The Potential of Chitosan and Its Derivatives in Prevention and Treatment of Age-Related Diseases
Abstract
:1. Introduction
2. Oxidative Stress
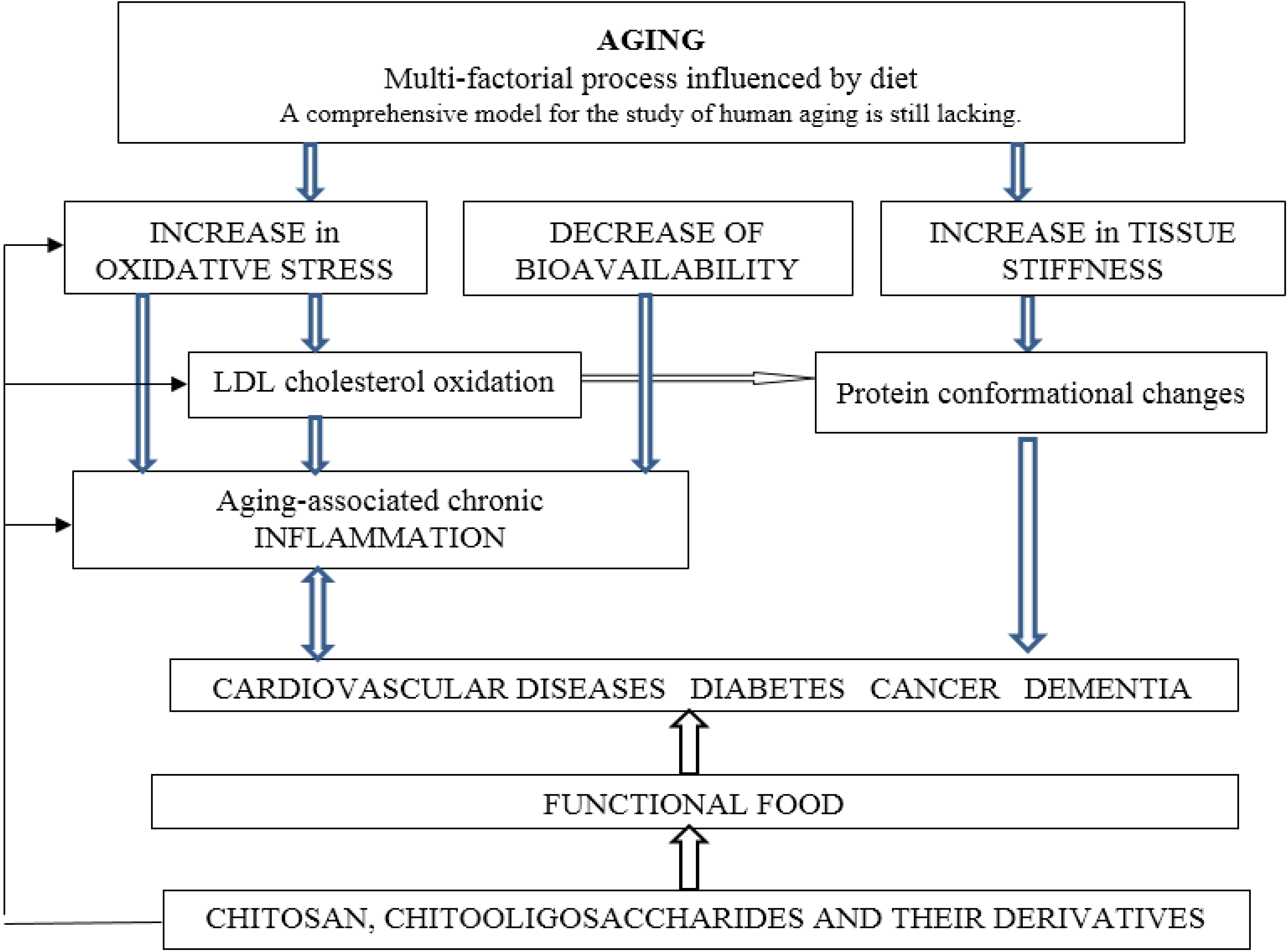
3. Inflammation
4. Diabetes Mellitus
5. Hypercholesterolemia
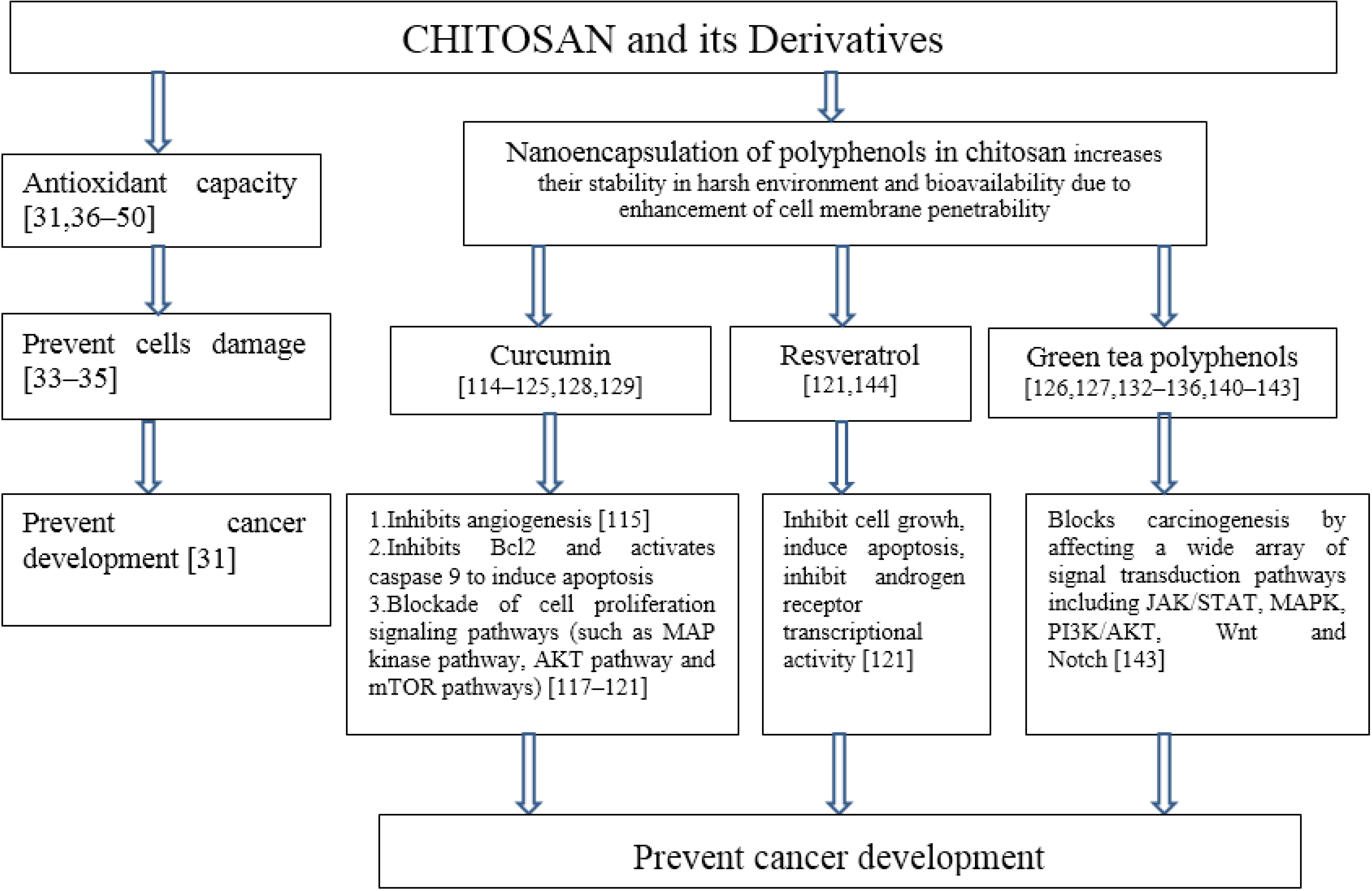
6. Cancer
7. Nanomedicine
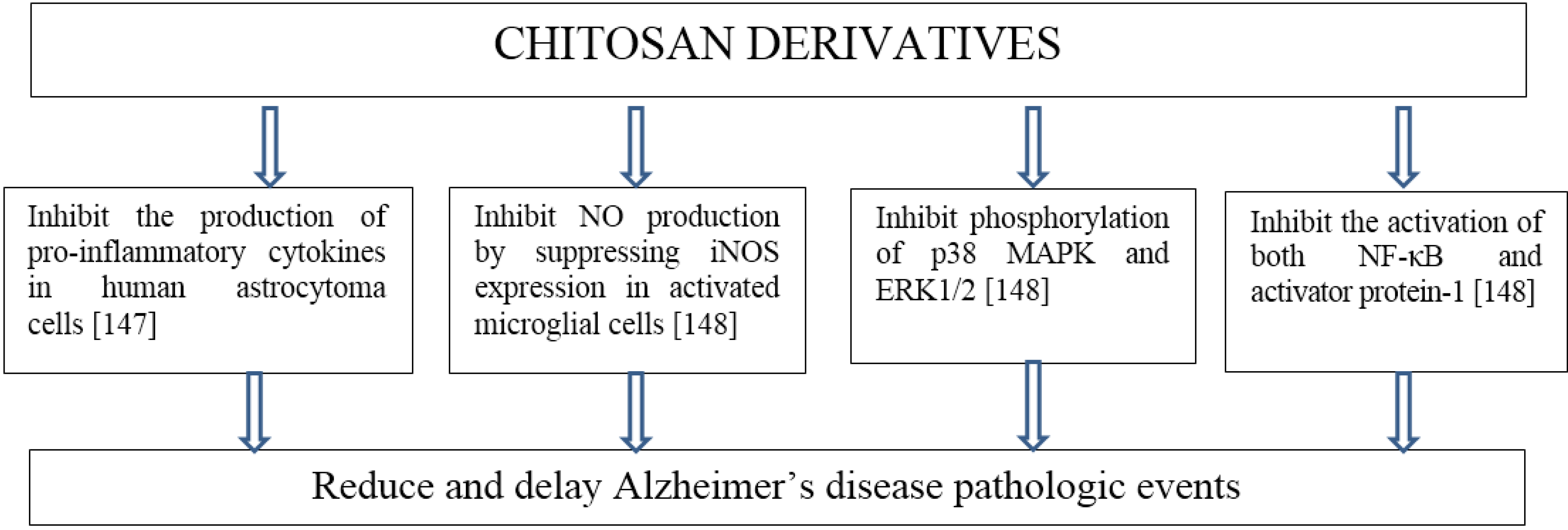
8. Neurodegenerative Diseases
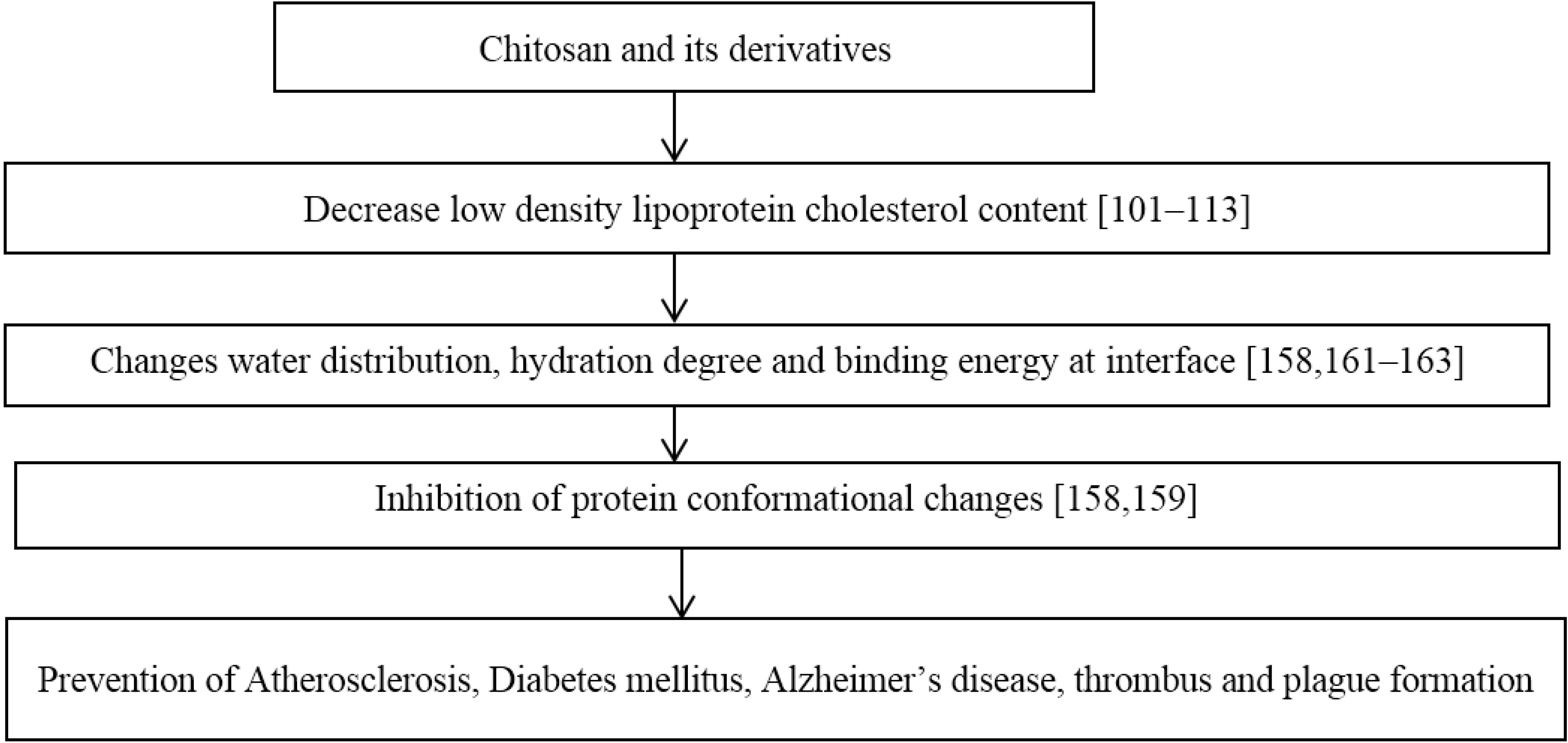
9. Protein Conformational Diseases
10. Chitosan Containing Food
10.1. Bread Containing Chitosan and Its Derivatives
10.2. Dairy Products Containing Chitosan and Its Derivatives
11. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2013; ST/ESA/SER.A/348; United Nations: New York, NY, USA, 2013. [Google Scholar]
- Brownie, S. Why are elderly individuals at risk of nutritional deficiency? Int. J. Nurs. Pract. 2006, 12, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.L.; Dumbrell, A.C. Nutrition and aging: assessment and treatment of compromised nutritional status in Frail elderly patients. Clin. Interv. Aging 2006, 1, 67–79. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Media centre. Available online: http://www.who.int/mediacentre/factsheets/fs312/en/ (accessed on 16 November 2014).
- Chandra, R.K. Nutrition and the immune system from birth to old age. Eur. J. Clin. Nutr. 2002, 56, 73–76. [Google Scholar] [CrossRef]
- Chandra, R.K. Nutrition and the immune system: An introduction. Am. J. Clin. Nutr. 1997, 66, 460–463. [Google Scholar]
- Chandra, R.K. Nutrition, immunity and infection: From basic knowledge of dietary manipulation of immune responses to practical application of ameliorating suffering and improving survival. Proc. Natl. Acad. Sci. USA 1996, 93, l4304–14307. [Google Scholar]
- Kirilenko, Y.K.; Dushkova, Z.G.; Cherkasova, E.I.; Sigilietov, A.E. Chitosan oligomer and ascorbic acid salt in compensation of deficiency of some micronutrients. In Advances in Chitin Science; Senel, S., Varum, K.M., Sumnu, M.M., Hincal, A.A., Eds.; TUBITAK: Antalya, Turkey, 2007; Volume 10. [Google Scholar]
- Shaw, A.C.; Joshi, S.; Greenwood, H.; Panda, A.; Lord, J.M. Aging of the innate immune system. Curr. Opin. Immunol. 2010, 22, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Perez de Heredia, F.; Jirillo, E.; Morabito, G.; Marcos, A.; Serafini, M. Functional foods and nutraceuticals as therapeutic tools for the treatment of diet-related diseases. Can. J. Physiol. Pharm. 2013, 91, 387–396. [Google Scholar] [CrossRef]
- Pae, M.; Meydani, S.N.; Wu, D. The role of nutrition in enhancing immunity in aging. Aging Dis. 2012, 3, 91–129. [Google Scholar] [PubMed]
- Maijó, M.; Clements, S.J.; Ivory, K.; Nicoletti, C.; Carding, S.R. Nutrition, diet and immunosenescence. Mech. Ageing Dev. 2014, 136–137, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, F.; Hu, M.; Ma, C.W.; Xiao, L.; Zhang, J.; Xiang, Y.; Huang, Z. Polysaccharides from medicinal herbs as potential therapeutics for aging and age-related neurodegeneration. Rejuvenation Res. 2014, 17, 201–204. [Google Scholar] [CrossRef] [PubMed]
- d’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef] [PubMed]
- Chitin and Chitosan Derivatives: Advances in Drug Discovery and Developments; Kim, S.K. (Ed.) CRC Press: Boca Raton, FL, USA, 2013; p. 527.
- Chitosan-Based Hydrogels: Functions and Applications; Yao, K.; Li, J.; Yao, F.; Yin, Y. (Eds.) CRC Press: Boca Raton, FL, USA, 2011; p. 241.
- Chitosan for Biomaterials I; Jayakumar, R.; Prabaharan, M.; Muzzarelli, R.A.A. (Eds.) Springer: Berlin Heidelberg, Germany, 2011; p. 243.
- Chitosan for Biomaterials II; Jayakumar, R.; Prabaharan, M.; Muzzarelli, R.A.A. (Eds.) Springer: Berlin Heidelberg, Germany, 2011; p. 223.
- Green Biorenewable Biocomposites: From Knowledge to Industrial Applications; Thakur, V.K.; Kessler, M.R. (Eds.) CRC Press: Boca Raton, USA, 2015; p. 568.
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloids 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Sudheesh Kumar, P.T.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahi, T.; Gu, W.; Li, B. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.J.; Park, R.D. Bioproduction of chitooligosaccharides: Present and perspectives. Mar. Drugs 2014, 12, 5328–5356. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Mattioli-Belmonte, M. Emerging biomedical applications of nano-chitins and nano-chitosans obtained via advanced eco-friendly technologies from marine resources. Mar. Drugs 2014, 12, 5468–5502. [Google Scholar] [CrossRef] [PubMed]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Ngo, D.H.; Kim, S.K. Antioxidant effects of chitin, chitosan and their derivatives. In Marine Carbohydrates: Fundamentals and Applications, Part B; Kim, S.-K., Ed.; Elsevier Inc.: Oxford, UK, 2014; pp. 15–31. [Google Scholar]
- Andriollo-Sanchez, M.; Hininger-Favier, I.; Meunier, N.; Venneria, E.; O’Connor, J.M.; Maiani, G.; Coudray, C.; Roussel, A.M. Age-related oxidative stress and antioxidant parameters in middle-aged and older European subjects: The ZENITH study. Eur. J. Clin. Nutr. 2005, 59, 58–62. [Google Scholar] [CrossRef]
- Abdollahi, M.; Moridani, M.Y.; Aruoma, O.I.; Mostafalou, S. Oxidative stress in aging. Oxidative Med. Cell. Longev. 2014, 2014, 876834:1–876834:2. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Saeidnia, S.; Abdollahi, M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol. Appl. Pharm. 2013, 273, 442–455. [Google Scholar] [CrossRef]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.I.; Hwang, S.G.; Park, N.G.; Jung, Y.R.; Shin, S.I.; Choi, S.D.; Park, D.K. Antioxidative effect of chitosan on chronic carbon tetrachloride induced hepatic injury in rats. Toxicology 2003, 187, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.S.; Taguchi, T.; Sakai, K.; Kikuchi, K.; Wang, M.W.; Miwa, I. Antioxidant activities of chitobiose and chitotriose. Biol. Pharm. Bull. 2003, 26, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xie, W.; Xu, P. Superoxide anion scavenging activity of graft chitosan derivatives. Carbohydr. Polym. 2004, 58, 379–382. [Google Scholar] [CrossRef]
- Huang, R.; Mendis, E.; Kim, S.K. Factors affecting the free radical scavenging behavior of chitosan sulfate. Int. J. Biol. Macromol. 2005, 36, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Du, Y.; Li, J.; Wei, Y.; Yao, P. Antioxidant activity of half N-acetylated water-soluble chitosan in vitro. Eur. Food Res. Technol. 2007, 225, 133–138. [Google Scholar] [CrossRef]
- Yen, M.T.; Tseng, Y.H.; Li, R.C.; Mau, J.L. Antioxidant properties of fungal chitosan from shiitake stipes. LWT-Food Sci. Technol. 2007, 40, 255–261. [Google Scholar] [CrossRef]
- Anraku, M.; Fujii, T.; Furutani, N.; Kadowaki, D.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Tomida, H. Antioxidant effects of a dietary supplement: Reduction of indices of oxidative stress in normal subjects by water-soluble chitosan. Food Chem, Toxicol. 2009, 47, 104–109. [Google Scholar] [CrossRef]
- Anraku, M.; Michihara, A.; Yasufuku, T.; Akasaki, K.; Tsuchiya, D.; Nishio, H.; Maruyama, T.; Otagiri, M.; Maezaki, Y.; Kondo, Y.; et al. The antioxidative and antilipidemic effects of different molecular weight chitosans in metabolic syndrome model rats. Biol. Pharm. Bull. 2010, 33, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Juneja, V.K. Review of antimicrobial and antioxidative activities of chitosans in food. J. Food Prot. 2010, 73, 1737–1761. [Google Scholar] [PubMed]
- Ngo, D.N. Chitin, Chitosan, and Their Derivatives against Oxidative Stress and Inflammation, and Some Applications. In Seafood Processing By-Products; Kim, S., Ed.; Springer: New York, NY, USA, 2014; pp. 389–405. [Google Scholar]
- Luo, Z.; Dong, X.; Ke, Q.; Duan, Q.; Shen, L. Chitooligosaccharides inhibit ethanol-induced oxidative stress via activation of Nrf2 and reduction of MAPK phosphorylation. Oncol. Rep. 2014, 32, 2215–2222. [Google Scholar] [PubMed]
- Anandan, R.; Ganesan, B.; Obulesu, T.; Mathew, S.; Kumar, R.S.; Lakshmanan, P.T.; Zynudheen, A.A. Dietary chitosan supplementation attenuates isoprenaline-induced oxidative stress in rat myocardium. Int. J. Biol. Macromol. 2012, 51, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Anandan, R.; Ganesan, B.; Obulesu, T.; Mathew, S.; Asha, K.K.; Lakshmanan, P.T.; Zynudheen, A.A. Antiaging effect of dietary chitosan supplementation on glutathione-dependent antioxidant system in young and aged rats. Cell Stress Chaperon 2013, 18, 121–125. [Google Scholar] [CrossRef]
- Qiao, Y.; Bai, X.F.; Du, Y.G. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011, 11, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Guo, H.; Sun, L.; Zhang, L.; Zhang, Y. Protective effects of sulfated chitooligosaccharides with different degrees of substitution in MIN6 cells. Int. J. Biol. Macromol. 2013, 52, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.S.; Hwang, J.W.; Han, Y.K.; Lee, J.S.; Kim, S.K.; Jeon, Y.J.; Moon, S.H.; Jeon, B.T.; Bahk, Y.Y.; et al. Sulfated chitosan oligosaccharides suppress LPS-induced NO production via JNK and NF-κB inactivation. Molecules 2014, 19, 18232–18247. [Google Scholar] [CrossRef] [PubMed]
- Tomida, H.; Fujii, T.; Furutani, N.; Michihara, A.; Yasufuku, T.; Akasaki, K.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Anraku, M. Antioxidant properties of some different molecular weight chitosans. Carbohydr. Res. 2009, 344, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xia, W. Effect of media milling on lipid-lowering and antioxidant activities of chitosan. Int. J. Biol. Macromol. 2015, 72, 1402–1405. [Google Scholar] [CrossRef]
- Lee, D.S.; Woo, J.Y.; Ahn, C.B.; Je, J.Y. Chitosan–hydroxycinnamic acid conjugates: Preparation, antioxidant and antimicrobial activity. Food Chem. 2014, 148, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Guo, X.W.; Li, L.; Wang, H.W.; Kim, W. The antioxidant property of chitosan green tea polyphenols complex induces transglutaminase activation in wound healing. J. Med. Food. 2013, 16, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wen, X.; Lu, J.; Kan, J.; Jin, C. Free radical mediated grafting of chitosan with caffeic and ferulic acids: Structures and antioxidant activity. Int. J. Biol. Macromol. 2014, 65, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, J.; Kan, J.; Tang, Y.; Jin, C. Preparation, characterization and antioxidant activity of phenolic acids grafted carboxymethyl chitosan. Int. J. Biol. Macromol. 2013, 62, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Jana, A.; Das, A.; Paul, T.; Mohapatra, P.K.D. Appraisal of antioxidant, anti-hemolytic and DNA shielding potentialities of chitosaccharides produced innovatively from shrimp shell by sequential treatment with immobilized enzymes. Food Chem. 2014, 158, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Eaton, P.; Nascimento, H.; Gião, M.S.; Ramos, Ó.S.; Belo, L.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X. Antioxidant activity of chitooligosaccharides upon two biological systems: Erythrocytes and bacteriophages. Carbohydr. Polym. 2010, 79, 1101–1106. [Google Scholar] [CrossRef]
- Ngo, D.N.; Kim, M.M.; Kim, S.K. Protective effects of aminoethyl-chitooligosaccharides against oxidative stress in mouse macrophage RAW 264.7 cells. Int. J. Biol. Macromol. 2012, 50, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, K.V.H.; Dharmesh, S.; Rao, K.S.J.; Tharanathan, R.N. Free radical-induced chitosan depolymerized products protect calf thymus DNA from oxidative damage. Carbohydr. Res. 2007, 342, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Trinh, M.D.L.; Ngo, D.H.; Tran, D.K.; Tran, Q.T.; Vo, T.S.; Dinh, M.H.; Ngo, D.N. Prevention of H2O2-induced oxidative stress in Chang liver cells by 4-hydroxybenzyl-chitooligomers. Carbohydr. Polym. 2014, 103, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Aggarwal, B.B. Transcription factor NF-κB: A sensor for smoke and stress signals. Ann. N. Y. Acad. Sci. 2005, 1056, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, G.; Rizzo, M.R.; Mazziotti, G.; Tagliamonte, M.R.; Gambardella, A.; Rotondi, M.; Carella, C.; Giugliano, D.; Varricchio, M.; D’Onofrio, F. Advancing age and insulin resistance: Role of plasma tumor necrosis factor-alpha. Am. J. Physiol. 1998, 275, E294–E299. [Google Scholar] [PubMed]
- Liu, H.T.; Li, W.M.; Li, X.Y.; Xu, Q.S.; Liu, Q.S.; Bai, X.F.; Yu, C.; Du, Y.G. Chitosan oligosaccharides inhibit the expression of interleukin-6 in lipopolysaccharide-induced human umbilical vein endothelial cells through p38 and ERK1/2 protein kinases. Basic Clin. Pharmacol. Toxicol. 2010, 106, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Liu, H.T.; Wei, P.; Xu, Q.S.; Bai, X.F.; Du, Y.G.; Yu, C. Chitosan oligosaccharides inhibit LPS-induced over-expression of IL-6 and TNF-α in RAW264.7 macrophage cells through blockade of mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways. Carbohydr. Polym. 2011, 84, 1391–1398. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Xu, Q.S.; Du, Y.G.; Xu, J. Chitosan oligosaccharides block LPS-induced O-GlcNAcylation of NF-κB and endothelial inflammatory response. Carbohydr. Polym. 2014, 99, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Q.; Wei, P.; Cheng, L.; Peng, Q.; Li, S.; Yin, H.; Du, Y. Chitosan oligosaccharides downregulate the expression of E-selectin and ICAM-1 induced by LPS in endothelial cells by inhibiting MAP kinase signaling. Int. J. Mol. Med. 2014, 33, 392–400. [Google Scholar] [PubMed]
- Huang, J.; Wang, R.; Liu, X.; Zeng, X.; Wei, M. Sulfochitosan inhibits P-selectin-mediated HL-60 leukocyte adhesion under flow conditions. Cell. Mol. Biol. Lett. 2013, 18, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Deng, J.; Yu, X.; Xu, Q.; Wu, H.; Pan, J. Modulation of pro-inflammatory mediators in LPS-stimulated human periodontal ligament cells by chitosan and quaternized chitosan. Carbohydr. Polym. 2013, 92, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Fang, I.M.; Yang, C.H.; Yang, C.M. Chitosan oligosaccharides attenuate ocular inflammation in rats with experimental autoimmune anterior uveitis. Mediat. Inflamm. 2014, 2014, 827847. [Google Scholar]
- Fang, I.M.; Yang, C.M.; Yang, C.H. Chitosan oligosaccharides prevented retinal ischemia and reperfusion injury via reduced oxidative stress and inflammation in rats. Exp. Eye Res. 2015, 130, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.L.; Yao, H.T.; Cheng, R.S.; Chiang, M.T. Chitosan reduces plasma adipocytokines and lipid accumulation in liver and adipose tissues and ameliorates insulin resistance in diabetic rats. J. Med. Food 2012, 15, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Bak, S.S.; Kim, S.K. Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. Int. J. Biol. Macromol. 2011, 49, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, Y.; Wu, G.; Xiao, Z.; Zhou, H.; Yu, X. Inhibitory effects of oligochitosan on TNF-α, IL-1β and nitric oxide production in lipopolysaccharide-induced RAW264.7 cells. Mol. Med. Rep. 2015, 11, 729–733. [Google Scholar] [PubMed]
- Mohamed, M.M. Effects of chitosan and wheat bran on serum leptin, TNF-α, lipid profile and oxidative status in animal model of non-alcoholic fatty liver. Aust. J. Basic Appl. Sci. 2011, 5, 1478–1488. [Google Scholar]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Hong, S.H.; Yoo, S.J.; Baek, K.S.; Jeon, Y.J.; Choung, S.Y. Differential effects of chitooligosaccharides on serum cytokine levels in aged subjects. J. Med. Food 2006, 9, 427–430. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wang, J. Chitooligosaccharides prevent osteopenia by promoting bone formation and suppressing bone resorption in ovariectomised rats: Possible involvement of COX-2. Nat. Prod. Res. 2015, 29, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Spindola, H.; de Sousa, V.; Alice Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-inflammatory activity of chitooligosaccharides in Vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, F.; Kim, S.K. Antidiabetic activities of chitosan and its derivatives: A mini review. In Marine Carbohydrates: Fundamentals and Applications, Part B; Kim, S., Ed.; Elsevier Inc.: Oxford, UK, 2014; pp. 15–31. [Google Scholar]
- Ericson, U.; Sonestedt, E.; Gullberga, B.; Hellstrand, S.; Hindy, G.; Wirfält, E.; Orho-Melander, M. High intakes of protein and processed meat associate with increased incidence of type 2 diabetes. Br. J. Nutr. 2013, 109, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., III. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Saghizadeh, M.; Ong, J.M.; Garvey, W.T.; Henry, R.R.; Kern, P.A. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J. Clin. Investig. 1996, 97, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Usami, M.; Tsuura, Y.; Ishida, H.; Seino, Y. Hypoglycemic and hypolipidemic effect of chitosan in normal and neonatal streptozotocin-induced diabetic mice. Biol. Pharm. Bull. 1995, 18, 1623–1625. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ito, M. Antidiabetic action of low molecular weight chitosan in genetically obese diabetic KK-Ay mice. Biol. Pharm. Bull. 2002, 25, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Chang, Y.H.; Chiang, M.T. Chitosan reduces gluconeogenesis and increases glucose uptake in skeletal muscle in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2010, 58, 5795–5800. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ahn, H.Y.; Kwak, J.H.; Shin, D.Y.; Kwon, Y.I.; Oh, C.G.; Lee, J.H. The effects of chitosan oligosaccharide (GO2KA1) supplementation on glucose control in subjects with prediabetes. Food Funct. 2014, 5, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Ha, K.S.; Lee, J.W.; Kim, Y.C.; Apostolidis, E.; Kwon, Y.I. The reduction effect of low molecular weight chitosan oligosaccharide (GO2KA1) on postprandial blood glucose levels in healthy individuals. Food Sci. Biotechnol. 2014, 23, 971–973. [Google Scholar] [CrossRef]
- Kim, J.G.; Jo, S.H.; Ha, K.S.; Kim, S.C.; Kim, Y.C.; Apostolidis, E.; Kwon, Y.I. Effect of long-term supplementation of low molecular weight chitosan oligosaccharide (GO2KA1) on fasting blood glucose and HbA1c in db/db mice model and elucidation of mechanism of action. BMC Complement. Altern. Med. 2014, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.X.; Rangaswamy, S.; Shen, S.; Dave, R.; Chang, Y.H.; Peterson, H.; Hodis, H.N.; Chisolm, G.M.; Sevanian, A. Arterial injury by cholesterol oxidation products causes endothelial dysfunction and arterial wall cholesterol accumulation. Arterioscl. Throm. Vas. 1998, 18, 1885–1894. [Google Scholar] [CrossRef]
- Sevanian, A.; Hodis, H.N.; Hwang, J.; McLeod, L.L.; Peterson, H. Characterization of endothelial cell injury by cholesterol oxidation products found in oxidized LDL. J. Lipid Res. 1995, 36, 1971–1986. [Google Scholar] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–149. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, G.W.; Rye, K.A.; Gamble, J.R.; Vadas, M.A.; Barter, P.J. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Ausar, S.F.; Morcillo, M.; León, A.E.; Ribotta, P.D.; Masih, R.; Vilaro Mainero, M.; Amigone, J.L.; Rubin, G.; Lescano, C.; Castagna, L.F.; et al. Improvement of HDL- and LDL-cholesterol levels in diabetic subjects by feeding bread containing chitosan. J. Med. Food 2003, 6, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Wuolijoki, E.; Hirvela, T.; Ylitalo, P. Decrease in serum LDL cholesterol with microcrystalline chitosan. Methods Find. Exp. Clin. Pharmacol. 1999, 21, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.S.; Sheu, W.H.H.; Lee, W.J.; Yao, H.T.; Chiang, M.T. Effect of chitosan on plasma lipoprotein concentrations in type 2 diabetic subjects with hypercholesterolemia. Diabetes Care 2000, 23, 1703–1704. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Deuchi, K.; Imasato, Y.; Shizukuishi, M.; Kobayashi, E. Mechanism for the inhibition of fat digestion by chitosan and for the synergistic effect of ascorbate. Biosci. Biotechnol. Biochem. 1995, 59, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Giglio, R.V.; Nikolic, D.; Patti, A.M.; Campanella, C.; Cocchi, M.; Katsiki, N.; Montalto, G. Effects of chitosan on plasma lipids and lipoproteins: A 4-month prospective pilot study. Angiology 2013, 65, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.; Xia, W. Hypocholesterolaemic effects of different chitosan samples in vitro and in vivo. Food Chem. 2008, 107, 419–425. [Google Scholar] [CrossRef]
- Choi, C.R.; Kim, E.K.; Kim, Y.S.; Je, J.Y.; An, S.H.; Lee, J.D.; Wang, J.H.; Ki, S.S.; Jeon, B.T.; Moon, S.H.; et al. Chitooligosaccharides decreases plasma lipid levels in healthy men. Int. J. Food Sci. Nutr. 2012, 63, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hong, E.K.; Ahn, J.; Kwak, H.S. Properties of nanopowdered chitosan and its cholesterol lowering effect in rats. Food Sci. Biotechnol. 2010, 19, 1457–1462. [Google Scholar] [CrossRef]
- Chiang, M.T.; Yao, H.T.; Chen, H.C. Effect of dietary chitosans with different viscosity on plasma lipids and lipid peroxidation in rats fed on a diet enriched with cholesterol. Biosci. Biotechnol. Biochem. 2000, 64, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Bokura, H.; Kobayashi, S. Chitosan decreases total cholesterol in women: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2003, 57, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, W.; Mamadouba, B.; Xia, W. A comparative study on hypolipidemic activities of high and low molecular weight chitosan in rats. Int. J. Biol. Macromol. 2012, 51, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T.U.; Shamsuddin, S.M.; Khan, M.A.; Rahman, M.M. Evaluation of Fat Binding Capacity of Gamma Irradiated Chitosan Extracted from Prawn Shell. Soft Mater. 2014, 12, 262–267. [Google Scholar] [CrossRef]
- Patti, A.M.; Katsiki, N.; Nikolic, D.; Al-Rasadi, K.; Rizzo, M. Nutraceuticals in lipid-lowering treatment a narrative review on the role of chitosan. Angiology 2014. [Google Scholar] [CrossRef]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine 2010, 6, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Zhang, Y.; Han, S.; Chen, Y.; Li, B.; Liao, M.; Chen, W.; Deng, X.; Zhao, J.; Huang, B. Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2010, 400, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Muthunarayanan, M.; Divyarani, V.V.; Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Curcumin-loaded biocompatible thermoresponsive polymeric nanoparticles for cancer drug delivery. J. Colloid Interface Sci. 2011, 360, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Zaki, N. Progress and problems in nutraceuticals delivery. J. Bioequivalence Bioavailab. 2014, 6, 75–77. [Google Scholar] [CrossRef]
- Tang, H.; Murphy, C.J.; Zhang, B.; Shen, Y.; van Kirk, E.A.; Murdoch, W.J.; Radosz, M. Curcumin polymers as anticancer conjugates. Biomaterials 2010, 31, 7139–7149. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Prajakta, D.; Ratnesh, J.; Chandan, K.; Suresh, S.; Grace, S.; Meera, V.; Vandana, P. Curcumin loaded pH-sensitive nanoparticles for the treatment of colon cancer. J. Biomed. Nanotechnol. 2009, 5, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer 2009, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mazzarino, L.; Loch-Neckel, G.; Bubniak, L.D.S.; Mazzucco, S.; Santos-Silva, M.C.; Borsali, R.; Lemos-Senna, E. Curcumin-loaded chitosan-coated nanoparticles as a new approach for the local treatment of oral cavity cancer. J. Nanosci. Nanotechnol. 2015, 15, 781–791. [Google Scholar] [CrossRef]
- Mazzarino, L.; Borsali, R.; Lemos-Senna, E. Mucoadhesive films containing chitosan-coated nanoparticles: A new strategy for buccal curcumin release. J. Pharm. Sci. 2014, 103, 3764–3771. [Google Scholar] [CrossRef] [PubMed]
- Mazzarino, L.; Otsuka, I.; Halila, S.; Bubniak Ldos, S.; Mazzucco, S.; Santos-Silva, M.C.; Lemos-Senna, E.; Borsali, R. Xyloglucan-block-Poly(ϵ-Caprolactone) Copolymer Nanoparticles Coated with Chitosan as Biocompatible Mucoadhesive Drug Delivery System. Macromol. Biosci. 2014, 14, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Mazzarino, L.; Coche-Guérente, L.; Lemos-Senna, E.; Borsali, R. On the mucoadhesive properties of chitosan-coated polycaprolactone nanoparticles loaded with curcumin using quartz crystal microbalance with dissipation monitoring. J. Biomed. Nanotechnol. 2014, 10, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Adhami, V.M.; Siddiqui, I.A.; Bharali, D.J.; Mousa, S.A.; Mukhtar, H. Abstract 5438: Oral administration of naturally occurring chitosan based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. In Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research, Chicago, IL, USA, 31 March–4 April 2012; AACR: Philadelphia, PA, USA, 2012. [Google Scholar]
- De Pace, R.C.; Liu, X.; Sun, M.; Nie, S.; Zhang, J.; Cai, Q.; Gao, W.; Pan, X.; Fan, Z.; Wang, S.; et al. Anticancer activities of (−)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J. Liposome Res. 2013, 23, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Deepa, N.; Chennazhi, K.P.; Lakshmanan, V.K.; Jayakumar, R. Combinatorial anticancer effects of curcumin and 5-fluorouracil loaded thiolated chitosan nanoparticles towards colon cancer treatment. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 2730–2743. [Google Scholar] [CrossRef]
- Anitha, A.; Sreeranganathan, M.; Chennazhi, K.P.; Lakshmanan, V.K.; Jayakumar, R. In vitro combinatorial anticancer effects of 5-fluorouracil and curcumin loaded N,O-carboxymethyl chitosan nanoparticles toward colon cancer and in vivo pharmacokinetic studies. Eur. J. Pharm. Biopharm. 2014, 88, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M. Chitosan-based nanoparticles for tumor-targeted drug delivery. Int. J. Biol. Macromol. 2015, 72, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; T Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the plasma exposure of (−)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur. J. Pharm. Sci. 2011, 44, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ting, Y.; Yang, X.; Tang, W.; Zeng, X.; Huang, Q. Nanochemoprevention by encapsulation of (−)-epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability. Chem. Commun. 2012, 48, 2421–2423. [Google Scholar] [CrossRef]
- Tang, D.W.; Yu, S.H.; Ho, Y.C.; Huang, B.Q.; Tsai, G.J.; Hsieh, H.Y.; Sung, H.W.; Mi, F.L. Characterization of tea catechins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocolloids 2013, 30, 33–41. [Google Scholar] [CrossRef]
- Oehlke, K.; Adamiuk, M.; Behsnilian, B.; Gräf, V.; Mayer-Miebach, E.; Walz, E.; Greine, R. Potential bioavailability enhancement of bioactive compounds using food-grade engineered nanomaterials: A review of the existing evidence. Food Funct. 2014, 5, 1341–1359. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.M.; Dorkoosh, F.A.; Avadi, M.R.; Weinhold, M.; Bayat, A.; Delie, F.; Gurny, R.; Larijani, B.; Rafiee-Tehrani, M.; Junginger, H.E. Permeation enhancer effect of chitosan and chitosan derivatives: Comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur. J. Pharm. Biopharm. 2008, 70, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Vllasaliu, D.; Exposito-Harris, R.; Heras, A.; Casettari, L.; Garnett, M.; Illum, L.; Stolnik, S. Tight junction modulation by chitosan nanoparticles: Comparison with chitosan solution. Int. J. Pharm. 2010, 400, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ha, J.; Zou, T.; Gu, L. Fabrication of coated bovine serum albumin (BSA)–epigallocatechin gallate (EGCG) nanoparticles and their transport across monolayers of human intestinal epithelial Caco-2 cells. Food Funct. 2014, 5, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nie, S.; Wang, S. Nanoencapsulation Enhances Epigallocatechin-3-gallate Stability and Its Antiatherogenic Bioactivities in Macrophages. J. Agric. Food Chem. 2013, 61, 9200–9209. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Xu, Y.Q.; Yin, J.F.; Jin, J.; Du, Q. Improving the Effectiveness of (−)-Epigallocatechin Gallate (EGCG) against Rabbit Atherosclerosis by EGCG-Loaded Nanoparticles Prepared from Chitosan and Polyaspartic Acid. J. Agric. Food Chem. 2014, 62, 12603–12609. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cao, L.; Zhang, L.; Wan, X.C. Preparation, characterization, and in vitro antitumor activity of folate conjugated chitosan coated EGCG nanoparticles. Food Sci. Biotechnol. 2014, 23, 569–575. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Roggio, A.M.; Pala, N.; Marceddu, S.; Lubinu, G.; Mariani, A.; Sechi, M. Effect of chitosan concentration on PLGA microcapsules for controlled release and stability of resveratrol. Int. J. Biol. Macromol. 2015, 72, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2012, 8, 131–168. [Google Scholar]
- Bieschke, J. Natural compounds may open new routes to treatment of amyloid diseases. Neurotherapeutics 2013, 10, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Sung, M.J.; Seo, S.B.; Yoo, S.J.; Lim, W.K.; Kim, H.M. Water-soluble chitosan inhibits the production of pro-inflammatory cytokine in human astrocytoma cells activated by amyloid beta peptide and interleukin-1beta. Neurosci. Lett. 2002, 321, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Ma, P.; Xu, Q.S.; Bai, Q.H.; Gu, J.G.; Xi, H.; Du, Y.G.; Yu, C. Chitosan oligosaccharides suppress production of nitric oxide in lipopolysaccharide-induced N9 murine microglial cells in vitro. Glycoconjugate J. 2012, 29, 285–295. [Google Scholar] [CrossRef]
- Howlett, G.J.; Moore, K.J. Untangling the role of amyloid in atherosclerosis. Curr. Opin. Lipidol. 2006, 17, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Herczenik, E.; Gebbink, M.F.B.G. Molecular and cellular aspects of protein misfolding and disease. FASEB J. 2008, 22, 2115–2133. [Google Scholar] [CrossRef] [PubMed]
- Korporaal, S.J.; Gorter, G.; van Rijn, H.J.; Akkerman, J.W. Effect of oxidation on the platelet-activating properties of low-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Herczenik, E.; Bouma, B.; Korporaal, S.J.A.; Strangi, R.; Zeng, Q.; Gros, P.; van Eck, M.; van Berkel, T.J.C.; Gebbink, M.F.B.G.; Akkerman, J.W.N. Activation of Human Platelets by Misfolded Proteins. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Davies, K.J.; Maiorino, M.; Parasassi, T.; Sevanian, A. Atherosclerosis: Another protein misfolding disease? Trends Mol. Med. 2002, 8, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R.; Tyagi, S.C.; Kerklo, M.M.; Nicolls, M.R. Type 2 diabetes mellitus as a conformational disease. JOP 2005, 6, 287–302. [Google Scholar] [PubMed]
- Stewart, C.R.; Tseng, A.A.; Mok, Y.F.; Staples, M.K.; Schiesser, C.H.; Lawrence, L.J.; Varghese, J.N.; Moore, K.J.; Howlett, G.J. Oxidation of low-density lipoproteins induces amyloid-like structures that are recognized by macrophages. Biochemistry 2005, 44, 9108–9116. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.A.; Badellino, K. β-Amyloid protein induces platelet aggregation and supports platelet adhesion. Biochem Biophys. Res. Commun. 1994, 205, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Laske, C.; Sopova, K.; Stellos, K. Platelet activation in alzheimer’s disease: From pathophysiology to clinical value. Curr. Vasc. Pharmacol. 2012, 10, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Stubbs, C.D. Hydration at the membrane protein-lipid interface. Biophys. J. 1992, 63, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Kabashima, M.; Namura, H.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Furutani, N.; Tomida, H. Antioxidant protection of human serum albumin by chitosan. Int. J. Biol. Macromol. 2008, 43, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Prestrelski, S.J.; Kenney, W.C.; Carpenter, J.F. Factors affecting short-term and long-term stabilities of proteins. Adv. Drug Deliv. Rev. 1993, 10, 1–28. [Google Scholar] [CrossRef]
- Lahm, D.; Lee, L.K.; Bettelheim, F.A. Age dependence of freezable and nonfreezable water content of normal human lenses. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1162–1165. [Google Scholar]
- Kerch, G.; Rustichelli, F.; Ausili, P.; Zicans, J.; Merijs Meri, R.; Glonin, A. Effect of chitosan on physical and chemical processes during bread baking and staling. Eur. Food Res. Technol. 2008, 226, 1459–1464. [Google Scholar] [CrossRef]
- Kerch, G.; Zicans, J.; Merijs Meri, R.; Stunda-Ramava, A.; Jakobsons, E. The use of thermal analysis in assessing the effect of bound water content and substrate rigidity on prevention of platelet adhesion. J. Therm. Anal. Calorim. 2015, 120, 533–539. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P.; Prinyawiwatkul, W.; Xu, Z. Applications of chitosan for improvement of quality and shelf life of foods: A review. J. Food Sci. 2007, 72, R87–R100. [Google Scholar] [CrossRef] [PubMed]
- Park, I.K.; Lee, Y.K.; Kim, M.J.; Kim, S.D. Effect of surface treatment with chitosan on shelf-life of baguette. J. Chitin Chitosan 2002, 7, 208–213. [Google Scholar]
- Ahn, D.H.; Choi, J.S.; Lee, H.Y.; Kim, J.Y.; Youn, S.K.; Park, S.M. Effects on preservation and quality of bread with coating high molecular weight chitosan. Korean J. Food Nutr. 2003, 16, 430–436. [Google Scholar]
- Park, I.K.; Lee, Y.K.; Kim, M.J.; Kim, S.D. Effect of surface treatment with chito-oligosaccharide on shelf-life of baguette. J. Chitin Chitosan 2002, 7, 214–218. [Google Scholar]
- Lee, H.Y.; Kim, S.M.; Kim, J.Y.; Youn, S.K.; Choi, J.S.; Park, S.M.; Ahn, D.H. Effect of addition of chitosan on improvement for shelf-life of bread. J. Korean Soc. Food Sci. Nutr. 2002, 31, 445–450. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, Y.C. Effect of carboxymethyl chitosan on quality of fermented pan bread. Korean J. Food Sci. Technol. 1997, 29, 96–100. [Google Scholar]
- Kerch, G.; Zicans, J.; Merijs Meri, R. The effect of chitosan oligosaccharides on bread staling. J. Cereal Sci. 2010, 52, 491–495. [Google Scholar] [CrossRef]
- Knorr, D. Functional properties of chitin and chitosan. J. Food Sci. 1982, 47, 593–595. [Google Scholar] [CrossRef]
- Lafarga, T.; Gallagher, E.; Walsh, D.; Valverde, J.; Hayes, M. Chitosan-containing bread made using marine shellfishery byproducts: Functional, bioactive, and quality assessment of the end product. J. Agric. Food Chem. 2013, 61, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Fadda, C.; Sanguinetti, A.M.; Del Caro, A.; Collar, C.; Piga, A. Bread staling: updating the view. Compr. Rev. Food Sci. Food Saf. 2014, 13, 473–492. [Google Scholar] [CrossRef]
- Lafarga, T.; Hayes, M.; Valverde, J.; Walsh, D.; Gallagher, E. Prawn chitosan containing bread: assessment of functional, bioactive and sensory qualities. J. Chitin Chitosan Sci. 2013, 1, 150–156. [Google Scholar] [CrossRef]
- Kerch, G.; Glonin, A.; Zicans, J.; Merijs Meri, R. A DSC study of the effect of ascorbic acid on bound water content and distribution in chitosan-enriched bread rolls during storage. J. Therm. Anal. Calorim. 2012, 108, 73–78. [Google Scholar] [CrossRef]
- Rakcejeva, T.; Rusa, K.; Dukalska, L.; Kerch, G. Effect of chitosan and chitooligosaccharide lactate on free lipids and reducing sugars content and on wheat bread firming. Eur. Food Res. Technol. 2011, 232, 123–128. [Google Scholar] [CrossRef]
- Kerch, G.; Glonin, A.; Zicans, J.; Merijs Meri, R. A DSC study of the effect of bread making methods on bound water content and redistribution in chitosan enriched bread. J. Therm. Anal. Calorim. 2012, 108, 185–189. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.C. The physico-chemical and sensory properties of milk with water soluble chitosan. Korean J. Food Sci. Technol. 2000, 32, 806–813. [Google Scholar]
- El-Sisi, A.S. Impact of replacement of gelatin with chitosan on the physicochemical properties of ice-milk. Int. J. Dairy Sci. 2015, 10, 26–33. [Google Scholar]
- Kwak, H.S.; Mijan, M.A.; Ganesan, P. Application of Nanomaterials, Nano- and Microencapsulation to Milk and Dairy Products, in Nano- and Microencapsulation for Foods; Kwak, H.-S., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2014. [Google Scholar] [CrossRef]
- Zanjani, M.A.K.; Mohammadi, N.; Ahari, H.; Tarzi, B.G.; Bakhoda, H. Effect of microencapsulation with chitosan coating on survival of Lactobacillus casei and Bifidobacterium bifidum in ice cream. Iran. J. Nutr. Sci. Food. Technol. 2014, 8, 125–134. [Google Scholar]
- Eduardo, M.F.; Correa De Mello, K.G.P.; Polakiewicz, B.; Da Silva Lannes, S.C. Evaluation of chocolate milk beverage formulated with modified chitosan. J. Agric. Sci. Technol. 2014, 16, 1301–1312. [Google Scholar]
- Tasneem, M.; Siddique, F.; Ahmad, A.; Farooq, U. Stabilizers: Indispensable substances in dairy products of high rheology. Crit. Rev. Food Sci. Nutr. 2014, 54, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Zagorska, J.; Pelnik, A.; Kerch, G. Effect of the addition of chitosans with different molecular structure on fermentation process and viscosity changes during sour cream storage. Biochem. Biophys. (BAB) 2013, 1, 13–21. [Google Scholar]
- Rajapaksha, D.S.W.; Kodithuwakku, K.A.H.T. Evaluation of chitosan for its inhibitory activity on post-acidification of set yoghurt under cold storage for 20 days. J. Chitin Chitosan 2014, 2, 16–20. [Google Scholar] [CrossRef]
- Seo, M.H.; Chang, Y.H.; Lee, S.; Kwak, H.S. The physicochemical and sensory properties of milk supplemented with ascorbic acid-soluble nano-chitosan during storage. Int. J. Dairy Technol. 2011, 64, 57–63. [Google Scholar] [CrossRef]
- Choi, H.J.; Ahn, J.; Kim, N.C.; Kwak, H.S. The Effects of Microencapsulated chitooligosaccharide on physical and sensory properties of the milk. Asian Aust. J. Anim. Sci. 2006, 19, 1347–1353. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H.C. Survival of probiotics encapsulated in chitosan-coated alginate beads in yoghurt from UHT-and conventionally treated milk during storage. LWT-Food Sci. Technol. 2006, 39, 177–183. [Google Scholar] [CrossRef]
- Seo, M.H.; Lee, S.Y.; Chang, Y.H.; Kwak, H.S. Physicochemical, microbial, and sensory properties of yogurt supplemented with nanopowdered chitosan during storage. J. Dairy Sci. 2009, 92, 5907–5916. [Google Scholar] [CrossRef] [PubMed]
- Altieri, C.; Scrocco, C.; Sinigaglia, M.; del Nobile, M.A. Use of chitosan to prolong mozzarella cheese shelf life. J. Dairy Sci. 2005, 88, 2683–2688. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerch, G. The Potential of Chitosan and Its Derivatives in Prevention and Treatment of Age-Related Diseases. Mar. Drugs 2015, 13, 2158-2182. https://doi.org/10.3390/md13042158
Kerch G. The Potential of Chitosan and Its Derivatives in Prevention and Treatment of Age-Related Diseases. Marine Drugs. 2015; 13(4):2158-2182. https://doi.org/10.3390/md13042158
Chicago/Turabian StyleKerch, Garry. 2015. "The Potential of Chitosan and Its Derivatives in Prevention and Treatment of Age-Related Diseases" Marine Drugs 13, no. 4: 2158-2182. https://doi.org/10.3390/md13042158
APA StyleKerch, G. (2015). The Potential of Chitosan and Its Derivatives in Prevention and Treatment of Age-Related Diseases. Marine Drugs, 13(4), 2158-2182. https://doi.org/10.3390/md13042158




