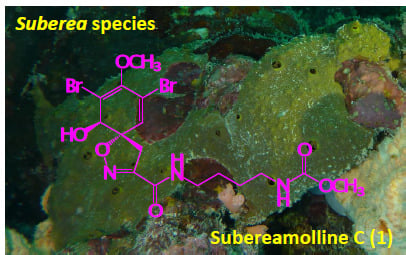
Bioactive Secondary Metabolites from the Red Sea Marine Verongid Sponge Suberea Species
Abstract
:1. Introduction
2. Results and Discussion
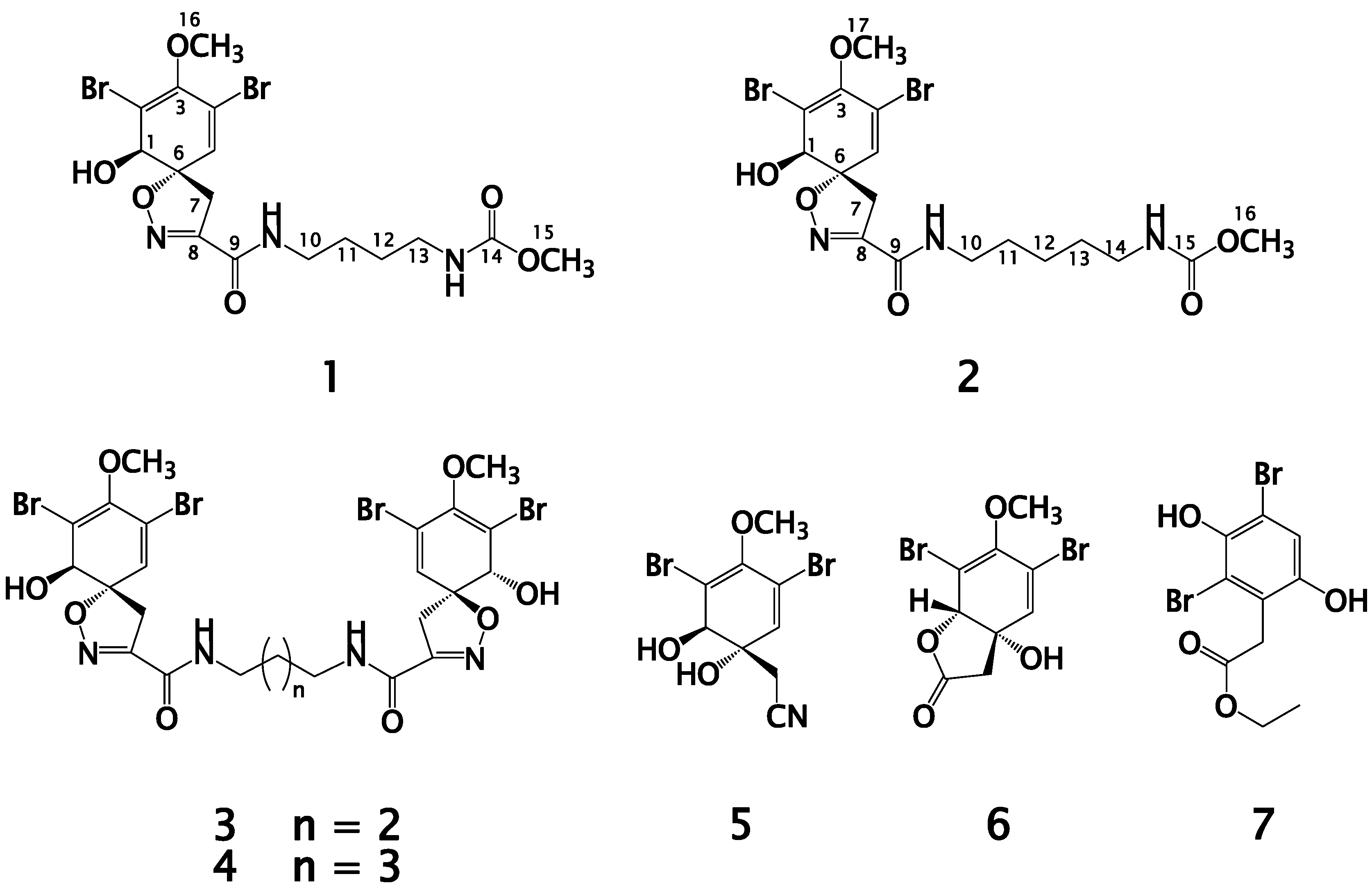
2.1. Purification of Compounds 1–7
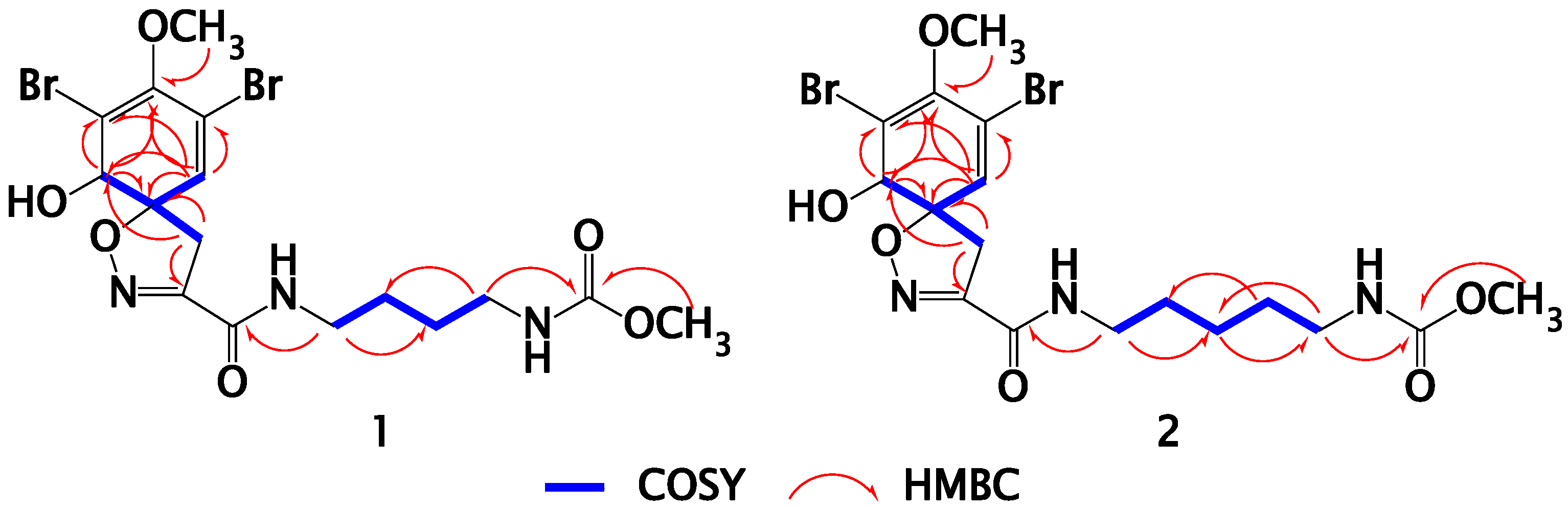
2.2. Structure Elucidation of Compound 1
| Position | δC (mult.) a | δH [mult., J (Hz)] | HMBC (H→C#) b |
|---|---|---|---|
| 1 | 75.5 (CH) | 4.07 (s) | H-5, H2-7 |
| 2 | 122.8 (qC) | H-5 | |
| 3 | 149.3 (qC) | H-1, H-5, H3-16 | |
| 4 | 114.2 (qC) | H-1, H-5 | |
| 5 | 132.3 (CH) | 6.42 (d, 0.6) | H-1, H2-7 |
| 6 | 92.3 (qC) | H-1, H-5, H2-7 | |
| 7 | 40.2 (CH2) | 3.76 (d, 18.0), 3.09 (d, 18.0) | H-1, H-5 |
| 8 | 155.3 (qC) | H2-7 | |
| 9 | 161.5 (qC) | H2-10 | |
| 10 | 40.1 (CH2) | 3.28 (t, 6.6) | H2-11, H2-12 |
| 11 | 27.7 (CH2) | 1.56 ( m) | H2-10, H2-12, H2-13 |
| 12 | 28.3 (CH2) | 1.50 (m) | H2-10, H2-13 |
| 13 | 41.3 (CH2) | 3.10 (t, 6.6) | |
| 14 | 159.6 (qC) | H2-13, H3-15 | |
| 15 | 52.4 (CH3) | 3.60 (s) | H2-13 |
| 16 | 60.4 (CH3) | 3.71 (s) |
2.3. Structure Elucidation of Compound 2
| Position | δC (mult.) | δH [mult., J (Hz)] | HMBC (H→C#) |
|---|---|---|---|
| 1 | 75.5 (CH) | 4.05 (s) | H-5, H2-7 |
| 2 | 122.8 (qC) | H-5 | |
| 3 | 149.3 (qC) | H-1, H-5, H3-17 | |
| 4 | 114.2 (qC) | H-1, H-5 | |
| 5 | 132.3 (CH) | 6.41 (d, 0.6) | H-1, H2-7 |
| 6 | 92.3 (qC) | H-1, H2-7 | |
| 7 | 40.2 (CH2) | 3.75 (d, 18.0), 3.09 (d, 18.0) | H-1, H-5 |
| 8 | 155.3 (qC) | H2-7 | |
| 9 | 161.5 (qC) | H2-10 | |
| 10 | 40.3 (CH2) | 3.26 (t, 7.2) | H2-11, H2-12 |
| 11 | 30.0 (CH2) | 1.56 (quin, 7.2) | H2-10 |
| 12 | 25.0 (CH2) | 1.34 (m) | H2-10, H2-13, H2-14 |
| 13 | 30.5 (CH2) | 1.49 (quin, 7.2) | H2-10, H2-14 |
| 14 | 41.6 (CH2) | 3.07 (t, 7.2) | |
| 15 | 159.6 (qC) | H2-14, H3-16 | |
| 16 | 52.4 (CH3) | 3.60 (s) | |
| 17 | 60.4 (CH3) | 3.71 (s) |
2.4. Structure Elucidation of Compounds 3–7
2.5. Biological Activities of the Isolated Compounds
| Compound | IC50 (μM) | |
|---|---|---|
| Antimigratory Activity (MDA-MB-231) | Antiproliferative Activity (HeLa Cells) | |
| 1 | >50 | >50 |
| 2 | >50 | >50 |
| 3 | >50 | 29 |
| 4 | NT | NT |
| 5 | NT | NT |
| 6 | 18.0 | >50 |
| 7 | >50 | 13.3 |
| S-Ethyl * | 43.4 | NT |
| Paclitaxel * | NT | 0.0017 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Biological Materials
3.3. Purification of Compounds 1–7
3.4. Biological Evaluation of the Compounds
3.4.1. Evaluation of the Antimigratory of 1–7 Using Wound Healing Assay
3.4.2. Evaluation of Antiproliferaive and Cytotoxic Activities against HeLa Cells
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed]
- Kernan, M.R.; Cambie, R.C.; Bergquist, P.R. Chemistry of sponges, VII. 11,19-Dideoxyfistularin 3 and 11-hydroxyaerothionin, bromotyrosine derivatives from Pseudoceratina durissima. J. Nat. Prod. 1990, 53, 615–622. [Google Scholar]
- Encarnacion, R.D.; Sandoval, E.; Malmastrom, J.; Christophersen, C. Calafianin, a bromotyrosine derivative from the marine sponge Aplysina gerardogreeni. J. Nat. Prod. 2000, 63, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Davis, R.A.; Shelper, T.; Sykes, M.L.; Avery, V.M.; Elofsson, M.; Sundin, C.; Quinn, R.J. Pseudoceramines A–D, new antibacterial bromotyrosine alkaloids from the marine sponge Pseudoceratina sp. Org. Biomol. Chem. 2011, 9, 6755–6760. [Google Scholar] [CrossRef] [PubMed]
- Kijjoa, A.; Watanadilok, R.; Sonchaeng, P.; Sawangwong, P.; Pedro, M.; Nascimento, M.S.J.; Silva, A.M.S.; Eaton, G.; Herz, W. Further halotyrosine derivatives from the marine sponge Suberea aff. praetensa. Z. Naturforsch. 2002, 57c, 732–738. [Google Scholar]
- Gunasekera, S.P.; Cross, S.S. Fistularin 3 and 11-Ketofistularin 3. Feline Leukemia virus active bromotyrosine metabolites from the marine sponge Aplysina archeri. J. Nat. Prod. 1992, 55, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.L.; Rodriguez, A.D. 11-Oxoaerothionin: A cytotoxic antitumor bromotyrosine-derived alkaloid from the Caribbean marine sponge Aplysina lacunosa. J. Nat. Prod. 1992, 55, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Koulman, A.; Proksch, P.; Ebel, R.; Beekman, A.C.; Uden, W.; Konings, A.W.T.; Pedersen, J.A.; Pras, N.; Woerdenbag, H.J. Cytoxicity and mode of action of aeroplysinin-1 and a related dienone from the sponge Aplysina aerophoba. J. Nat. Prod. 1996, 59, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Han, S.; Lee, H.S.; Kang, J.S.; Yun, J.; Sim, C.J.; Shin, H.J.; Lee, J.S. Cytotoxic psammaplysin analogues from a Suberea sp. marine sponge and the role of the spirooxepinisoxazoline in their activity. J. Nat. Prod. 2013, 76, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, M.S.; Carroll, A.R.; Wessling, D.; Jobling, M.; Avery, V.M.; Davis, R.A.; Feng, Y.; Xue, Y.; Oster, L.; Fex, T.; et al. Clavatadine A, a natural product with selective recognition and irreversible inhibition of factor XIa. J. Med. Chem. 2008, 51, 3583–3587. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Youssef, D.T.A.; Sulaiman, M.; Behery, F.A.; Foudah, A.I.; el Sayed, K.A. Subereamolline A as a potent breast cancer migration, invasion and proliferation inhibitor and bioactive dibrominated alkaloids from the Red Sea sponge Pseudoceratina arabica. Mar. Drugs 2012, 10, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Bamane, F.H.; Badr, J.M.; Youssef, D.T.A. Brominated arginine-derived alkaloids from the Red Sea sponge Suberea mollis. J. Nat. Prod. 2011, 74, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shoer, M.I.; Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Habib, A.M. Bioactive brominated metabolites from the Red Sea sponge Suberea mollis. J. Nat. Prod. 2008, 71, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Khalifa, S.I.; Mesbah, M.K.; van Soest, R.W.M.; Youssef, D.T.A. Subereaphenol A, a new cytotoxic and antimicrobial dibrominated phenol from the Red Sea sponge Suberea mollis. Nat. Prod. Commun. 2008, 3, 219–222. [Google Scholar]
- Abbas, A.T.; el-Shitany, N.A.; Shaala, L.A.; Ali, S.S.; Azhar, E.I.; Abdel-Dayem, U.A.; Youssef, D.T.A. Red Sea Suberea mollis sponge extract protects against CCl4-induced acute liver injury in rats via an antioxidant mechanism. Evid. Based. Complement. Alternat. Med. 2014. [Google Scholar] [CrossRef]
- Badr, J.M.; Shaala, L.A.; Abou-Shoer, M.I.; Tawfik, M.A.; Habib, A.M. Bioactive brominated metabolites from the Red Sea sponge Pseudoceratina arabica. J. Nat. Prod. 2008, 71, 1472–1474. [Google Scholar] [CrossRef] [PubMed]
- Debitus, C.; Guella, G.; Mancini, I.; Waikedre, J.; Guemas, J-P.; Nicolas, J.L.; Pietra, F. Quinolones from a bacterium and tyrosine metabolites from its host sponge, Suberea creba from the Coral Sea. J. Mar. Biotechnol. 1998, 6, 136–141. [Google Scholar] [PubMed]
- Bowden, B.F.; McCool, B.J.; Willis, R.H. Lihouidine, a novel spiro polycyclic aromatic alkaloid from the marine sponge Suberea n. sp. (Apysinellidae, Verongida). J. Org. Chem. 2004, 69, 7791–7793. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.; Jonsson, E.N.; Ebel, R.; Hartman, M.S.; Holman, T.R.; Crews, P. Probing sponge-derived terpenoids for human 15-lipoxygenase inhibitors. J. Org. Chem. 2001, 66, 6847–6851. [Google Scholar] [CrossRef] [PubMed]
- Kijjoa, A.; Watanadilok, R.; Sonchaeng, P.; Silva, A.M.S.; Eaton, G.; Herz, W. 11,17-Dideoxyagelorin A and B, new bromotyrosine derivates and analogs from the marine sponge Suberea aff. praetensa. Z. Naturforsch. C 2001, 56c, 1116–1119. [Google Scholar]
- Tsuda, M.; Sakuma, Y.; Kobayashi, J. Suberedamines A and B, new bromotyrosine alkaloids from a sponge Suberea species. J. Nat. Prod. 2001, 64, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Kubota, T.; Tsuda, M.; Watanabe, K.; Fromont, J.; Kobayashi, J. Ma’edamines A and B, cytotoxic bromotyrosine alkaloids with a unique 2(1H)pyrazinone ring from sponge Suberea sp. Tetrahedron 2000, 56, 8107–8110. [Google Scholar] [CrossRef]
- Andersen, R.J.; Faulkner, D.J. A novel antibiotic from a sponge of the genus Verongia. Tetrahedron Lett. 1973, 14, 1175–1178. [Google Scholar] [CrossRef]
- Venkateswarlu, Y.; Rao, M.R.; Venkatesham, U.A. A new dibromotyrosine-derived metabolite from the sponge Psammaplysilla purpurea. J. Nat. Prod. 1998, 61, 1388–1389. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Costantino, V.; Fattorusso, E.; Magno, S.; Mangoni, A. Chemistry of Verongida sponges, II. Constituents of the Caribbean sponge Aplysina fistularis forma fulva. J. Nat. Prod. 1994, 57, 705–712. [Google Scholar] [CrossRef]
- Albrizio, S.; Ciminiello, P.; Fattorusso, E.; Magno, S.; Pansini, M. Chemistry of Verongida sponges. I. Constituents of the Caribbean sponge Pseudoceratina crassa. Tetrahedron 1994, 50, 783–788. [Google Scholar] [CrossRef]
- Minale, L.; Sodano, G.; Chan, W.R.; Chen, A.M. Aeroplysinin-2, a dibromolactone from marine sponges Aplysina (Verongia) aerophoba and Ianthella sp. J. Chem. Soc. Chem. Commun. 1972, 674–675. [Google Scholar] [CrossRef]
- Shearman, J.W.; Myers, R.M.; Brenton, J.D.; Ley, S.V. Total syntheses of subereamollines A and B. Org. Biomol. Chem. 2011, 9, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.A.; Mohyeldin, M.M.; Foudah, A.I.; Akl, M.R.; Nazzal, S.M.; Meyer, S.A.; Liu, Y.-Y.; el Sayed, K.A. Marine natural products-inspired phenylmethylene hydantoins with potent in vitro and in vivo antitumor activities via suppression of Brk and FAK signaling. Org. Biomol. Chem. 2014, 12, 5295–5303. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Risinger, A.L.; Jackson, E.M.; Polin, L.A.; Helms, G.L.; LeBoeuf, D.A.; Joe, P.A.; Hopper-Borge, E.; Ludueña, R.F.; Kruh, G.D.; Mooberry, S.L. The taccalonolides: Microtubule stabilizers that circumvent clinically relevant taxane resistance mechanisms. Cancer Res. 2008, 68, 8881–8888. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Sulaiman, M.; Khedr, A. Bioactive Secondary Metabolites from the Red Sea Marine Verongid Sponge Suberea Species. Mar. Drugs 2015, 13, 1621-1631. https://doi.org/10.3390/md13041621
Shaala LA, Youssef DTA, Badr JM, Sulaiman M, Khedr A. Bioactive Secondary Metabolites from the Red Sea Marine Verongid Sponge Suberea Species. Marine Drugs. 2015; 13(4):1621-1631. https://doi.org/10.3390/md13041621
Chicago/Turabian StyleShaala, Lamiaa A., Diaa T. A. Youssef, Jihan M. Badr, Mansour Sulaiman, and Alaa Khedr. 2015. "Bioactive Secondary Metabolites from the Red Sea Marine Verongid Sponge Suberea Species" Marine Drugs 13, no. 4: 1621-1631. https://doi.org/10.3390/md13041621
APA StyleShaala, L. A., Youssef, D. T. A., Badr, J. M., Sulaiman, M., & Khedr, A. (2015). Bioactive Secondary Metabolites from the Red Sea Marine Verongid Sponge Suberea Species. Marine Drugs, 13(4), 1621-1631. https://doi.org/10.3390/md13041621