Enhanced Control of Bladder-Associated Tumors Using Shrimp Anti-Lipopolysaccharide Factor (SALF) Antimicrobial Peptide as a Cancer Vaccine Adjuvant in Mice
Abstract
:1. Introduction
2. Results
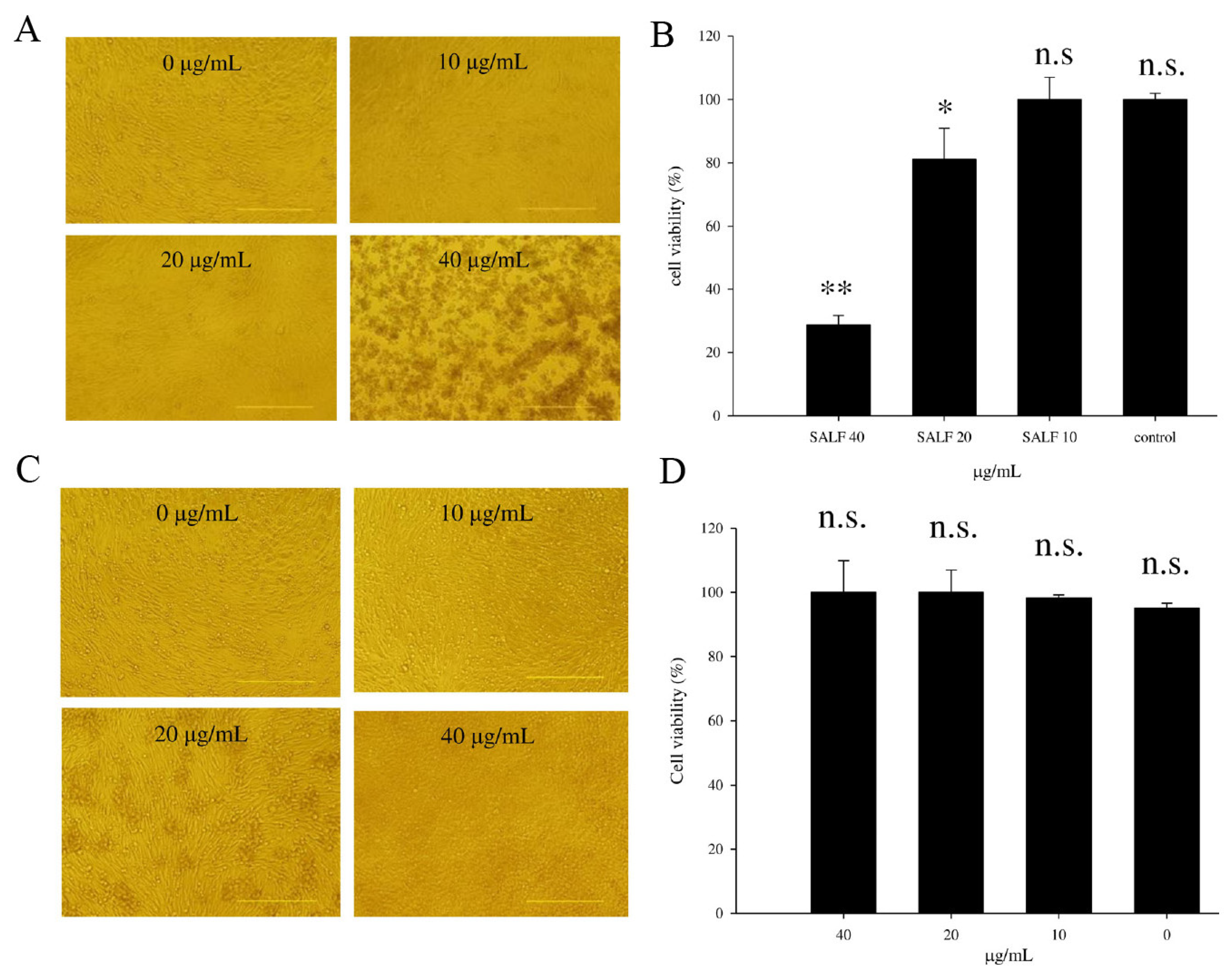
2.1. SALF Inhibits Murine Bladder Tumor (MBT)-2 Cells
2.2. Co-Treatment with SALF and Inactivated MBT-2 Lysate Promoted Innate IL-1β Production in Mouse Macrophages
2.3. SALF in Conjunction with Inactivated MBT-2 Lysate Modulates Tumor-Associated Chemokines and Cytokines


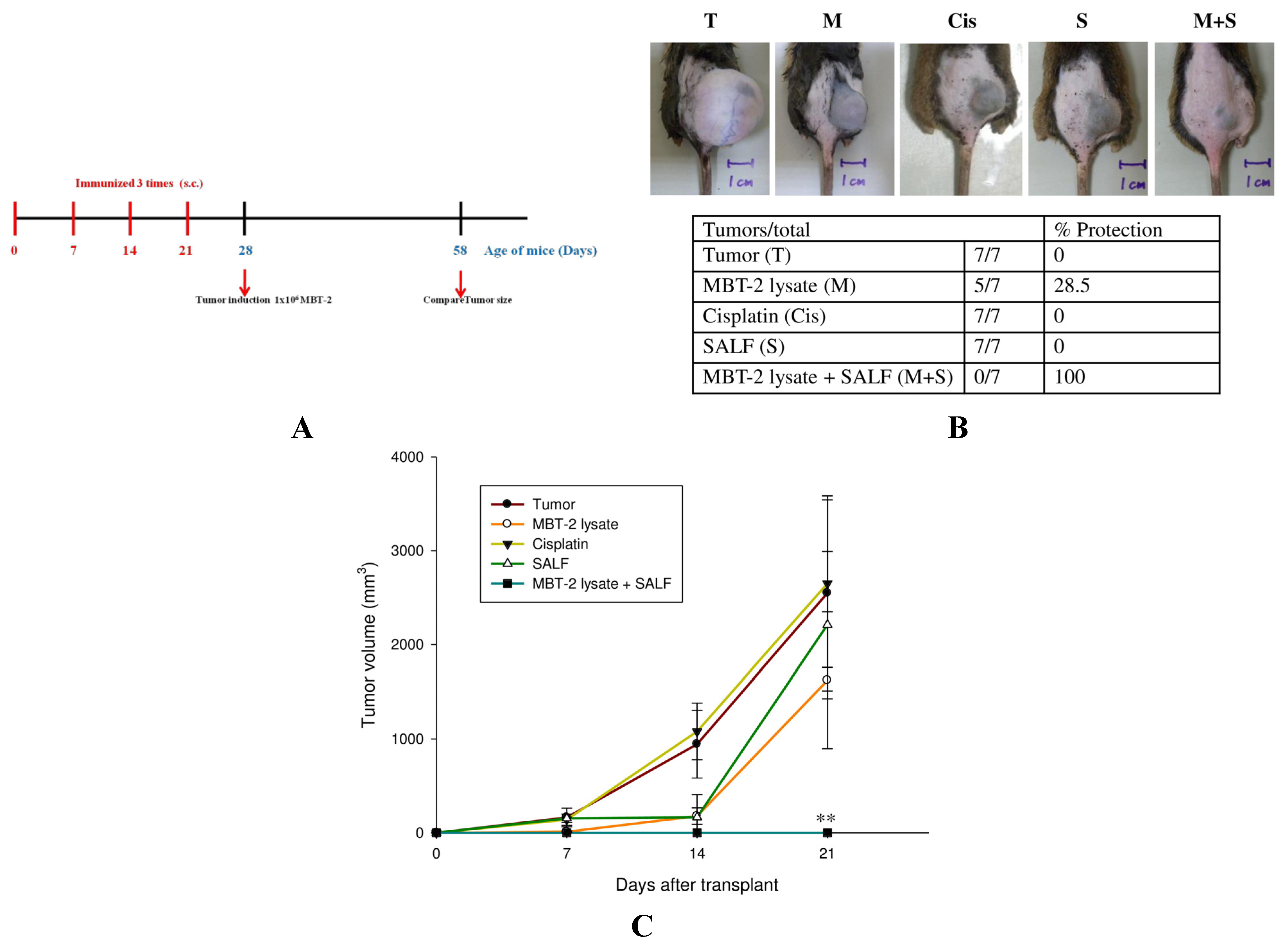
2.4. Tumor Formation Is Attenuated by Immunization with SALF and Inactivated MBT-2 Lysate
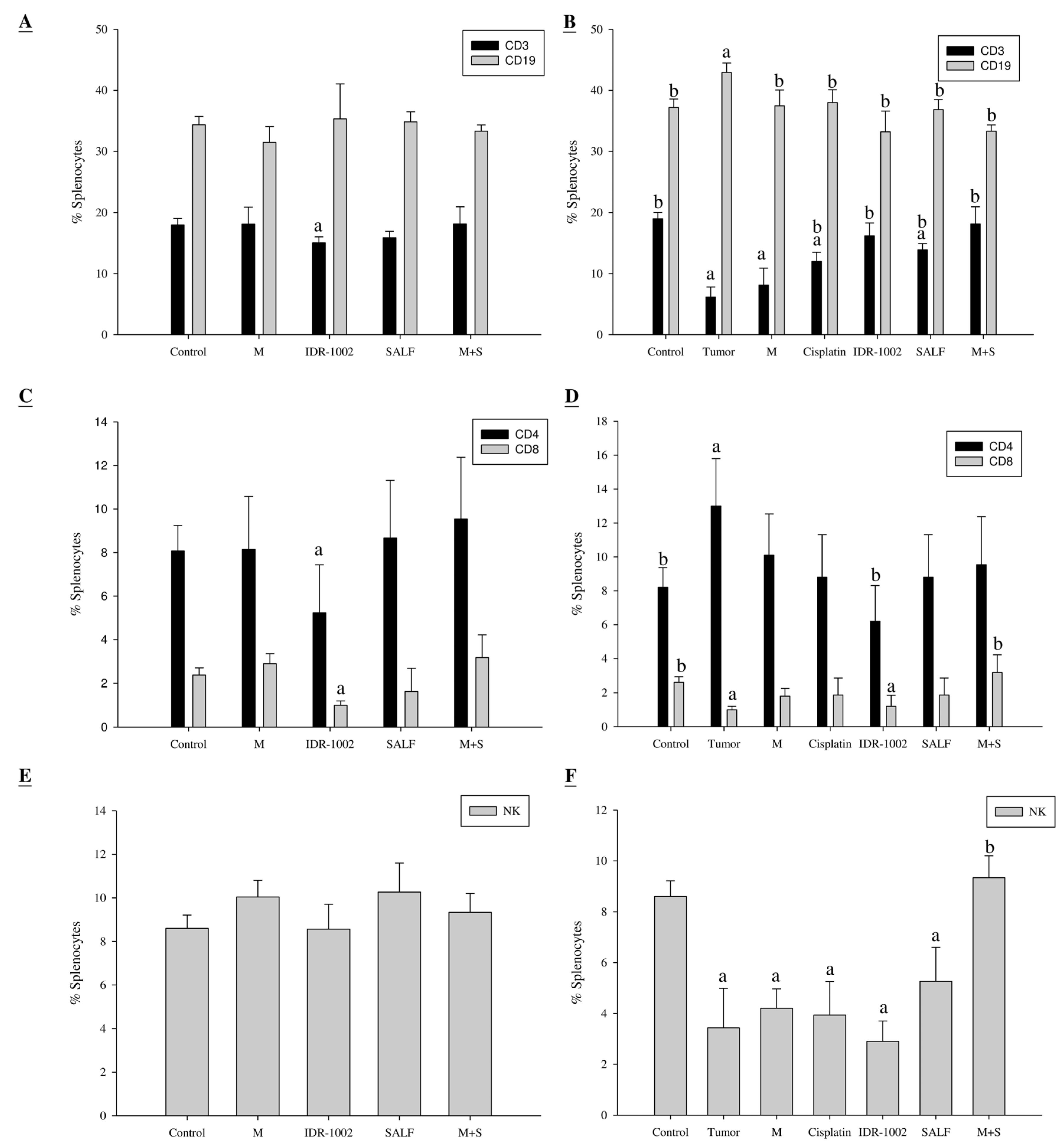
2.5. Spleen Cell Activation is Modulated by Immunization with SALF and Inactivated MBT-2 Lysate
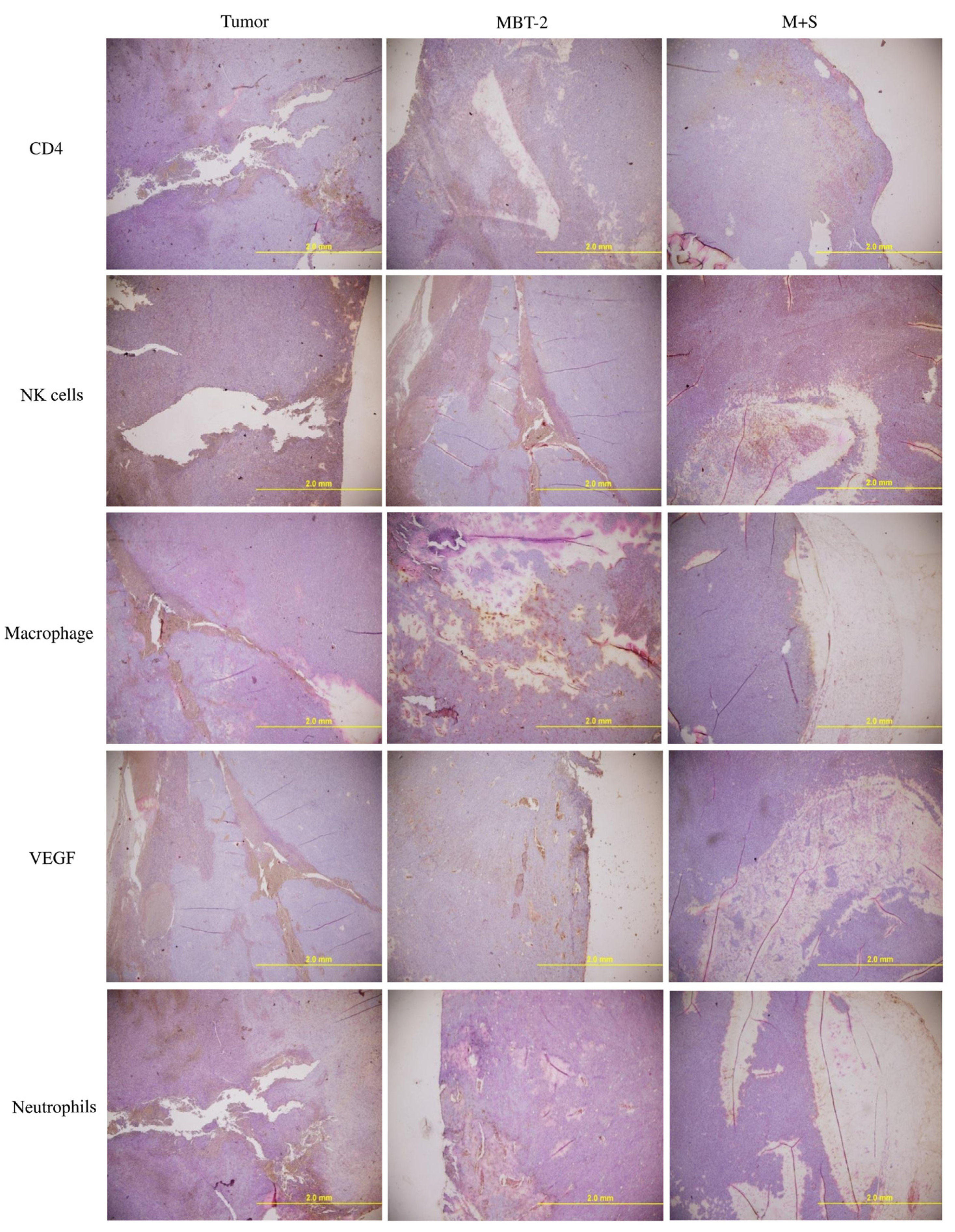
2.6. Immunization with SALF and Inactivated MBT-2 Lysate Enhanced Infiltration of Immune Cells into the Tumor Site
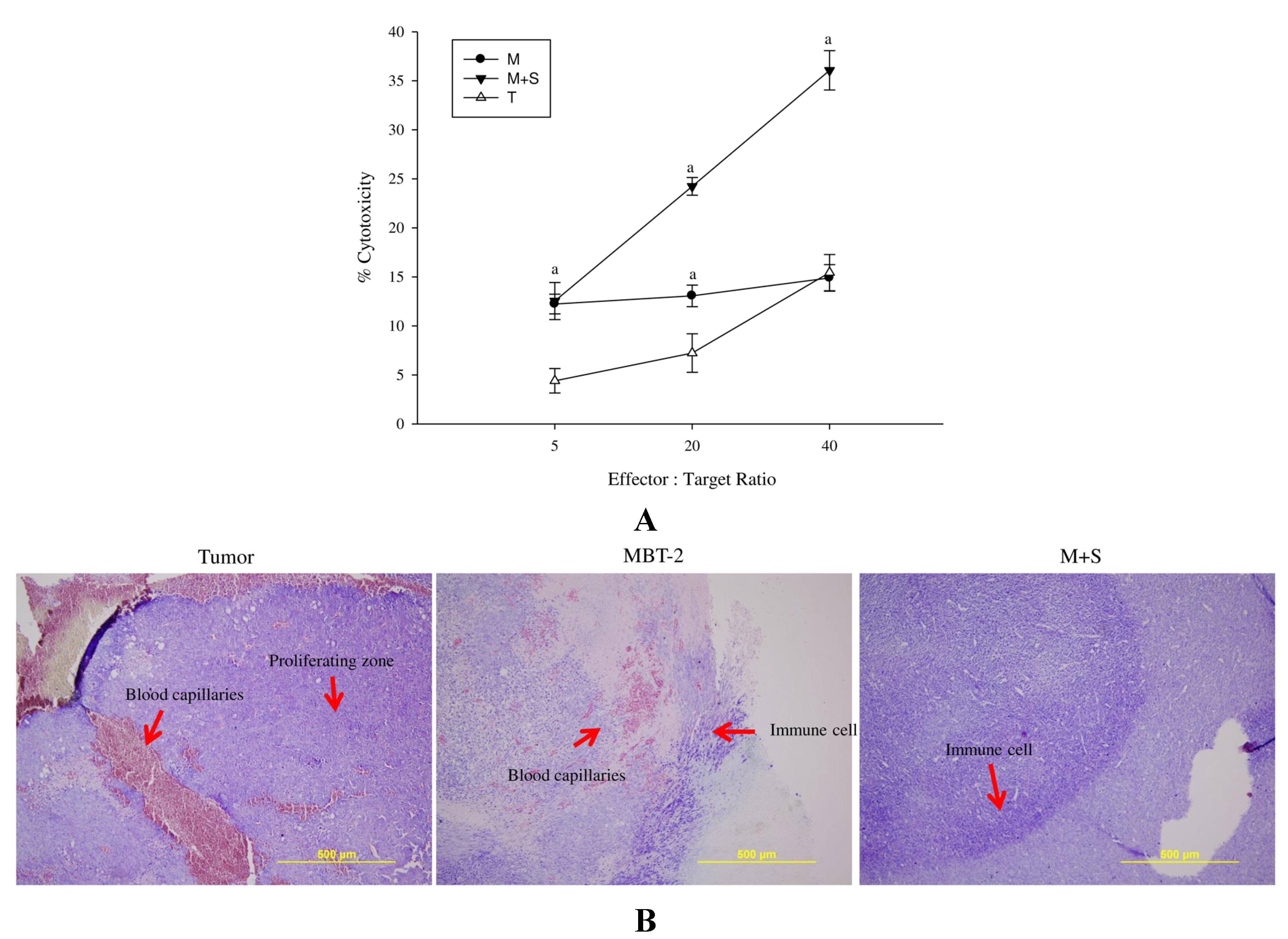
2.7. Immunization with SALF and Inactivated MBT-2 Lysate Activates Tumor-Associated Immune Cells
3. Discussion
4. Experimental Section
4.1. Cells and Mice
4.2. Peptides, Chemicals, and Reagents
4.3. Cell Proliferation
4.4. Preparation of Tumor Vaccines and Immunization
4.5. CTL Assay
4.6. Tumor Growth Analysis
4.7. Immunohistochemistry
4.8. SALF-Induced Cytokine Production in Vitro
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aketagawa, J.; Miyata, T.; Ohtsubo, S.; Nakamura, T.; Morita, T.; Hayashida, H.; Miyata, T.; Iwanaga, S.; Takao, T.; Shimonishi, Y. Primary structure of limulus anticoagulant anti lipopolysaccharide factor. J. Biol. Chem. 1986, 261, 7357–7365. [Google Scholar] [PubMed]
- Supungul, P.; Klinbunga, S.; Pichyangkura, R.; Jitrapakdee, S.; Hirono, I.; Aoki, T.; Tassanakajon, A. Identification of immune-related genes in hemocytes of black tiger shrimp (Penaeus monodon). Mar. Biotechnol. 2002, 4, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Somboonwiwat, K.; Marcos, M.; Tassanakajon, A.; Klinbunga, S.; Aumelas, A.; Romestand, B.; Gueguen, Y.; Boze, H.; Moulin, G.; Bachère, E. Recombinant expression and anti-microbial activity of anti-lipopolysaccharide factor (ALF) from the black tiger shrimp Penaeus monodon. Dev. Comp. Immunol. 2005, 29, 841–851. [Google Scholar] [CrossRef] [PubMed]
- De la Vega, E.; O’Leary, N.A.; Shockey, J.E.; Robalino, J.; Payne, C.; Browdy, C.L.; Warr, G.W.; Gross, P.S. Anti-lipopolysaccharide factor in Litopenaeus vannamei (LvALF): A broad spectrum antimicrobial peptide essential for shrimp immunity against bacterial and fungal infection. Mol. Immunol. 2008, 45, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Boze, H.; Chemardin, P.; Padilla, A.; Moulin, G.; Tassanakajon, A.; Pugnière, M.; Roquet, F.; Destoumieux-Garzón, D.; Gueguen, Y.; et al. NMR structure of rALF-Pm3, an anti-lipopolysaccharide factor from shrimp: Model of the possible lipid A-binding site. Biopolymers 2009, 91, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Tharntada, S.; Ponprateep, S.; Somboonwiwat, K.; Liu, H.; Söderhäll, I.; Söderhäll, K.; Tassanakajon, A. Role of anti-lipopolysaccharide factor from the black tiger shrimp, Penaeus monodon, in protection from white spot syndrome virus infection. J. Gen. Virol. 2009, 90, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.J.; Wu, Y.C.; Chen, J.Y.; Chi, S.C. Antimicrobial peptides (AMP) with antiviral activity against fish nodavirus. Fish Shellfish. Immunol. 2010, 28, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Prapavorarat, A.; Pongsomboon, S.; Tassanakajon, A. Identification of genes expressed in response to yellow head virus infection in the black tiger shrimp, Penaeus monodon, by suppression subtractive hybridization. Dev. Comp. Immunol. 2010, 34, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y.; Chao, T.T.; Chen, J.C.; Chen, J.Y.; Liu, W.C.; Lin, C.H.; Kuo, C.M. Shrimp (Penaeus monodon) anti-lipopolysaccharide factor reduces the lethality of Pseudomonas aeruginosa sepsis in mice. Int. Immunopharmacol. 2007, 7, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Lin, S.B.; Chen, J.C.; Hui, C.F.; Chen, J.Y. Shrimp anti-lipopolysaccharide factor peptide enhances the antitumor activity of cisplatin in vitro and inhibits HeLa cells growth in nude mice. Peptides 2010, 31, 1019–1025. [Google Scholar] [CrossRef]
- Caffo, O.; Veccia, A.; Fellin, G.; Russo, L.; Mussari, S.; Galligioni, E. Trimodality treatment in the conservative management of infiltrating bladder cancer: A critical review of the literature. Crit. Rev. Oncol. Hematol. 2013, 86, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Sadick, M.; Schoenberg, S.O.; Hoermann, K.; Sadick, H. Current oncologic concepts and emerging techniques for imaging of head and neck squamous cell cancer. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2012, 11. [Google Scholar] [CrossRef]
- Thundimadathil, J. Cancer treatment using peptides: Current therapies and future prospects. J. Amino Acids 2012, 2012, 967347. [Google Scholar] [CrossRef] [PubMed]
- Aina, O.H.; Sroka, T.C.; Chen, M.L.; Lam, K.S. Therapeutic cancer targeting peptides. Biopolymers 2002, 66, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Brayer, J.B.; Pinilla-Ibarz, J. Developing strategies in the immunotherapy of leukemias. Cancer Control. 2013, 20, 49–59. [Google Scholar] [PubMed]
- Iida, N.; Nakamoto, Y.; Baba, T.; Kakinoki, K.; Li, Y.Y.; Wu, Y.; Matsushima, K.; Kaneko, S.; Mukaida, N. Tumor cell apoptosis induces tumor-specific immunity in a CC chemokine receptor 1- and 5-dependent manner in mice. J. Leukoc. Biol. 2008, 84, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.L.; Foreman, O.; Bosio, C.M.; Anver, M.R.; Elkins, K.L. Interleukin-6 is essential for primary resistance to Francisella tularensis live vaccine strain infection. Infect. Immun. 2013, 81, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Miller, M.; Stojanovic, A.; Garbi, N.; Cerwenka, A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J. Exp. Med. 2012, 209, 2351–2365. [Google Scholar] [CrossRef] [PubMed]
- Bellmann-Weiler, R.; Schroll, A.; Engl, S.; Nairz, M.; Talasz, H.; Seifert, M.; Weiss, G. Neutrophil gelatinase-associated lipocalin and interleukin-10 regulate intramacrophage Chlamydia pneumoniae replication by modulating intracellular iron homeostasis. Immunobiology 2012, 218, 969–978. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vendramini-Costa, D.B.; Carvalho, J.E. Molecular link mechanisms between inflammation and cancer. Curr. Pharm. Des. 2012, 18, 3831–3852. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.D.; Houghton, A.M. Tumor-associated neutrophils: New targets for cancer therapy. Cancer Res. 2011, 71, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Wilke, C.M.; Kryczek, I.; Chen, G.Y.; Kao, J.; Núñez, G.; Zou, W. Interleukin-10 ablation promotes tumor development, growth, and metastasis. Cancer Res. 2012, 72, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Disis, M.L. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 2005, 54, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Fruh, K.; Yang, Y. Antigen presentation by MHC class I and its regulation by interferon gamma. Curr. Opin. Immunol. 1999, 11, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.G.; Khammanivong, V.; Liu, W.J.; Leggatt, G.R.; Frazer, I.H.; Fernando, G.J. Antigen-specific CD4+ T-cell help is required to activate a memory CD8+ T cell to a fully functional tumor killer cell. Cancer Res. 2002, 62, 6438–6441. [Google Scholar] [PubMed]
- Terme, M.; Fridman, W.H.; Tartour, E. NK cells from pleural effusions are potent antitumor effector cells. Eur. J. Immunol. 2013, 43, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Kipriyanov, S.M.; Cochlovius, B.; Schäfer, H.J.; Moldenhauer, G.; Bähre, A.; le Gall, F.; Knackmuss, S.; Little, M. Synergistic antitumor effect of bispecific CD19 × CD3 and CD19 × CD16 diabodies in a preclinical model of non-Hodgkin’s lymphoma. J. Immunol. 2002, 169, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, Z.; Tian, Z.; Xie, Y.; Xin, F.; Jiang, R.; Kan, H.; Song, W. The biological effects of individual-level PM2.5 exposure on systemic immunity and inflammatory response in traffic policemen. Occup. Environ. Med. 2013, 70, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Vacchelli, E.; Martins, I.; Eggermont, A.; Fridman, W.H.; Galon, J.; Sautès-Fridman, C.; Tartour, E.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: Peptide vaccines in cancer therapy. Oncoimmunology 2012, 1, 1557–1576. [Google Scholar] [CrossRef] [PubMed]
- Buonaguro, L.; Petrizzo, A.; Tornesello, M.L.; Buonaguro, F.M. Translating tumor antigens into cancer vaccines. Clin. Vaccine Immunol. 2011, 18, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Lin, S.B.; Lee, S.C.; Lin, C.C.; Hui, C.F.; Chen, J.Y. Antimicrobial peptide of an anti-lipopolysaccharide factor modulates of the inflammatory response in RAW264.7 cells. Peptides 2010, 31, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Hui, C.F.; Chen, J.Y.; Wu, J.L. The antimicrobial peptide, shrimp anti-lipopolysaccharide factor (SALF), inhibits proinflammatory cytokine expressions through the MAPK and NF-kappaB pathways in Trichomonas vaginalis adherent to HeLa cells. Peptides 2012, 38, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Pan, C.Y.; Hui, C.F.; Chen, J.Y.; Wu, J.L. Shrimp anti-lipopolysaccharide factor (SALF), an antimicrobial peptide, inhibits proinflammatory cytokine expressions through the MAPK and NF-kappaB pathways in LPS-induced HeLa cells. Peptides 2013, 40, 42–48. [Google Scholar] [CrossRef]
- Soloway, M.S. Intravesical and systemic chemotherapy of murine bladder cancer. Cancer Res. 1977, 37, 2918–2929. [Google Scholar] [PubMed]
- Rajanbabu, V.; Pan, C.Y.; Lee, S.C.; Lin, W.J.; Lin, C.C.; Li, C.L.; Chen, J.Y. Tilapia hepcidin 2–3 peptide modulates lipopolysaccharide-induced cytokines and inhibits tumor necrosis factor-alpha through cyclooxygenase-2 and phosphodiesterase 4D. J. Biol. Chem. 2010, 285, 30577–30586. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.Q.; Yu, Y.L.; Shen, Z.J.; Zhou, X.L.; Chen, S.W.; Liao, G.D.; Zhang, Y. Antitumor effects of human interferon-alpha 2b secreted by recombinant Bacillus Calmette-Guerin vaccine on bladder cancer cells. J. Zhejiang Univ. Sci. B 2012, 13, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhou, X.M. A model of orthotopic murine bladder (MBT-2) tumor implants. Urol. Res. 1997, 25, 179–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, S.A.; Tsai, M.H.; Wu, F.T.; Hsiang, A.; Chen, Y.L.; Lei, H.Y.; Tzai, T.S.; Leung, H.W.; Jin, Y.T.; Hsieh, C.L.; et al. Induction of antitumor immunity with combination of HER2/neu DNA vaccine and interleukin 2 gene-modified tumor vaccine. Clin. Cancer Res. 2000, 6, 4381–4388. [Google Scholar] [PubMed]
- Hikosaka, S.; Hara, I.; Miyake, H.; Hara, S.; Kamidono, S. Antitumor effect of simultaneous transfer of interleukin-12 and interleukin-18 genes and its mechanism in a mouse bladder cancer model. Int. J. Urol. 2004, 11, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Chou, B.; Hiromatsu, K.; Okano, S.; Ishii, K.; Duan, X.; Sakai, T.; Murata, S.; Tanaka, K.; Himeno, K. Antiangiogenic tumor therapy by DNA vaccine inducing aquaporin-1-specific CTL based on ubiquitin-proteasome system in mice. J. Immunol. 2012, 189, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-N.; Rajanbabu, V.; Pan, C.-Y.; Chan, Y.-L.; Chen, J.-Y.; Wu, C.-J. Enhanced Control of Bladder-Associated Tumors Using Shrimp Anti-Lipopolysaccharide Factor (SALF) Antimicrobial Peptide as a Cancer Vaccine Adjuvant in Mice. Mar. Drugs 2015, 13, 3241-3258. https://doi.org/10.3390/md13053241
Huang H-N, Rajanbabu V, Pan C-Y, Chan Y-L, Chen J-Y, Wu C-J. Enhanced Control of Bladder-Associated Tumors Using Shrimp Anti-Lipopolysaccharide Factor (SALF) Antimicrobial Peptide as a Cancer Vaccine Adjuvant in Mice. Marine Drugs. 2015; 13(5):3241-3258. https://doi.org/10.3390/md13053241
Chicago/Turabian StyleHuang, Han-Ning, Venugopal Rajanbabu, Chieh-Yu Pan, Yi-Lin Chan, Jyh-Yih Chen, and Chang-Jer Wu. 2015. "Enhanced Control of Bladder-Associated Tumors Using Shrimp Anti-Lipopolysaccharide Factor (SALF) Antimicrobial Peptide as a Cancer Vaccine Adjuvant in Mice" Marine Drugs 13, no. 5: 3241-3258. https://doi.org/10.3390/md13053241
APA StyleHuang, H.-N., Rajanbabu, V., Pan, C.-Y., Chan, Y.-L., Chen, J.-Y., & Wu, C.-J. (2015). Enhanced Control of Bladder-Associated Tumors Using Shrimp Anti-Lipopolysaccharide Factor (SALF) Antimicrobial Peptide as a Cancer Vaccine Adjuvant in Mice. Marine Drugs, 13(5), 3241-3258. https://doi.org/10.3390/md13053241





