1. Introduction
Marine sponges (Porifera) are primitive filter-feeders, yet they represent the richest and best studied sources of novel marine bioactive natural products [
1]. Furthermore, sponges are considered to be producers of the highest sterol diversity amongst all animal phyla, presenting molecules with unique functionalization and structures, many with no terrestrial analogues [
1,
2,
3]. The role of sterols in sponges is primarily functional, with sterols as constituents of cell membranes, and a secondary role as metabolic precursors for the production of diverse steroid classes [
4,
5,
6]. Highly functionalized steroids are a growing group of metabolites that exhibit interesting biological and pharmacological properties, including ichthyotoxic, antihistaminic, cytotoxic, and antiviral activities [
4,
5].
Polymastia boletiformis (Lamarck, 1814, family Polymastiidae, order Hadromerida), is a brightly coloured orange-yellow fistulose sponge that grows on upper rock faces in the sublittoral zone and is widespread on the coasts of Britain and Ireland. Although not extensively studied, the genus
Polymastia has been a source of various new fatty acids [
7], carotenoids [
8], and steroid compounds [
9,
10], including the antibiotic polymastiamides A–F [
11,
12]. Polymastiamides are the first examples of natural steroids with a side chain containing an amide bond linking the steroid part to a non-proteinaceous amino acid.
There has been a considerable increase in the frequency of fungal infections during the past decades.
Cladosporium cucumerinum has been known as an important phytopathogen that causes scab disease in many commercial vegetables, resulting in significant losses [
13].
Candida albicans, one of the most commonly encountered human pathogens, causes a variety of difficult-to-cure mucosal, skin, and systemic infections [
14]. The declining number and efficacy of existing antifungal agents, the increased occurrence of systemic fungal infections, and rapid emergence of drug resistance underline the necessity for the discovery of new antifungal drug leads.
In the course of our ongoing investigations within the Beaufort Marine Biodiscovery Research program aimed at identification of novel bioactive metabolites from Irish marine resources [
15], we have undertaken a chemical study of the Irish marine sponge
P. boletiformis, as it displayed antifungal activity in initial screening studies. This report describes the bioactivity-guided isolation and structure elucidation of two new, minor sulfated steroid-amino acid conjugates,
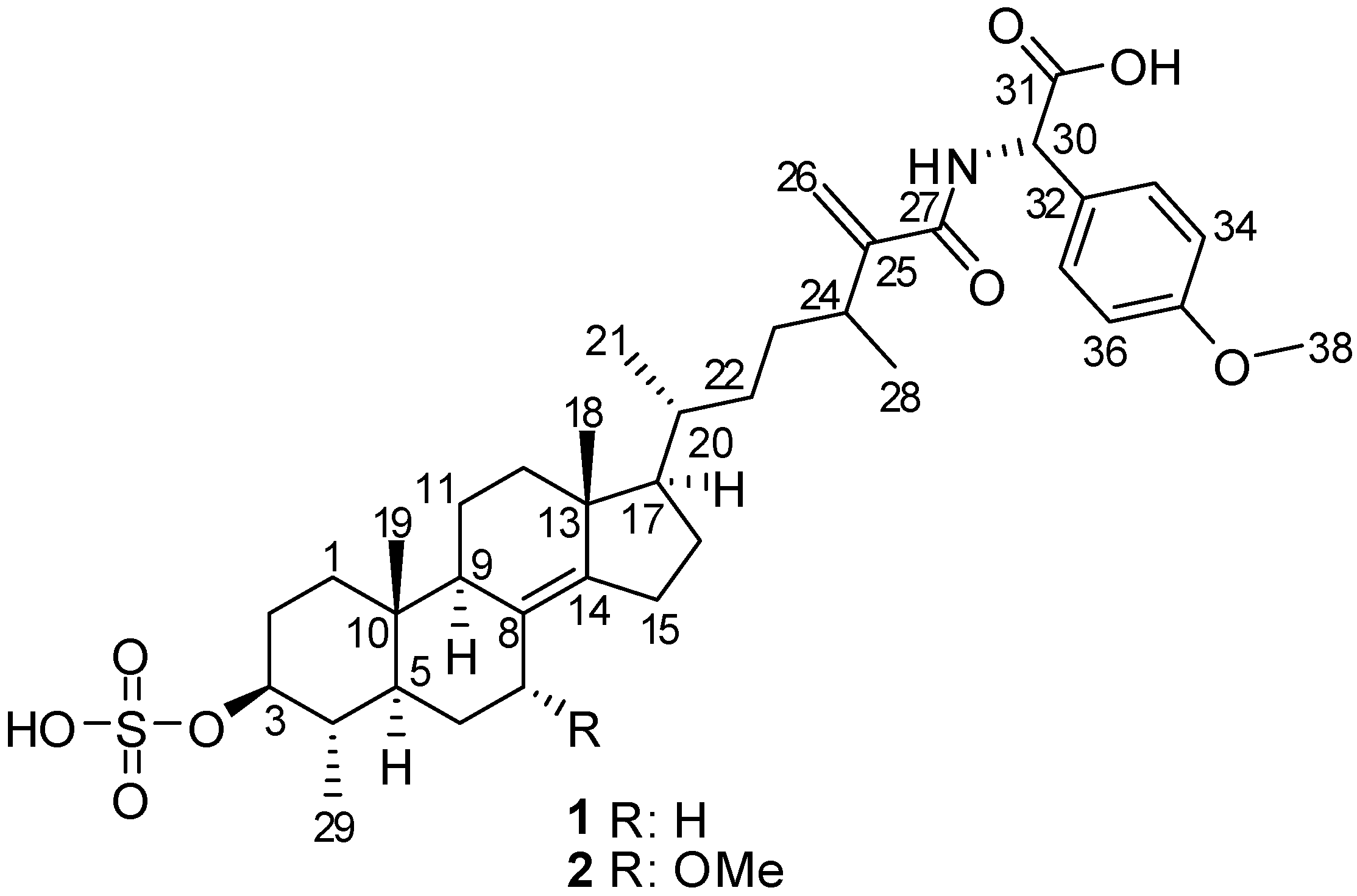
1, and its 7-methoxy derivative,
2 (
Figure 1). Both compounds feature linkage of the non-proteinaceous α-amino acid
p-methoxyphenylglycine with 4,24-dimethyl-3-
O-sulfocholest-8(14),25(26)-dien-27-oic acid, via an amide bond. The molecular structures of the natural products were established on the basis of 1D and 2D NMR, IR, UV, HRMS, and experimental and calculated ECD, optical rotation, and
13C chemical shift data. The isolated metabolites were evaluated for their antifungal activity and were found to exhibit moderate antifungal activity against
C. cucumerinum, while compound
2 was also active against
C. albicans.
Figure 1.
Structures of compounds 1 and 2.
Figure 1.
Structures of compounds 1 and 2.
2. Results and Discussion
P. boletiformis specimens were collected by SCUBA from Roskeeda, Co. Galway (Ireland) and the CH2Cl2/MeOH extract of the freeze-dried sponge was subjected to a modified Kupchan partitioning scheme to yield n-hexane, CHCl3, and aqueous MeOH subextracts. Bioassay-guided separation of the antifungal aqueous MeOH-soluble fraction, which included a combination of C18 reversed-phase flash column chromatography (RP-FCC), Sephadex LH-20 size-exclusion chromatography, and repeated C18 reversed-phase (RP) HPLC purifications, yielded compounds 1 and 2.
Metabolite
1 was obtained as a white amorphous powder with
+45.9 (
c 0.03, MeOH). NMR data combined with the [M − H]
− ion at
m/z 684.3570 in the HRESIMS of
1 suggested a molecular formula of C
38H
55NO
8S (calcd for C
38H
54NO
8S, 684.3576; Δ 0.75 ppm) requiring twelve indices of hydrogen deficiency. The structural characterization of compound
1 was established on the basis of extensive 1D and 2D NMR studies of data acquired in both DMSO-
d6 and methanol-
d4 (
Table 1 and
Supplementary Table S1). In DMSO-
d6, both
1H and
13C-NMR spectra showed resonances typical of a steroid, displaying shielded resonances for two tertiary methyl groups (δ
H 0.64, δ
C 13.7; δ
H 0.78, δ
C 18.1) and three secondary methyls (δ
H 0.86, δ
C 19.0; δ
H 0.87, δ
C 15.5; δ
H 0.99, δ
C 19.7). Additional resonances accounting for one oxygenated methine (δ
H 3.53, δ
C 80.1), a tetrasubstituted double bond (δ
C 125.9, 141.4), a terminal olefinic methylene (δ
H 5.53 and 5.17, δ
C 113.9 and δ
C 151.4), a
p-disubstituted benzene moiety (δ
H 6.74, δ
C 112.7; δ
H 7.18, δ
C 127.5; δ
C 134.9; δ
C 157.5), two carbonyl groups (δ
C 166.7 and δ
C 170.4), and one aromatic methoxy functionality (δ
H 3.67, δ
C 55.0) suggested that
1 was a steroid with an atypical, modified side chain. These functional groups also accounted for eight indices of hydrogen deficiency, with the remaining four assembling a typical four-ring steroid core. Furthermore,
1H and
13C-NMR data of
1 (
Table 1) suggested the presence of a sulfate group based on deshielded resonances of the H-3/C-3 oxygenated methine (δ
H 3.53, δ
C 80.1) relative to a typical hydroxylated methine [
16,
17]. This was also supported by strong absorptions at 1214 and 1055 cm
−1, characteristic of a sulfate functional group, present in the IR spectrum. An amide group was also evident due to the appearance of the resonance for a labile NH (δ
H 7.78) function in DMSO-
d6, in addition to the broad IR absorption band centred at 3416 cm
−1 (OH, NH stretch), and the intense bands observed at 1591 and 1548 cm
−1. Analysis of the COSY and HMBC data led to the assignment of the ABCD steroid ring system in
1 (
Figure 2). The COSY cross peaks between all neighbouring protons from H
2-1 to H-7 assisted the assignment of rings A and B. HMBC correlations of angular methyl protons H
3-19 with C-1, C-5, C-9, and C-10; of H-6α, Η-7β, and H-9 with C-8; of H-7β with C-9, along with the cross peaks of H-6β and H-9 with C-10; and of H-1β, H-4, Η-6β, H-7β, and H
3-29 with C-5, validated the structure of the decalin AB rings. From the COSY spectrum it was possible to differentiate two discrete spin systems in the C and D rings; H-9/H
2-11/H
2-12 and H
2-15/H
2-16. HMBC correlations from the H
3-18 methyl singlet to C-12, C-13, C-14, and C-17 and from C-13 to H-11β, Η-15β, Η-16α, and H-17 established the connectivity of C-12 and C-17 through the quaternary C-13. Diagnostic HMBC cross peaks between the olefinic C-14 and H-7α, H-9, H-12β, H
2-15, and H
2-16, along with correlations between C-8 and H-9 and H-15α assembled the ABCD steroidal rings. The COSY spectrum revealed useful information concerning the side chain. COSY correlations among all adjacent protons from C-17 to C-24, along with the long range coupling of methine H-24 with the sp
2 methylene H-26b established the connection of spin system (i) C
H17-C
H20(C
H321)-C
H222-C
H223-C
H24(C
H328)-C25(C
H226) of the side chain. Two more spin systems were evident within the side chain: (ii) N
H-C
H30; and (iii) C
H33/37-C
H34/36. Fragments (i) and (ii) could be connected on the basis of the HMBC correlations between C-27 and both terminal olefinic methylenes H
2-26, as well as those between the amide proton NH and C-27, C-30, and C-31. The cross peaks in the HMBC spectrum between H-30 and C-31 clearly established the position of the carboxylic acid group at C-30. Complementary HMBC correlations between C-30 and H-33/37 and between H-30 and the aromatic carbons C-32 and C-33/37 validated the attachment of the
p-disubstituted benzene moiety at the end of the side chain, thus connecting fragments (i), (ii), and (iii). Long-range heteronuclear couplings between H
3-38 and C-35 secured the position of the methoxy group in the benzene ring and further established the gross structure for metabolite
1. The amide linkage connecting the steroid side chain and
d-cysteinolic acid in carolisterols A–C from the starfish
Styracaster caroli is another rare example of marine natural products containing a steroidal and an amino acid component [
18].
Table 1.
1H (600 MHz) and 13C (150 MHz) NMR chemical shifts of compound 1 and polymastiamide A (polyA) 7a in DMSO-d6, δ in ppm, J values in Hz.
Table 1.
1H (600 MHz) and 13C (150 MHz) NMR chemical shifts of compound 1 and polymastiamide A (polyA) 7a in DMSO-d6, δ in ppm, J values in Hz.
| No. | 1H (δ) m (J) | 13C (δ) m |
|---|
| 1 | polyA | δΗ(1) − δΗ(polyA) | 1 | polyA | δC(1) − δC(polyA) |
|---|
| 1 | 1.59 m
1.03 m | 1.60 m
1.03 m | –0.01
- | 35.8 t | 35.8 t | - |
| 2 | 2.10 m
1.28 m | 2.10 m
1.28 m | -
- | 28.2 t | 28.1 t | +0.1 |
| 3 | 3.53 ddd 10.9, 10.9, 4.4 | 3.53 m | - | 80.1 d | 80.1 d | - |
| 4 | 1.21 m | 1.21 m | - | 37.2 d | 37.1 d | +0.1 |
| 5 | 0.87 m | 0.89 m | −0.02 | 50.7 d | 50.7 d | - |
| 6 | 1.65 m
0.90 m | 1.64 m
0.87 m | +0.01
+0.03 | 24.7 t | 24.7 t | - |
| 7 | 2.32 br. dd 12.6, 3.7
1.65 d | 2.32 m
1.63 m | -
+0.02 | 29.2 t | 29.2 t | - |
| 8 | - | - | - | 125.9 s | 125.8 s | +0.1 |
| 9 | 1.61 m | 1.60 m | +0.01 | 48.7 d | 48.7 d | - |
| 10 | - | - | | 36.9 s | 36.8 s | +0.1 |
| 11 | 1.55 m
1.41 m | 1.55 m
1.40 m | -
+0.01 | 19.5 t | 19.5 t | - |
| 12 | 1.87 ddd 12.2, 3.2, 3.2
1.04 m | 1.85 m
1.85 m | +0.02
−0.81 | 36.9 t | 36.9 t | - |
| 13 | - | - | - | 42.2 s | 42.1 s | +0.1 |
| 14 | - | - | - | 141.4 s | 141.4 s | - |
| 15 | 2.18 br. dd 16.7, 10.2
2.11 m | 2.16 m
2.08 m | +0.02
+0.03 | 25.3 t | 25.3 t | - |
| 16 | 1.71 dddd 13.1, 9.6, 7.3, 2.3
1.28 m | 1.69 m
1.25 m | +0.02
+0.03 | 26.6 t | 26.5 t | +0.1 |
| 17 | 1.02 m | 0.97 m | +0.05 | 56.3 d | 56.3 d | - |
| 18 | 0.78 s | 0.76 s | +0.02 | 18.1 q | 18.0 q | +0.1 |
| 19 | 0.64 s | 0.63 s | +0.01 | 13.7 q | 13.6 q | +0.1 |
| 20 | 1.37 m | 1.34 m | +0.03 | 34.1 d | 33.9 d | +0.2 |
| 21 | 0.86 d 6.6 | 0.83 d 6.5 | +0.03 | 19.0 q | 18.9 q | +0.1 |
| 22 | 1.33 m
0.98 m | 1.32 m
0.96 m | +0.01
+0.02 | 32.5 t | 32.5 t | - |
| 23 | 1.48 dddd 12.4, 12.0, 6.7, 4.3
1.15 dddd 12.0, 10.6, 6.6, 4.8 | 1.46 m
1.13 m | +0.02
+0.02 | 31.3 t | 31.3 t | - |
| 24 | 2.50 m | 2.54 m | +0.04 | 35.0 d | 34.9 d | +0.1 |
| 25 | - | - | - | 151.4 s | 149.5 s | +1.9 |
| 26 | 5.53 s
5.17 s | 5.61 s
5.21 s | −0.08
−0.04 | 113.9 t | 115.8 t | −1.9 |
| 27 | - | - | - | 166.7 s | 168.5 s | −1.8 |
| 28 | 0.99 d 6.9 | 0.98 d 6.8 | +0.01 | 19.7 q | 19.6 q | +0.1 |
| 29 | 0.87 d 6.3 | 0.87 d 6.2 | - | 15.5 q | 15.5 q | - |
| 30 | 4.60 d 5.8 | 5.35 d 7.6 | −0.75 | 58.2 d | 55.6 d | +2.6 |
| 31 | - | - | - | 170.4 s | 172.2 s | −1.8 |
| 32 | - | - | - | 134.9 s | 129.3 s | +5.6 |
| 33 | 7.18 d 8.6 | 7.31 d 8.7 | −0.13 | 127.5 d | 129.1 d | −1.6 |
| 34 | 6.74 d 8.6 | 6.88 d 8.7 | −0.14 | 112.7 d | 113.6 d | −0.9 |
| 35 | - | - | - | 157.5 s | 158.8 s | −1.3 |
| 36 | 6.74 d 8.6 | 6.88 d 8.7 | −0.14 | 112.7 d | 113.6 d | −0.9 |
| 37 | 7.18 d 8.6 | 7.31 d 8.7 | −0.13 | 127.5 d | 129.1 d | −1.6 |
| 38 | 3.67 s | 3.72 s | −0.05 | 55.0 q | 55.1 q | −0.1 |
| NH | 7.78 d 5.8 | 8.48 d 7.5 | −0.70 | - | - | |
Figure 2.
Key HMBC (arrows) and COSY (bold line) correlations observed in 1.
Figure 2.
Key HMBC (arrows) and COSY (bold line) correlations observed in 1.
The relative configurations of the steroidal ring stereogenic centres of
1 were deduced from NOE studies and
J values in the
1H NMR spectra. The NOESY data indicated that
1 contained the common 5α/10β/9α/13β steroid nucleus (
Figure 3). Key NOE correlations of angular methyl protons H
3-19 with Η-4, as well as cross peaks of H-3 with Η-5 and H
3-29, and of H-5 with H-9 established the
trans-fusion of the AB decalin ring system, indicating that H-4 and H
3-19 were cofacial and β-oriented, while H
3-29, H-3, H-5, and H-9 were cofacially α-oriented. H-3 was designated axial based on a large vicinal coupling constant (
J = 10.9 Hz), and the NOE observed between H-3 and H-5. The NOE correlations from Η
3-18 to both H
3-19 and H-20, and an additional cross peak between H-17 and H-21 established the relative configurations of the remaining stereocentres of rings C and D, and the β-orientation of the side chain. However, the NOESY data did not allow definition of the relative configurations of the C-24 and C-30 stereogenic centres of the freely rotating side chain.
Comparison of the gross NMR data of
1 with those reported for polymastiamide A obtained from a
P. boletiformis specimen from Norway by Kong and Anderson in 1993 [
11] indicated similar planar structures. The NMR data in DMSO-
d6 (
Table 1) displayed similar
1H and
13C chemical shifts, for all carbons and protons in the rigid steroid portion (ABCD rings). Notable differences were observed in the chemical shifts of the carbons and protons in the proximity of the chiral environment of C-24 (C-24 to C-27) and C-30 (especially C-30 to C-32). Both
1H and
13C-NMR chemical shift values of
1 were solvent dependent (
Table 1,
Supplementary Table S1). For example, in methanol-
d4, H-30 appeared at δ
H 5.25 in
1, comparable to that of polymastiamide A (δ
H 5.35) in DMSO-
d6. The above-mentioned data pointed out the necessity for a detailed investigation of the absolute configuration at stereocentres C-24 and C-30.
Figure 3.
Relative configurations and key NOESY correlations observed within the steroid portion of 1 and 2.
Figure 3.
Relative configurations and key NOESY correlations observed within the steroid portion of 1 and 2.
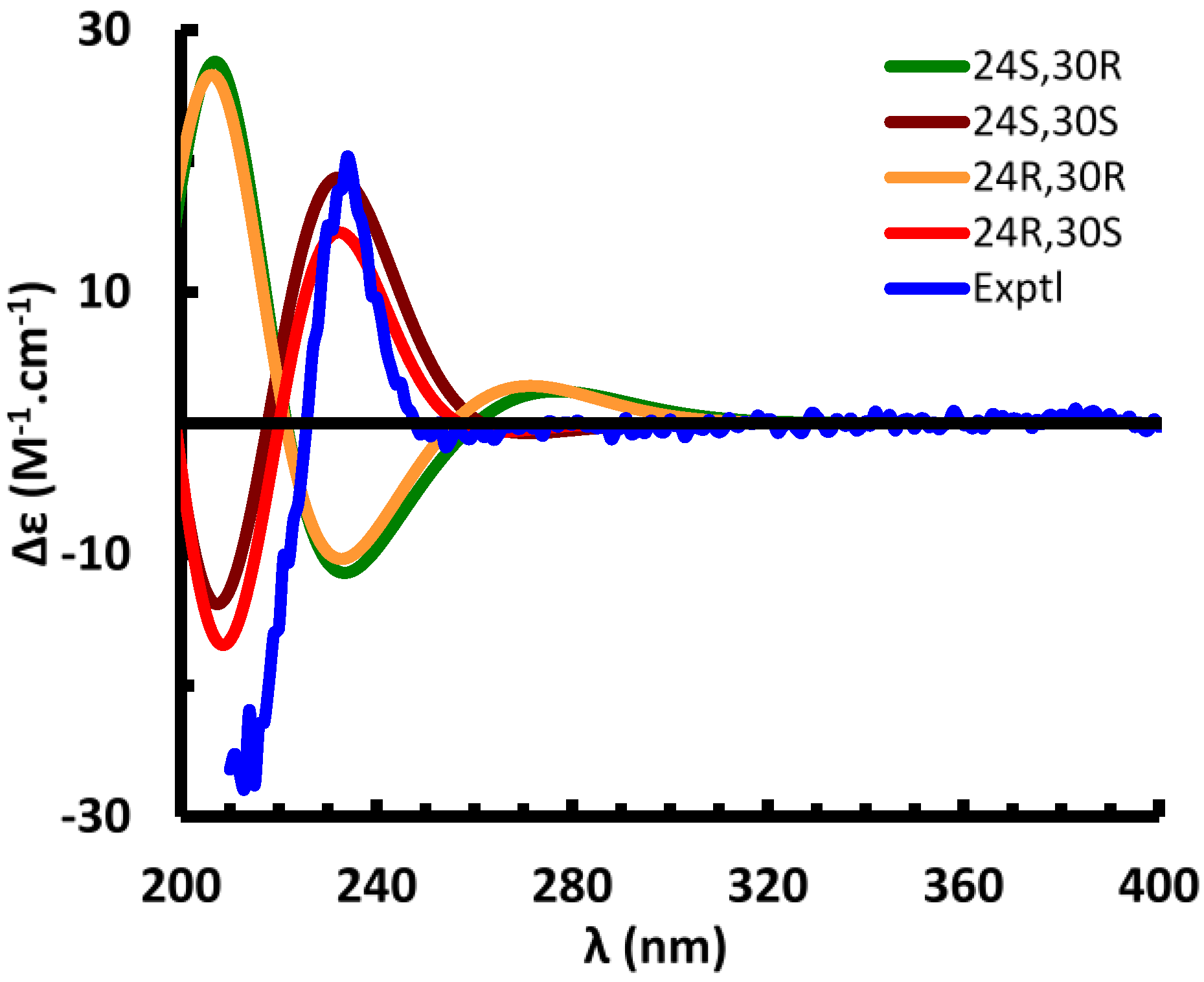
Suitable crystals of
1 could not be obtained. The absolute configuration of the side chain was therefore investigated by comparing the experimental electronic circular dichroism (ECD) spectrum of
1 to the ECD spectra predicted from quantum mechanical calculations for the four possible diastereomers. The ECD spectra [
19,
20,
21,
22] were simulated to assign the absolute configuration (AC) of
1 by using the Gaussian 09 software packages [
23]. The configurations of the 4-methoxyphenylglycine moiety and C-24 were assigned as
S- or
R-, denoted as (24
R,30
R)-, (24
R,30
S)-, (24
S,30
R)-, and (24
S,30
S)-
1, respectively. The OPLS-2005 force field in MacroModel [
24] was employed to perform the conformational random search with an energy window of 130 kJ/mol, and yielded a total of 591 conformers for (24
S,30
R)-
1 and 546 conformers for (24
S,30
S)-
1 (
Supplementary Figure S1). Fourteen of the (24
S,30
S)-
1 (energy cut-off, 20 kJ/mol) and 44 of the (24
S,30
R)-
1 conformers (energy cut-off, 15 kJ/mol) were included for the geometric optimization, followed by harmonic vibrational frequency computation to confirm these as energy minima. Nine of the (24
S,30
S)-
1 conformers were relocated at the B3LYP/6-31G** level in the gas phase, among which conformer 7 (
Supplementary Table S3 and Figure S2) was calculated to dominantly occupy 97.5% of the conformational itinerary. The ECD spectrum of the predominant conformer was simulated at the B3LYP/6-31G** level in the gas phase and matched very well with the experimental spectrum (
Figure 4). Conformer 1 of (24
S,30
R)
-1 occupies 96.7% of the conformational itinerary and its computed ECD spectrum was opposite to the experimental spectrum (
Figure 4). Optimized geometries of the predominant conformers of (24
S,30
S)- and (24
S,30
R)-
1 show strong intramolecular hydrogen bonding between the hydroxy group of the carboxylic moiety and the C-3 sulfate group. The distance of (C3-O-SO
2-)O…H(-O-C31) was optimized as 1.519 and 1.469 Å for (24
S,30
S)- and (24
S,30
R)
-1, respectively (
Supplementary Figure S2). The C25-C27(O)-N-C30-C31(O)-O-H…O-S-O “backbone” in (24
S,30
S)-
1 is coplanar, thus delocalized (π
1), with the delocalized 4-methoxyphenyl group (π
2) perpendicular to the plane. Molecular orbital analysis of (24
S,30
S)-
1 indicated the experimentally observed positive Cotton effect (CE) at 234 nm corresponds to the electronic transitions at 230.9 and 230.2 nm (
Supplementary Table S4) from Orb182 (π
C-8=C-14,
Supplementary Figure S3) to Orb187 (π*
2), and from Orb183 (π
2) to Orb188 (π*
2). The negative CE at 213 nm results predominantly from the transition at 201 nm from Orb182 (π
C-8=C-14) to Orb189 (π*
C-8=C-14), and the transitions at 216 and 213 nm from Orb180 to Orb187, and from Orb181 to Orb188. The similar CEs result from the electronic transitions between π
1, π
2, π
C-8=C-14, and the unoccupied orbitals, but did not permit definition of the absolute configuration at C-24. This is confirmed by the finding that the calculated ECD spectra of (24
R,30
S)- and (24
R,30
R)-
1 are similar to those of (24
S,30
S)- and (24
S,30
R)-
1, respectively (
Figure 4). An attempt to differentiate these pairs by calculation of their specific rotations was also unsuccessful. The calculated specific rotations of (24
S,30
R)- and (24
R,30
R)-
1 are −62.9 and −73.5 at the B3LYP/6-311++G**//B3LYP/6-31G** level in the gas phase, and those of (24
R,30
S)- and (24
S,30
S)-
1 are 96.4 and 96.2, respectively [
25].
Figure 4.
Experimental and calculated electronic circular dichroism (ECD) spectra of compound 1.
Figure 4.
Experimental and calculated electronic circular dichroism (ECD) spectra of compound 1.
The comparison of quantum chemical calculated
13C NMR chemical shifts with the experimental data was considered as a useful tool to assist the assignment of absolute configuration of chiral molecules [
26]. However, due to the large size of compound
1, the computation of
13C NMR shielding constants of the above conformers failed when using the GIAO technique at the mPW1PW91-SCRF (PCM, MeOH)/6-311++G** level of theory [
27]. The calculated
13C NMR chemical shifts of (24
R,30
S)
-1 and (24
S,30
S)
-1 at the B3LYP/6-31G** level in the gas phase show very high similarity, differing less than 2.4 ppm and roughly match the experimental data (
Supplementary Table S5). Even though the calculated chemical shifts of C-28 differ by 4.7 ppm, both match the experimental value. The noticeable difference is that the calculated chemical shifts of C-24 differ by 5.3 ppm, whereas that in (24
S,30
S)
-1 was calculated as 43.1 ppm that was closer to the experimental value of 35.0 ppm than that in (24
R,30
S)
-1 which was computed as 48.4 ppm, implying that most likely the absolute configuration at C-24 is (24
S).
In summary, the ECD study provided unambiguous confirmation of the (30
S) absolute configuration, but could not differentiate between (24
R) and (24
S) configurations. Calculation of the specific rotations of the four diastereomers similarly failed to differentiate the (24
R) and (24
S) configurations. The (30
S)-configuration matches with the reported assignment for polymastiamide A that resulted from hydrolysis of the amino acid residue (
p-hydroxyphenylglycine) from the desulfated methyl ester of the compound by Marfey’s method [
11]. The
l-configuration of the amino acid residue in polymastiamide A dictated (30
S) configuration at C-30 while the configuration of C-24 remained unidentified [
11]. Compound
1 and polymastiamide A have similar specific rotations (
+45.9 for
1, (
+67.4 for polymastiamide A), both acquired in MeOH. Based on these data we conclude that
1 and polymastiamide A differ at the C-24 stereogenic centre; however, the C-24 absolute configuration remains undefined in both compounds.
The molecular formula for the optically active (
+33.8,
c 0.04, MeOH) metabolite
2, C
39H
57NO
9S, was derived from NMR data and the HRESIMS ion at
m/z 714.3674 ([M − H]
−, calcd for C
39H
56NO
9S, 714.3681; Δ 1.07 ppm). Comparison of the molecular formula of
2 with that of
1 revealed that
2 contained a methoxymethine rather than a methylene functionality. The
1H and
13C-NMR resonances in both DMSO-
d6 and methanol-
d4, fully assigned through COSY, HSQC, and HMBC experiments (
Table 2 and
Supplementary Table S2) indicated that the only difference between the two molecules was the presence of a C-7 methoxy group in
2 (δ
H 3.94, δ
C 73.3). The COSY couplings from H-7 to both H-6α and H-6β, and the HMBC correlations from H-7 to C-5, C-6, C-9 and C-14, and from C-7 to H-6α, H-9 and C7-OMe confirmed the position of the methoxy group at C-7. Once the planar structure of
2 was defined, its relative configuration was established on the basis of 2D NMR NOESY to be the same as that of
1 (
Figure 3). The NOESY correlations between H-7 and H-6β, between H-6β and H
3-19, and between C7-OCH
3 and H-5 and H-9 confirmed the α-axial orientation of the methoxy group. The similarity of the experimental ECD spectra of compounds
1 and
2 indicates they have the same absolute configurations for all stereogenic centres, with the exception of the (7
R)-configuration of
2.
Table 2.
1H (600 MHz) and 13C (150 MHz) NMR chemical shifts of compound 2 in DMSO-d6, δ in ppm, J values in Hz.
Table 2.
1H (600 MHz) and 13C (150 MHz) NMR chemical shifts of compound 2 in DMSO-d6, δ in ppm, J values in Hz.
| No. | 1H (δ) m (J) | 13C (δ) m |
|---|
| 1 | 1.56 m
1.02 m | 35.6 t |
| 2 | 2.09 dm 12.2
1.28 m | 28.1 t |
| 3 | 3.50 ddd 11.2, 10.0, 4.8 | 80.2 d |
| 4 | 1.20 m | 36.8 d |
| 5 | 1.22 m | 44.1 d |
| 6 | 1.83 m
1.07 m | 30.4 t |
| 7 | 3.94 br. s | 73.3 d |
| 8 | - | 124.5 s |
| 9 | 1.87 m | 43.4 d |
| 10 | - | 37.1 s |
| 11 | 1.56 m
1.36 m | 19.8 t |
| 12 | 1.89 m
1.04 m | 36.6 t |
| 13 | - | 42.8 s |
| 14 | - | 147.9 s |
| 15 | 2.37 ddd 17.6, 9.5, 8.7
2.21 br. dd 17.6, 12.4 | 25.0 t |
| 16 | 1.76 m
1.31 m | 26.4 t |
| 17 | 1.05 m | 56.6 d |
| 18 | 0.80 s | 17.4 q |
| 19 | 0.63 s | 12.9 q |
| 20 | 1.38 m | 34.1 d |
| 21 | 0.89 d 6.4 | 19.0 q |
| 22 | 1.36 m
0.97 m | 32.5 t |
| 23 | 1.49 m
1.17 m | 31.5 t |
| 24 | 2.50 m | 35.0 d |
| 25 | - | 151.3 s |
| 26 | 5.53 s
5.17 br. s | 114.0 t |
| 27 | - | 166.7 s |
| 28 | 1.00 d 6.9 | 19.0 q |
| 29 | 0.84 d 5.8 | 15.4 q |
| 30 | 4.57 d 5.0 | 58.2 d |
| 31 | - | 170.5 s |
| 32 | - | 134.9 s |
| 33 | 7.18 d 8.4 | 127.5 d |
| 34 | 6.74 d 8.4 | 112.7 d |
| 35 | - | 157.5 s |
| 36 | 6.74 d 8.4 | 112.7 d |
| 37 | 7.18 d 8.4 | 127.5 d |
| 38 | 3.69 s | 55.0 q |
| 39 | 3.03 s | 53.5 q |
| NH | 7.78 d 5.0 | - |
Compounds
1 and
2 were evaluated for their antifungal activity against
Cladosporium cucumerinum and
Candida albicans, and for their antibacterial activity against
Staphylococcus aureus. The assays demonstrated that
1 and
2 showed moderate inhibitory effects on
C. cucumerinum at 60 and 30 μg/disc, respectively. The observed diameters of their inhibition zones were 8.0 and 10.1 mm, respectively. Compound
2 was also found to possess significant activity at 100 μg/disc against
C. albicans, displaying an inhibition zone diameter of 9.8 mm. The diameters of inhibition zones of the positive controls used in the assays, CuSO
4 and nystatin, were 16.8 and 25.8 mm, respectively. No significant activity was demonstrated against
S. aureus. Polymastiamide A was reported by Kong
et al., to be active against both human pathogens
S. aureus and
C. albicans [
11].