Diphlorethohydroxycarmalol Inhibits Interleukin-6 Production by Regulating NF-κB, STAT5 and SOCS1 in Lipopolysaccharide-Stimulated RAW264.7 Cells
Abstract
:1. Introduction
2. Results and Discussion
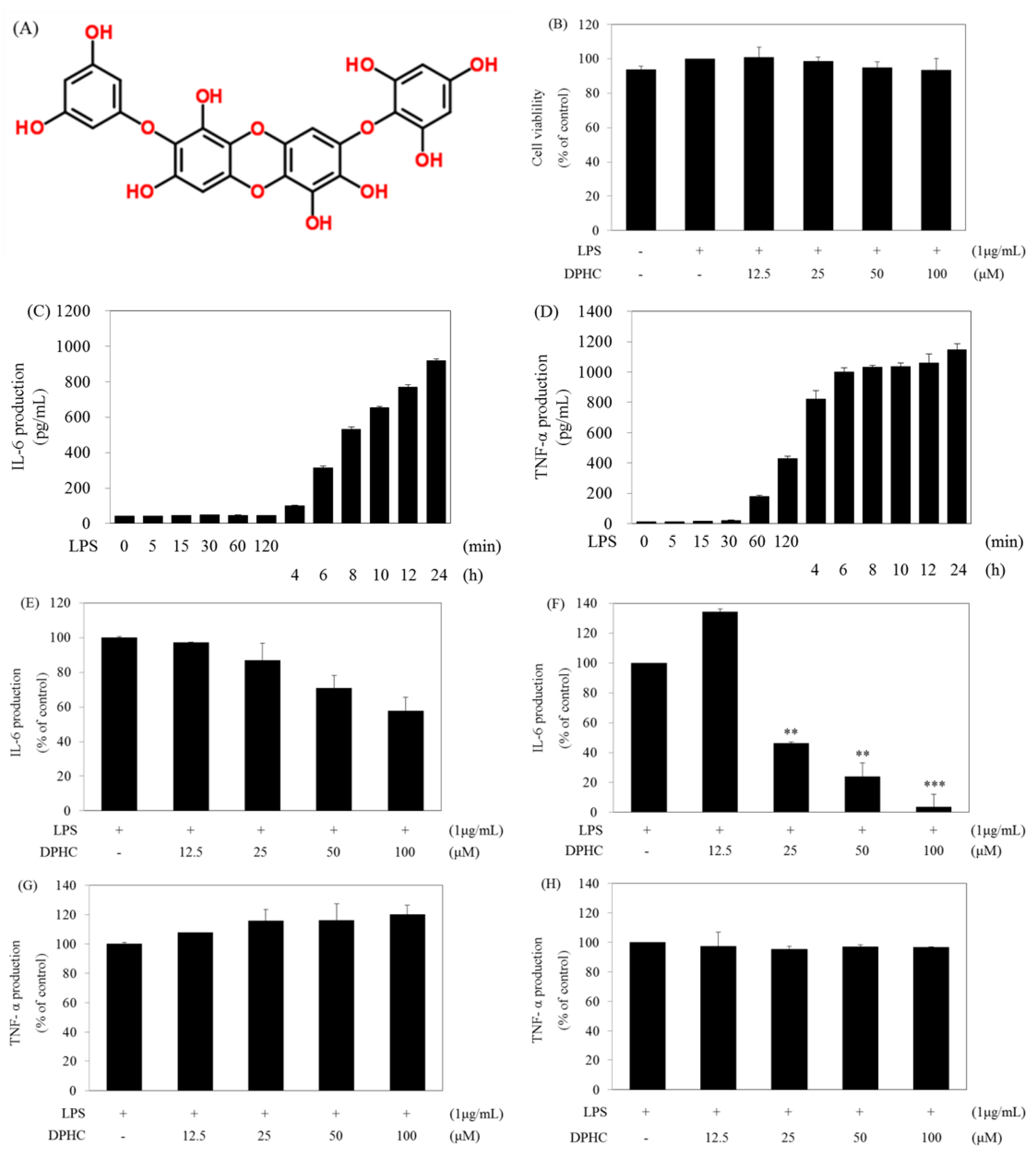
2.1. DPHC Selectively Inhibits LPS-Induced IL-6 Production in RAW264.7 Cells
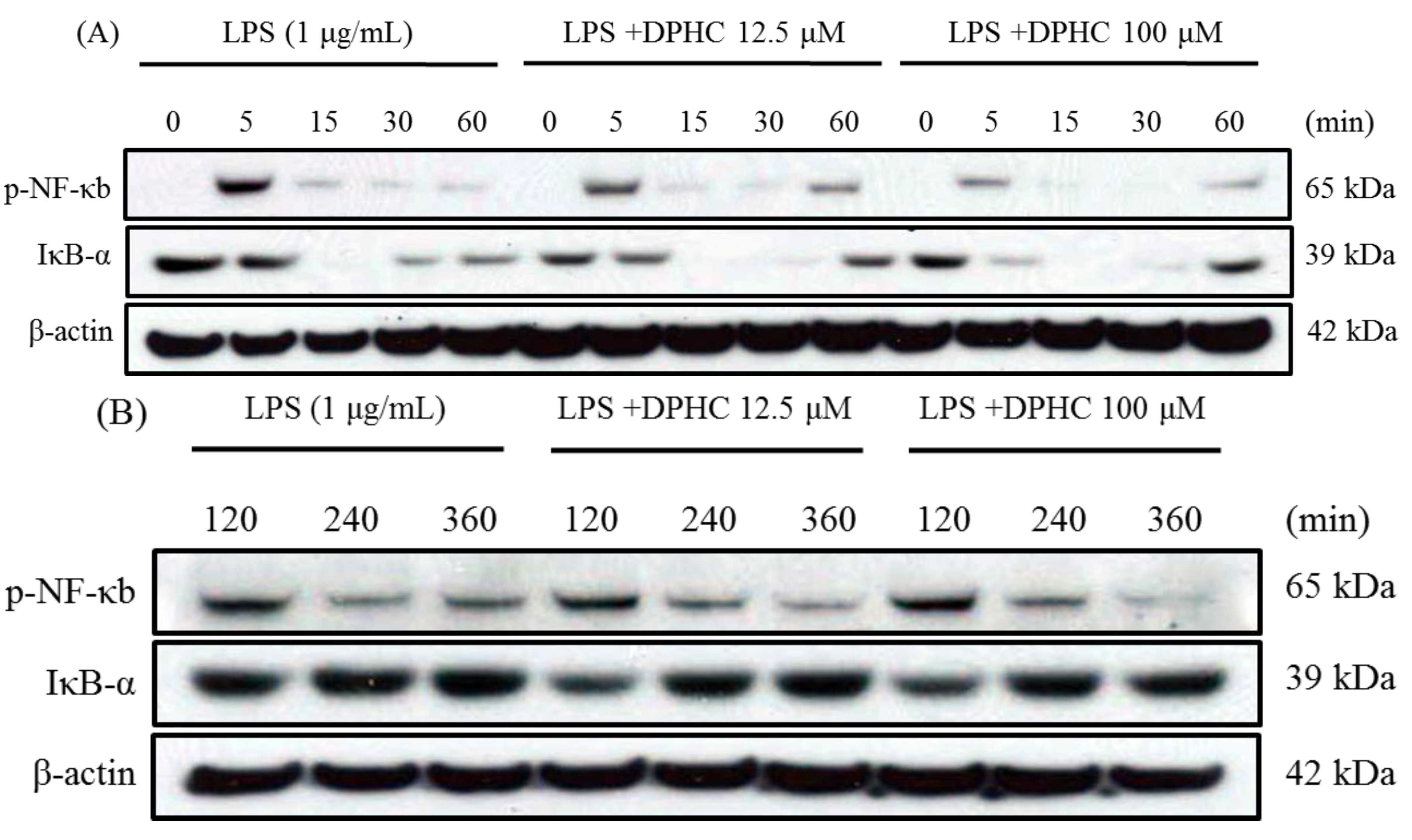
2.2. DPHC Inhibits the Activation and Nuclear Translocation of NF-κB in LPS-Stimulated RAW264.7 Cells

2.3. DPHC Does Not Affect the MAPK Pathway in LPS-Stimulated RAW264.7 Cells
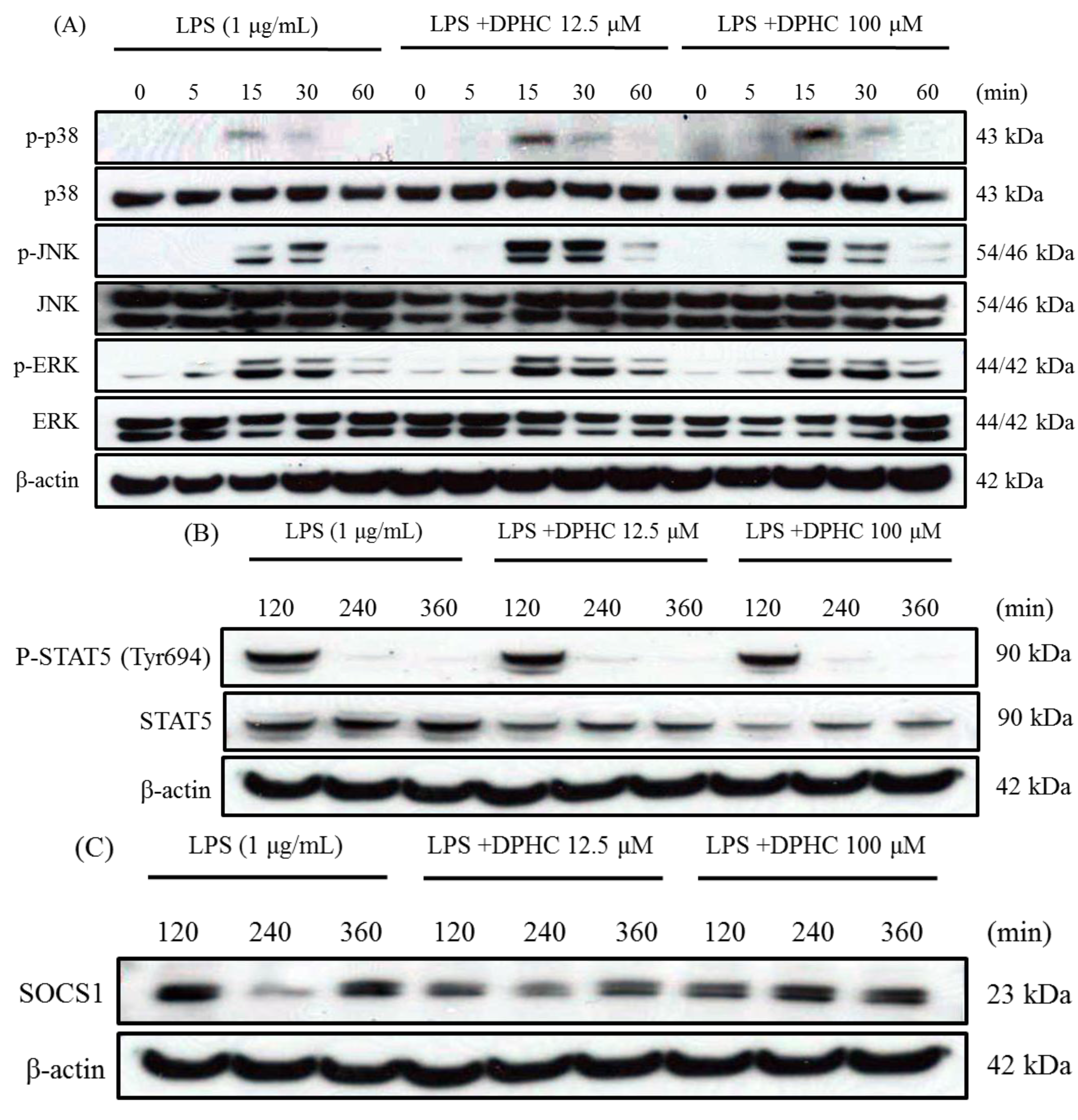
2.4. DPHC Inhibits STAT5 and SOCS1 in LPS-Stimulated RAW264.7 Cells
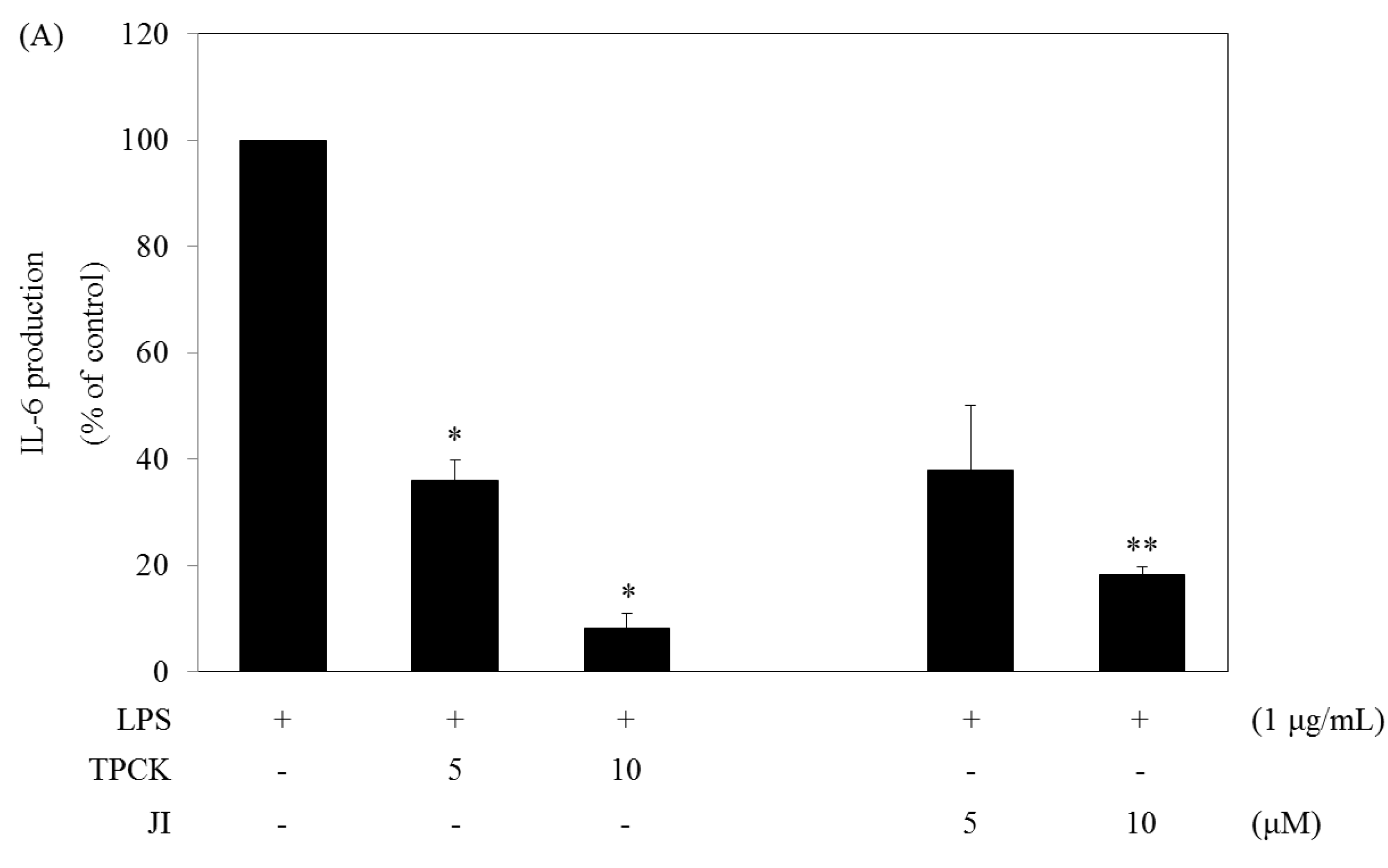
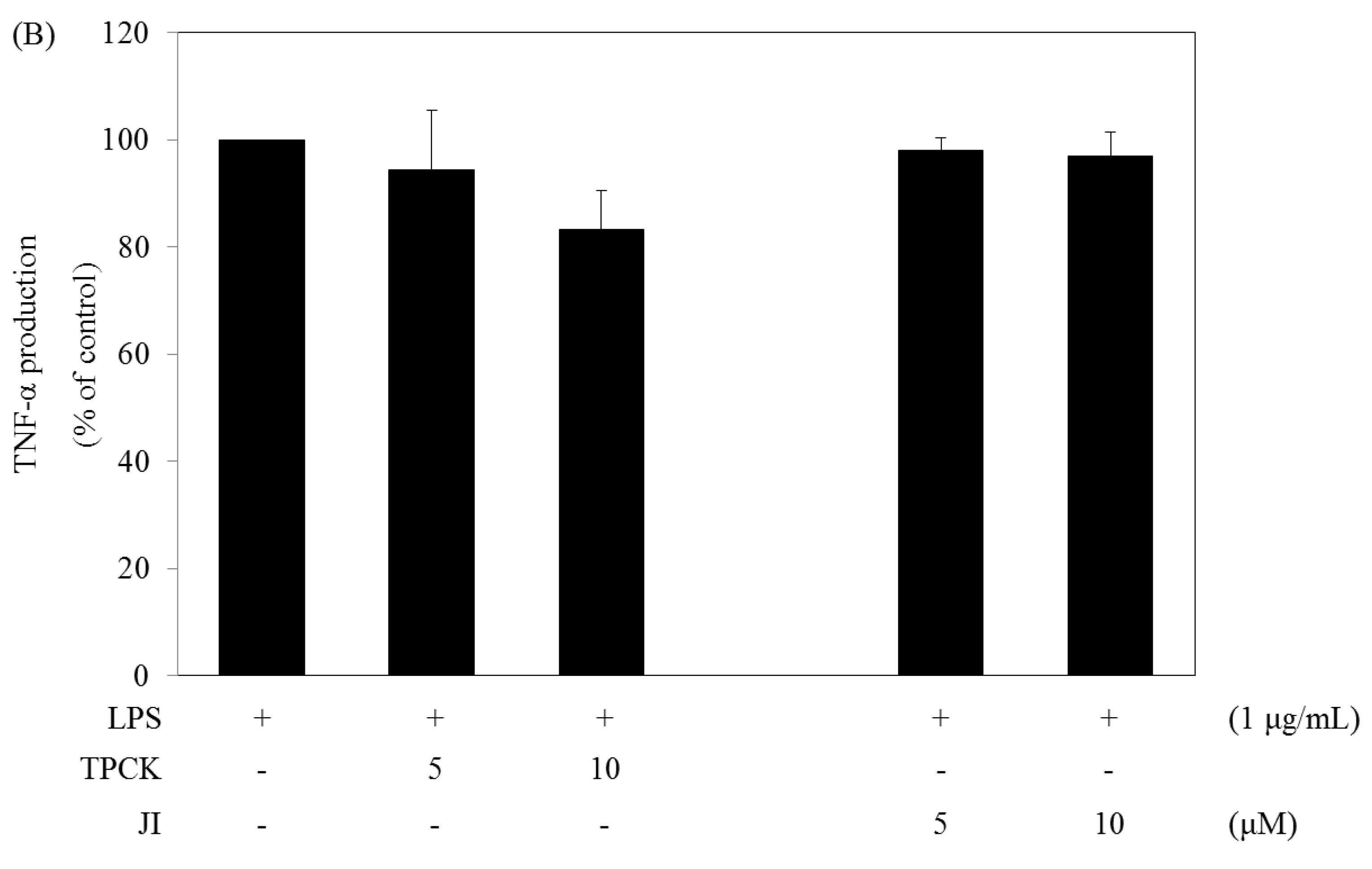
2.5. N-Tosyl-L-phenylalanine Chloromethyl Ketone and JI Selectively Reduce the Production of IL-6
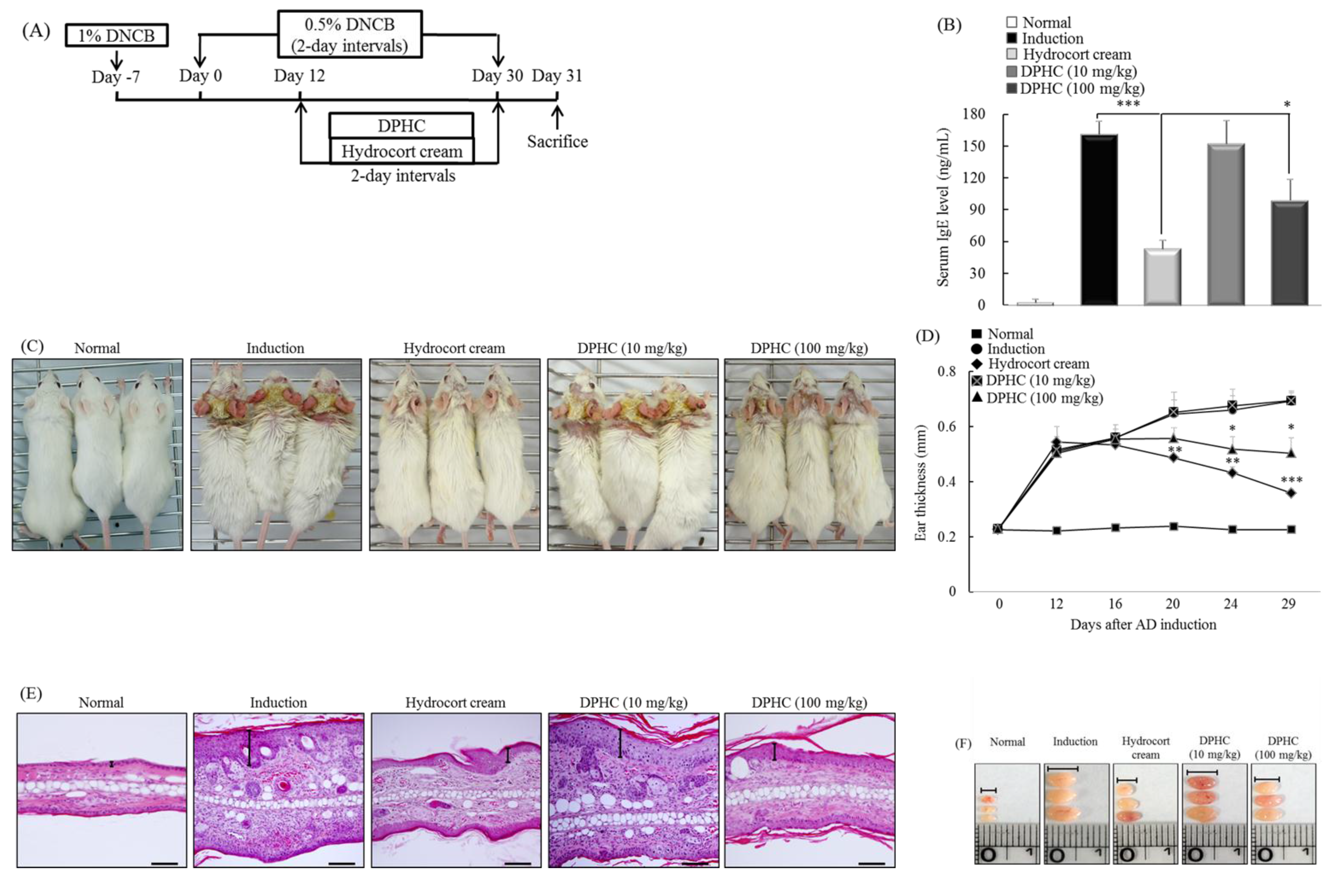
2.6. DPHC Suppresses the Development of Experimental Atopic Dermatitis
3. Experimental Section
3.1. Reagents
3.2. Experimental Animals
3.3. DPHC Isolation
3.4. Cell Culture
3.5. Cell Viability
3.6. ELISA
3.7. Western Blot Analysis
3.8. Confocal Laser Scanning Microscopy Analysis
3.9. Disease Models
3.10. Macroscopic Edema and Histological Evaluation
3.11. Statistical Analysis
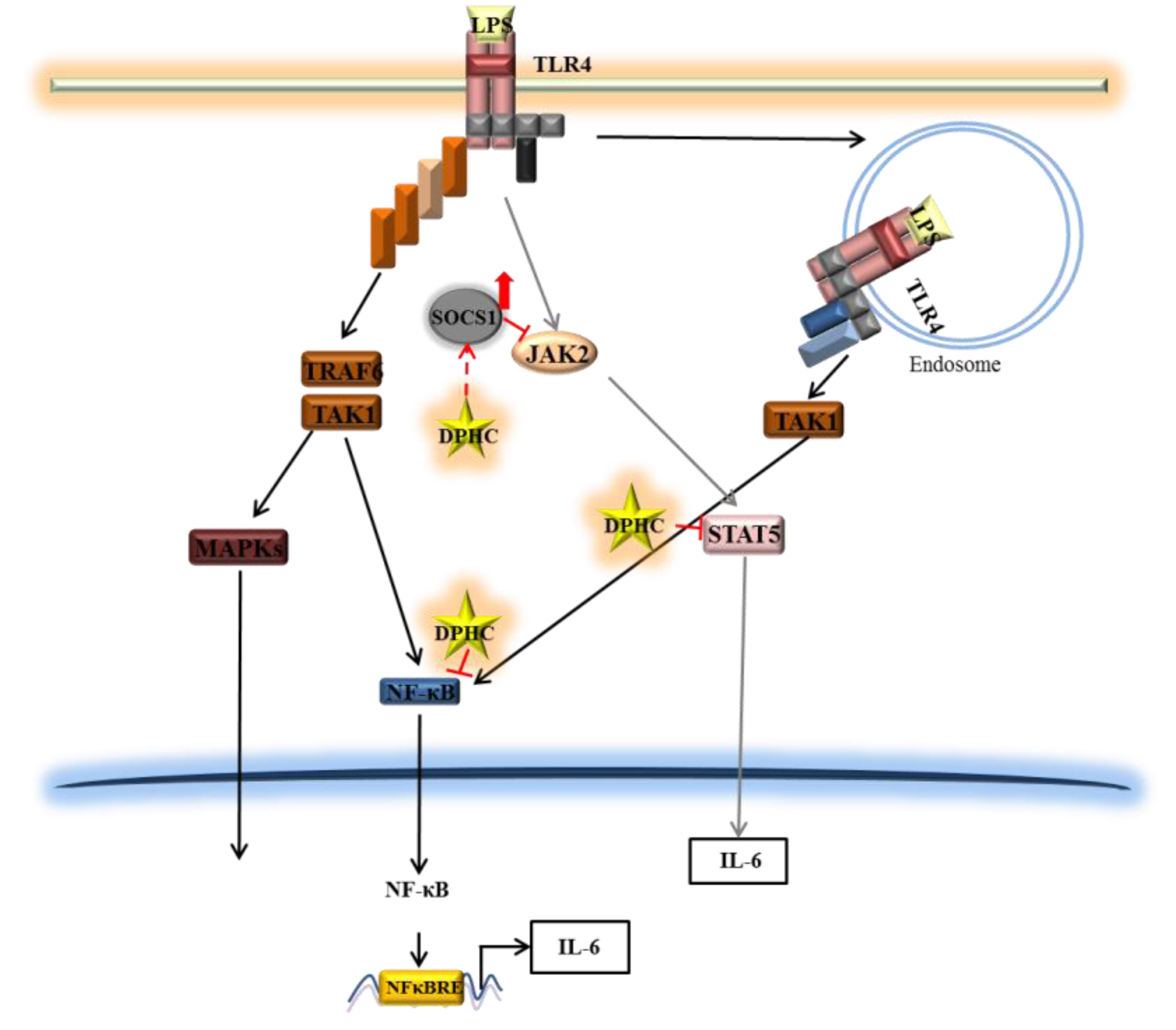
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hohki, S.; Ohguro, N.; Haruta, H.; Nakai, K.; Terabe, F.; Serada, S.; Fujimoto, M.; Nomura, S.; Kawahata, H.; Kishimoto, T.; et al. Blockade of Interleukin-6 Signaling Suppresses Experimental Autoimmune Uveoretinitis by the Inhibition of Inflammatory Th17 Responses. Exp. Eye Res. 2010, 91, 162–170. [Google Scholar]
- Hack, C.E.; de Groot, E.R.; Felt-Bersma, R.J.; Nuijens, J.H.; Strack van Schijndel, R.J.; Eerenberg-Belmer, A.J.; Thijs, L.G.; Aarden, L.A. Increased Plasma Levels of Interleukin-6 in Sepsis. Blood 1989, 74, 1704–1710. [Google Scholar] [PubMed]
- Takeda, K.; Akira, S. Toll-Like Receptors in Innate Immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.A.; Dorf, M.E. Differential Regulation of Interleukin-6, Macrophage Inflammatory Protein-1, and JE/MCP-1 Cytokine Expression in Macrophage Cell Lines. Cell. Immunol. 1991, 135, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting Edge: Toll-Like Receptor 4 (TLR4)-Deficient Mice are Hyporesponsive to Lipopolysaccharide: Evidence for TLR4 as the Lps Gene Product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar] [PubMed]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The Structural Basis of Lipopolysaccharide Recognition by the TLR4-MD-2 Complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-Like Receptor Signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J.C.; Medzhitov, R. Phosphoinositide-Mediated Adaptor Recruitment Controls Toll-Like Receptor Signaling. Cell 2006, 125, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 Signal Transduction Pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, I.M. NF-kappaB Regulation in the Immune System. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB Activation by Reactive Oxygen Species: Fifteen Years Later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Baltimore, D. NF-Kappa B: Ten Years After. Cell 1996, 87, 13–20. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Murray, P.J. Cytokine Signaling Modules in Inflammatory Responses. Immunity 2008, 28, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Shuai, K.; Liu, B. Regulation of JAK-STAT Signalling in the Immune System. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, C.; Sriram, S.; Muthian, G.; Bright, J.J. Signaling through JAK2-STAT5 Pathway is Essential for IL-3-Induced Activation of Microglia. Glia 2004, 45, 188–196. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, W.J.; van der Zanden, E.P.; The, F.O.; Bijlsma, M.F.; van Westerloo, D.J.; Bennink, R.J.; Berthoud, H.R.; Uematsu, S.; Akira, S.; van den Wijngaard, R.M.; et al. Stimulation of the Vagus Nerve Attenuates Macrophage Activation by Activating the Jak2-STAT3 Signaling Pathway. Nat. Immunol. 2005, 6, 844–851. [Google Scholar]
- Darnell, J.E., Jr. STATs and Gene Regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.; Ropke, C. Suppressors of Cytokine Signalling: SOCS. APMIS 2002, 110, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Starr, R.; Willson, T.A.; Viney, E.M.; Murray, L.J.; Rayner, J.R.; Jenkins, B.J.; Gonda, T.J.; Alexander, W.S.; Metcalf, D.; Nicola, N.A.; et al. A Family of Cytokine-Inducible Inhibitors of Signalling. Nature 1997, 387, 917–921. [Google Scholar]
- Naka, T.; Narazaki, M.; Hirata, M.; Matsumoto, T.; Minamoto, S.; Aono, A.; Nishimoto, N.; Kajita, T.; Taga, T.; Yoshizaki, K.; et al. Structure and Function of a New STAT-Induced STAT Inhibitor. Nature 1997, 387, 924–929. [Google Scholar]
- Kimura, A.; Naka, T.; Muta, T.; Takeuchi, O.; Akira, S.; Kawase, I.; Kishimoto, T. Suppressor of Cytokine Signaling-1 Selectively Inhibits LPS-Induced IL-6 Production by Regulating JAK-STAT. Proc. Natl. Acad. Sci. USA 2005, 102, 17089–17094. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Heo, S.J.; Han, S.C.; Lee, H.J.; Kang, G.J.; Kang, H.K.; Hyun, J.W.; Koh, Y.S.; Yoo, E.S. Anti-Inflammatory Effect of Sargachromanol G Isolated from Sargassum Siliquastrum in RAW 264.7 Cells. Arch. Pharm. Res. 2012, 35, 1421–1430. [Google Scholar] [CrossRef]
- Chae, D.; Manzoor, Z.; Kim, S.C.; Kim, S.; Oh, T.H.; Yoo, E.S.; Kang, H.K.; Hyun, J.W.; Lee, N.H.; Ko, M.H.; et al. Apo-9′-Fucoxanthinone, Isolated from Sargassum Muticum, Inhibits CpG-Induced Inflammatory Response by Attenuating the Mitogen-Activated Protein Kinase Pathway. Mar. Drugs 2013, 11, 3272–3287. [Google Scholar]
- Heo, S.J.; Hwang, J.Y.; Choi, J.I.; Han, J.S.; Kim, H.J.; Jeon, Y.J. Diphlorethohydroxycarmalol Isolated from Ishige Okamurae, a Brown Algae, a Potent Alpha-Glucosidase and Alpha-Amylase Inhibitor, Alleviates Postprandial Hyperglycemia in Diabetic Mice. Eur. J. Pharmacol. 2009, 615, 252–256. [Google Scholar] [CrossRef]
- Heo, S.J.; Hwang, J.Y.; Choi, J.I.; Lee, S.H.; Park, P.J.; Kang, D.H.; Oh, C.; Kim, D.W.; Han, J.S.; Jeon, Y.J.; et al. Protective Effect of Diphlorethohydroxycarmalol Isolated from Ishige Okamurae Against High Glucose-Induced-Oxidative Stress in Human Umbilical Vein Endothelial Cells. Food Chem. Toxicol. 2010, 48, 1448–1454. [Google Scholar]
- Lee, H.J.; Maeng, K.; Dang, H.T.; Kang, G.J.; Ryou, C.; Jung, J.H.; Kang, H.K.; Prchal, J.T.; Yoo, E.S.; Yoon, D. Anti-Inflammatory Effect of Methyl Dehydrojasmonate (J2) is Mediated by the NF-kappaB Pathway. J. Mol. Med. (Berl.) 2011, 89, 83–90. [Google Scholar] [CrossRef]
- Lee, H.J.; Dang, H.T.; Kang, G.J.; Yang, E.J.; Park, S.S.; Yoon, W.J.; Jung, J.H.; Kang, H.K.; Yoo, E.S. Two Enone Fatty Acids Isolated from Gracilaria Verrucosa Suppress the Production of Inflammatory Mediators by Down-Regulating NF-kappaB and STAT1 Activity in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Arch. Pharm. Res. 2009, 32, 453–462. [Google Scholar] [CrossRef]
- Feng, D.; Ling, W.H.; Duan, R.D. Lycopene Suppresses LPS-Induced NO and IL-6 Production by Inhibiting the Activation of ERK, p38MAPK, and NF-kappaB in Macrophages. Inflamm. Res. 2010, 59, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Joo, Y.E. Theaflavin Inhibits LPS-Induced IL-6, MCP-1, and ICAM-1 Expression in Bone Marrow-Derived Macrophages through the Blockade of NF-kappaB and MAPK Signaling Pathways. Chonnam Med. J. 2011, 47, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Levin, T.A.; Ownby, D.R.; Smith, P.H.; Peterson, E.L.; Williams, L.K.; Ford, J.; Young, P.; Johnson, C.C. Relationship between Extremely Low Total Serum IgE Levels and Rhinosinusitis. Ann. Allergy Asthma Immunol. 2006, 97, 650–652. [Google Scholar] [CrossRef] [PubMed]
- De Vries, I.J.; Langeveld-Wildschut, E.G.; van Reijsen, F.C.; Bihari, I.C.; Bruijnzeel-Koomen, C.A.; Thepen, T. Nonspecific T-Cell Homing during Inflammation in Atopic Dermatitis: Expression of Cutaneous Lymphocyte-Associated Antigen and Integrin alphaE beta7 on Skin-Infiltrating T Cells. J. Allergy Clin. Immunol. 1997, 100, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Debes, G.F.; Bonhagen, K.; Wolff, T.; Kretschmer, U.; Krautwald, S.; Kamradt, T.; Hamann, A. CC Chemokine Receptor 7 Expression by effector/memory CD4+ T Cells Depends on Antigen Specificity and Tissue Localization during Influenza A Virus Infection. J. Virol. 2004, 78, 7528–7535. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Kim, J.P.; Jung, W.K.; Lee, N.H.; Kang, H.S.; Jun, E.M.; Park, S.H.; Kang, S.M.; Lee, Y.J.; Park, P.J.; et al. Identification of Chemical Structure and Free Radical Scavenging Activity of Diphlorethohydroxycarmalol Isolated from a Brown Alga, Ishige Okamurae. J. Microbiol. Biotechnol. 2008, 18, 676–681. [Google Scholar]
- Zou, Y.; Qian, Z.J.; Li, Y.; Kim, M.M.; Lee, S.H.; Kim, S.K. Antioxidant Effects of Phlorotannins Isolated from Ishige Okamurae in Free Radical Mediated Oxidative Systems. J. Agric. Food Chem. 2008, 56, 7001–7009. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, N.-J.; Han, S.-C.; Kang, G.-J.; Koo, D.-H.; Koh, Y.-S.; Hyun, J.-W.; Lee, N.-H.; Ko, M.-H.; Kang, H.-K.; Yoo, E.-S. Diphlorethohydroxycarmalol Inhibits Interleukin-6 Production by Regulating NF-κB, STAT5 and SOCS1 in Lipopolysaccharide-Stimulated RAW264.7 Cells. Mar. Drugs 2015, 13, 2141-2157. https://doi.org/10.3390/md13042141
Kang N-J, Han S-C, Kang G-J, Koo D-H, Koh Y-S, Hyun J-W, Lee N-H, Ko M-H, Kang H-K, Yoo E-S. Diphlorethohydroxycarmalol Inhibits Interleukin-6 Production by Regulating NF-κB, STAT5 and SOCS1 in Lipopolysaccharide-Stimulated RAW264.7 Cells. Marine Drugs. 2015; 13(4):2141-2157. https://doi.org/10.3390/md13042141
Chicago/Turabian StyleKang, Na-Jin, Sang-Chul Han, Gyeoung-Jin Kang, Dong-Hwan Koo, Young-Sang Koh, Jin-Won Hyun, Nam-Ho Lee, Mi-Hee Ko, Hee-Kyoung Kang, and Eun-Sook Yoo. 2015. "Diphlorethohydroxycarmalol Inhibits Interleukin-6 Production by Regulating NF-κB, STAT5 and SOCS1 in Lipopolysaccharide-Stimulated RAW264.7 Cells" Marine Drugs 13, no. 4: 2141-2157. https://doi.org/10.3390/md13042141
APA StyleKang, N.-J., Han, S.-C., Kang, G.-J., Koo, D.-H., Koh, Y.-S., Hyun, J.-W., Lee, N.-H., Ko, M.-H., Kang, H.-K., & Yoo, E.-S. (2015). Diphlorethohydroxycarmalol Inhibits Interleukin-6 Production by Regulating NF-κB, STAT5 and SOCS1 in Lipopolysaccharide-Stimulated RAW264.7 Cells. Marine Drugs, 13(4), 2141-2157. https://doi.org/10.3390/md13042141




