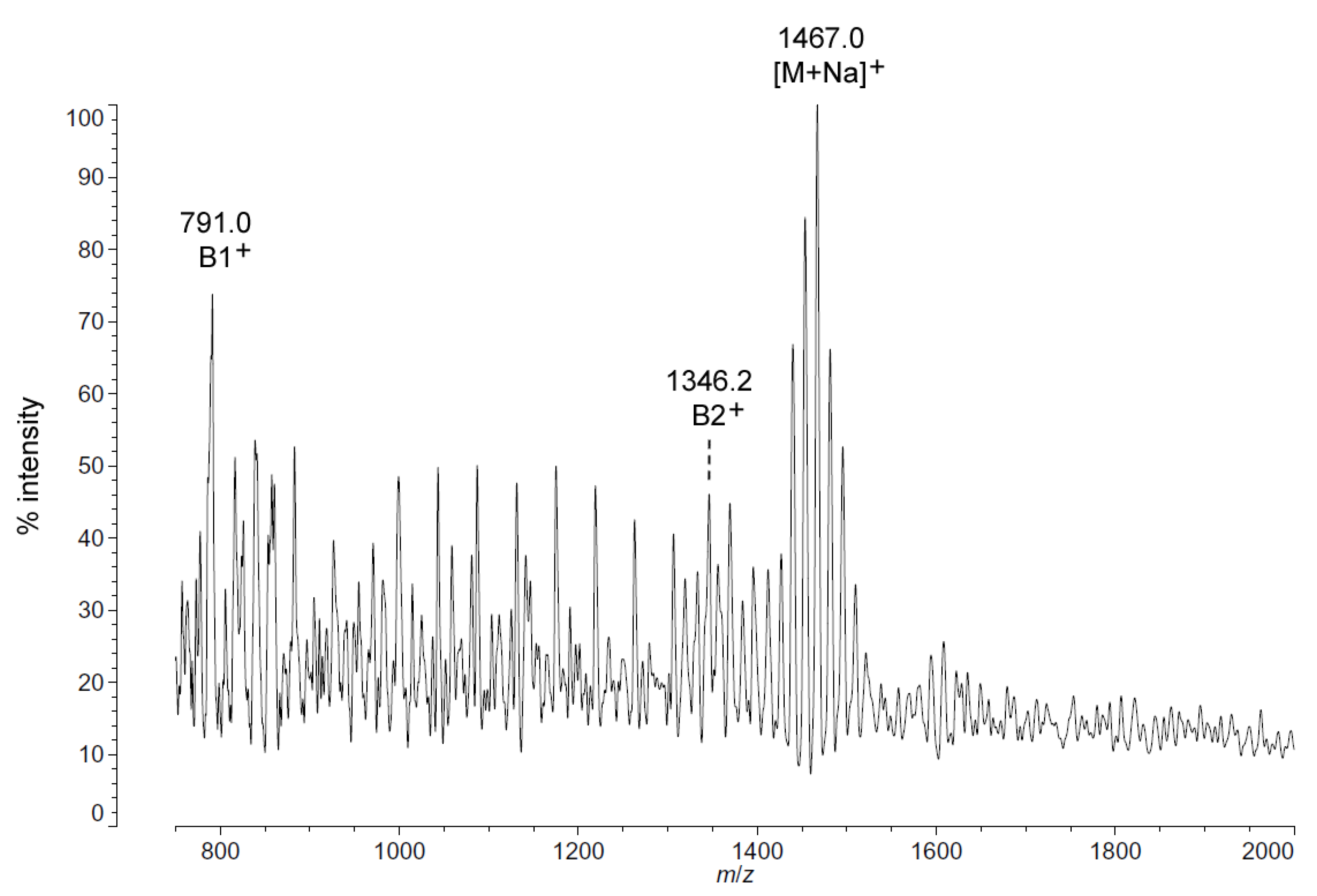
2.3. Structural Characterization of C. hornerae Lipid A at 4 °C and 15 °C
Negative ion MALDI-TOF spectra of lipid A from another obligate psychrophile,
C. hornerae, also shows a very similar overall pattern and general range of acyl length variants to that of
C. piezophila (
Figure 6). There is a slight difference in that the most abundant [M − H]
− ion of
C. hornerae, at
m/
z 1441.9, is ~28 mu smaller than that of
C. piezophila and corresponds instead to a predicted molecular mass of 1442.79 Da. However, the general range of the
C. hornerae variants seen in
Figure 6 and the acyl content assessed by FAME GC-MS (
Table 2) are similar to that of
C. piezophila. This suggests that the difference in average size of lipid A between these species is due to minor alterations in enzymatic specificity for greater use of shorter acyl units, rather than a significant shift in the acyl content.
As with
C. piezophila, the positive ion MALDI-TOF spectrum of lipid A from
C. hornerae confirms the proposed molecular masses with those of the B2
+ and [M + Na]
+ clusters, centered on
m/
z of 1346.2 and 1467.0, respectively (
Figure 7), and informs about the acyl distribution of the molecule through the mass difference of 555.2 mu between the B1
+ and B2
+ ion clusters (loss of a diacylglucosamine fragment). Furthermore, as with
C. piezophila, the relative abundance of 3-OH undecanoyl methyl ester and tetradecanoyl methyl ester in the FAME GC-MS of
C. hornerae suggests that these are the most abundant acyl units in the molecule on average (
Figure 4C), with greater or lesser acyl units in combination to account for all of the acyl length variants seen in
Figure 6.
Figure 6.
Negative ion MALDI-TOF mass spectra of
C. hornerae lipid A at 4 °C and 15 °C. Lipid A was prepared as described in the Materials and Methods. Spectral data were collected by Shimadzu Axima Confidence MS with a power setting of 75–80 and pulsed extraction at 2000 Da as the average of at least 1000 profiles in the linear mode in offset comparison by temperature (
A) and as a stack of the main peak region (
B). Peak interpretation is shown on the spectra and in
Table 1.
Figure 6.
Negative ion MALDI-TOF mass spectra of
C. hornerae lipid A at 4 °C and 15 °C. Lipid A was prepared as described in the Materials and Methods. Spectral data were collected by Shimadzu Axima Confidence MS with a power setting of 75–80 and pulsed extraction at 2000 Da as the average of at least 1000 profiles in the linear mode in offset comparison by temperature (
A) and as a stack of the main peak region (
B). Peak interpretation is shown on the spectra and in
Table 1.
Figure 7.
Positive ion MALDI-TOF mass spectra of
C. hornerae lipid A at 4 °C. Lipid A was prepared as described in the Materials and Methods. Spectral data were collected by Shimadzu Axima Confidence MS with a power setting of 75, pulsed extraction, and 2000 profiles in the linear mode. Peak interpretation is shown on the spectra and in
Table 1.
Figure 7.
Positive ion MALDI-TOF mass spectra of
C. hornerae lipid A at 4 °C. Lipid A was prepared as described in the Materials and Methods. Spectral data were collected by Shimadzu Axima Confidence MS with a power setting of 75, pulsed extraction, and 2000 profiles in the linear mode. Peak interpretation is shown on the spectra and in
Table 1.
2.5. Discussion
We find that the facultative (Psychrotolerant) species
P. cryohalolentis synthesizes lipid A with a dominant hexa-acyl cluster with at least six significant lipid A structures varying from each other by single methylene (CH
2) units at 25 °C and minor clusters of the penta-acyl and hepta-acyl forms (
Figure 1; and also [
17]). These variations are generated by use of a wide range of acyl chain lengths, including C11:0–C13:0 3-OH acyl units in primary linkage and C10:0–C15:0 in secondary linkage. The structural form represented in
Figure 4A shows the most likely acyl lengths, on average, which would generate the most abundant structure (1601.99 Da), though a diversity of discrete acylation forms is likely to populate each of the observed molecular weights. In contrast to the obligate psychrophiles characterized in this work,
P. cryohalolentis lipid A displays modulation of the acyl elements of lipid A structure with varying growth temperature. Presumably, these changes are the result of a combination of temperature-dependent changes in the activity or transcription of enzymes synthesizing fatty acids and/or lipid A, as has been noted in the fluidity adaptation of other bacterial species in response to temperature changes [
13,
14].
The
P. cryohalolentis K5 genome (
http://www.ncbi.nlm.nih.gov) encodes one homolog of the lipid A acyltransferases LpxD, LpxL and LpxM, while containing two homologs of LpxA, suggesting that the 3 and 3′ primary acyl positions of the molecule might be particularly subject to variability in acyl usage as temperature changes. This is analogous to the switch from LpxL to LpxP activity in
E. coli at low temperature, which is responsible for replacing dodecanoyl (C12:0) acyl units with hexadecenoyl (C16:1) ones during cold shock [
10,
14]. These changes to overall lipid A structure with descending temperature, while subtle enough that 1601.99 Da is still the predominant molecular weight at all temperatures, have distinct and significant effects on many of the flanking acyl variants of
P. cryohalolentis lipid A (
Figure 1). The first of these effects is the near-elimination of odd-length acyl chains in lipid A and in phospholipids at cold temperature (seen in both the absence of single-methylene flankers of the most abundant peak in
Figure 1 and
Figure 2 and also in the absence of most of the odd-chain residues from the FAME GC-MS results in
Table 2), and the second is a significant shift toward a shorter overall average of the acyl units at cold temperature, which demonstrates a structural trend in fluidity adaptation of psychrophilic lipid A with temperature. While the metabolic benefit of changes to the odd-chain population of acyl units in
P. cryohalolentis with temperature is unclear, the appearance of this effect in the acyl residues of both lipid A and phospholipids suggests that this is happening at the level of fatty acid synthesis. The trend in overall shortening of the lipid A acyl units seen in the MALDI-TOF data is, however, a homeoviscous adaptation of the lipid A acyl moiety in response to the challenge to membrane fluidity posed by cold growth temperatures, similar to well-characterized shifts in the acyl fluidity of membrane phospholipids in psychrophiles and other organisms [
8,
9,
24]. While it is tempting to speculate that the existence of two LpxA homologs in
P. cryohalolentis implies a molecular mechanism for the strong change in odd-chain acyl incorporation with temperature, the substrate specificities of these homologs remains to be investigated and will be an interesting aspect of future work in psychrophilic lipid A. It will also be fruitful to see if ultra-cold growth of these psychrophiles (at −5 to −10 °C) alters
P. cryohalolentis further or alters
Colwellia or
P. marina lipid A. However, growth at such temperatures requires specialized equipment beyond the capacity of the current investigation.
Figure 4B,C demonstrates the most likely average acyl length and distribution of lipid A from
C. piezophila and
C. hornerae, two organisms cultured from the sea ice-water interface of Arctic fast ice [
25]. Both of these organisms are obligate psychrophiles (they cannot grow above 15 °C). In addition, the
C. piezophila strain BRX10.3 described here is remarkable in that while it is psychrophilic, it grows at nominal atmospheric pressure and up to 15 °C, both of which are traits that distinguish it from the type strain of the
C. piezophila species, which is an obligate barophile [
22]. Both of these
Colwellia strains demonstrate an astonishing diversity of lipid A acyl forms, with a main penta-acyl cluster of at least eight significant forms (and perhaps as many as fourteen, including minor ones), in addition to lesser clusters of at least five tetra-acyl and five hexa-acyl variants (
Figure 3). In
C. piezophila, the most abundant molecular structure is a penta-acyl variant with a mass of 1471.85 Da. Unexpectedly, this mass (and its characteristic positive ion fragments seen in
Figure 5) can only be reasonably explained in light of known lipid A biosynthetic pathways by inclusion of normal (non-hydroxylated) fatty acids in primary linkage to the carbohydrates, a variation previously only known to occur in the presence of tetradecanoyl residues at the 3 and 3′ position of
Chlamydia trachomatis lipid A [
26,
27], due to the unusual acyl specificity of
C. trachomatis LpxA [
23]. FAME GC-MS shows that tetradecanoyl residues are also the most abundant non-hydroxylated acyl unit in
C. piezophila lipid A; therefore, this chain length is shown in primary linkage in the proposed structure in
Figure 4B. In this structure, the non-hydroxylated residues are placed at the 3 and 3′ positions in light of the known positioning of this acyl unit in
C. trachomatis. Alternative structural explanations of
C. piezophila lipid A that would place the non-hydroxylated acyl units solely in their typical acyl-oxyacyl positions can be ruled out by the fact that a mass of 1471.85 Da requires two 3-OH-acyl units and three non-hydroxylated acyl units, a biosynthetic impossibility if none of the primary linkages are non-hydroxylated chains. Furthermore, the dominant B1
+ ion mass of
m/
z 820.1 (
Figure 5 and
Table 1) shows that two non-hydroxylated units (and one hydroxy-acyl unit) are present on the non-reducing (glucosamine II) end of the molecule.
The minor hexa-acyl components of
C. piezophila lipid A represent the addition of another non-hydroxylated acyl unit with an average gain in total acyl length of nine carbons for the most abundant peak seen at
m/
z 1611.7 (
Figure 3). However, it is unlikely that this is the result of the simple addition of a nonanoyl unit to the penta-acyl structure. This hexa-acyl cluster likely involves some biasing of the size of other acyl residues toward shorter lengths, particularly substitution of tetradecanoyl residues for dodecanoyl and/or tridecanoyl ones, which, unlike nonanoyl residues, are present in the FAME GC-MS analysis of
C. piezophila (
Table 2). To complete the most likely average structure corresponding to the most abundant molecular form at 1470.84 Da shown in
Figure 4B, a fifth acyl unit of dodecanoyl length is placed in acyl-oxyacyl linkage on glucosamine II, consistent with the spectral evidence described above. In light of the presence of an LpxL/P homolog rather than an LpxM homolog in the
C. piezophila genome (
http://www.ncbi.nlm.nih.gov), this secondary acyl residue is placed at the
N-linked (2′) position of the structure. The final variation in
C. piezophila lipid A structure, the loss of an acyl unit to yield the minor tetra-acyl cluster seen at
m/
z of 1285.8, is explained by the removal of one of the hydroxy-acyl units in primary linkage. On average, the mass difference is that of a 3-OH undecanoyl residue, which is the most abundant hydroxy-acyl residue seen in the
C. piezophila FAME GC-MS analysis (
Table 2). This alteration is likely to be the result of a lipid A deacylase similar in activity to PagL or LpxO from other Gram-negative species [
28,
29]; however, no homolog of the
O-acyl specific PagL or LpxO deacylases is present in the
C. piezophila genome. This suggests that the hydroxy-acyl unit removed in this process is on the amide-linked position of the reducing sugar (indicated in
Figure 4 with a hashed bond rather than a solid one) and that the ability to synthesize the tetra-acyl structure of
Colwellia is due to an uncharacterized 2-
N-acyl lipid A deacylase.
Analysis of
C. hornerae lipid A (
Figure 4C) is identical in almost all respects to that of
C. piezophila, with the exception that the average size of
C. hornerae is ~28 mu (two methylene units) smaller than the corresponding structures in
C. piezophila, suggesting a shift in the substrate specificity of one or more of the
C. hornerae acyltransferases in comparison to those of
C. piezophila(see
Figure 5 and
Figure 6). Comparison of
C. piezophila and
C. hornerae to related mesophilic gamma-proteobacteria, such as
E. coli (
Figure S1) or
Vibrio species [
30,
31], shows that the
Colwellia lipid A contains generally shorter acyl units, much like
P. cryohalolentis in comparison to its close relative,
Acinetobacter baumannii [
17].
These comparisons suggest that in the case of obligate psychrophiles, evolutionary adaptation to cold environments has constitutively altered the structure of lipid A in these organisms to use more fluid acyl chains than their mesophilic relatives, rather than relying on metabolic responses to tune the fluidity of the outer membrane to temperature. Facultative psychrotolerant organisms, such as P. cryohalolentis, conversely, require greater capability to respond to and thrive in a wider range of temperatures. The ability of this species to metabolically alter the type and length of its lipid A acyl units is likely to be an aspect of this greater adaptability, increasing the functional temperature range of the outer membrane in these organisms. Thus, psychrotolerant species, if not the obligate psychrophiles, join the ranks of those organisms capable of metabolic changes to the fluidity of lipid A acyl moiety of the outer membrane.