The Marine-Derived Kinase Inhibitor Fascaplysin Exerts Anti-Thrombotic Activity
Abstract
:1. Introduction
2. Results
2.1. Effect of Fascaplysin on Platelet Viability and PI3K Signaling
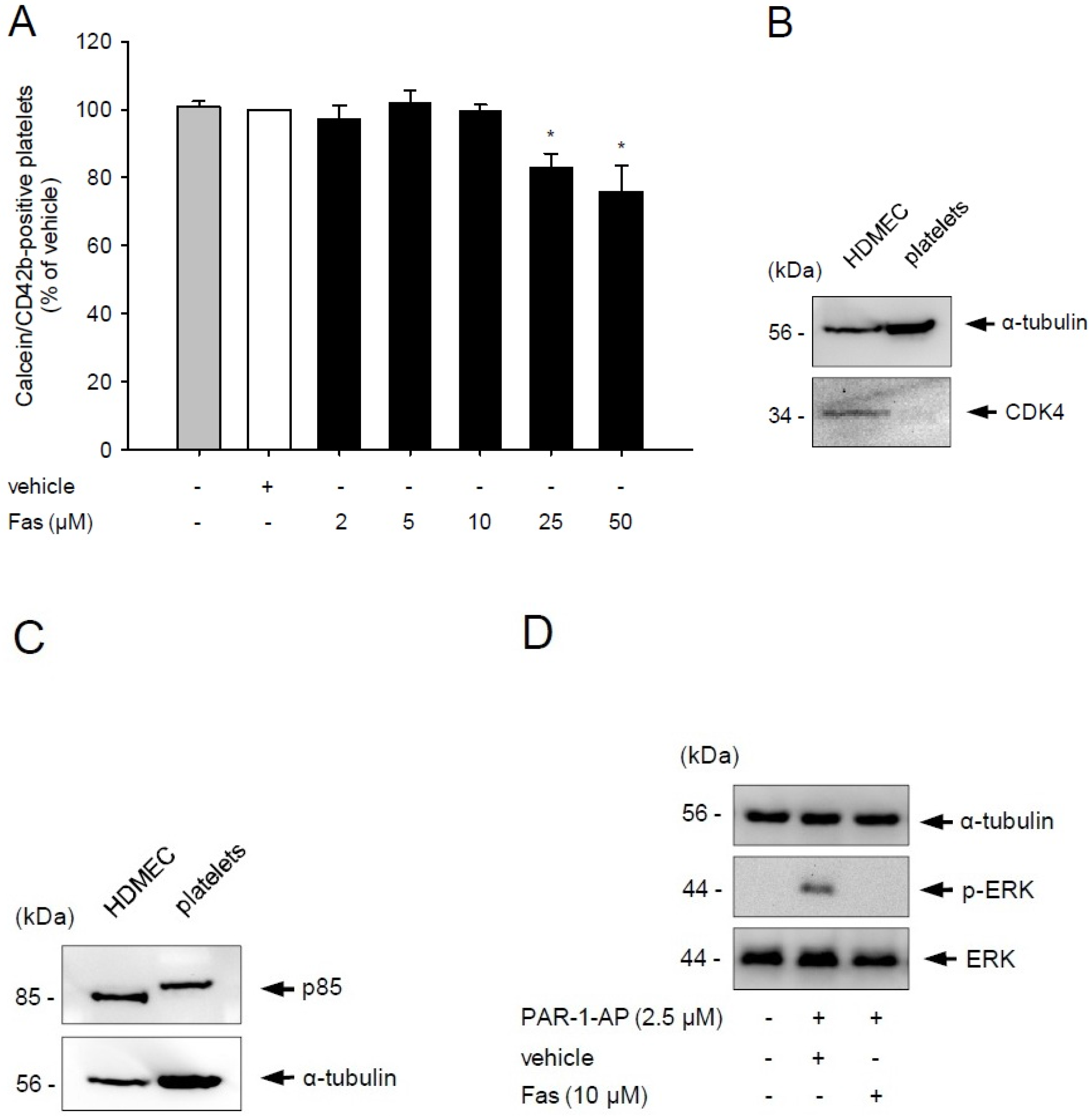
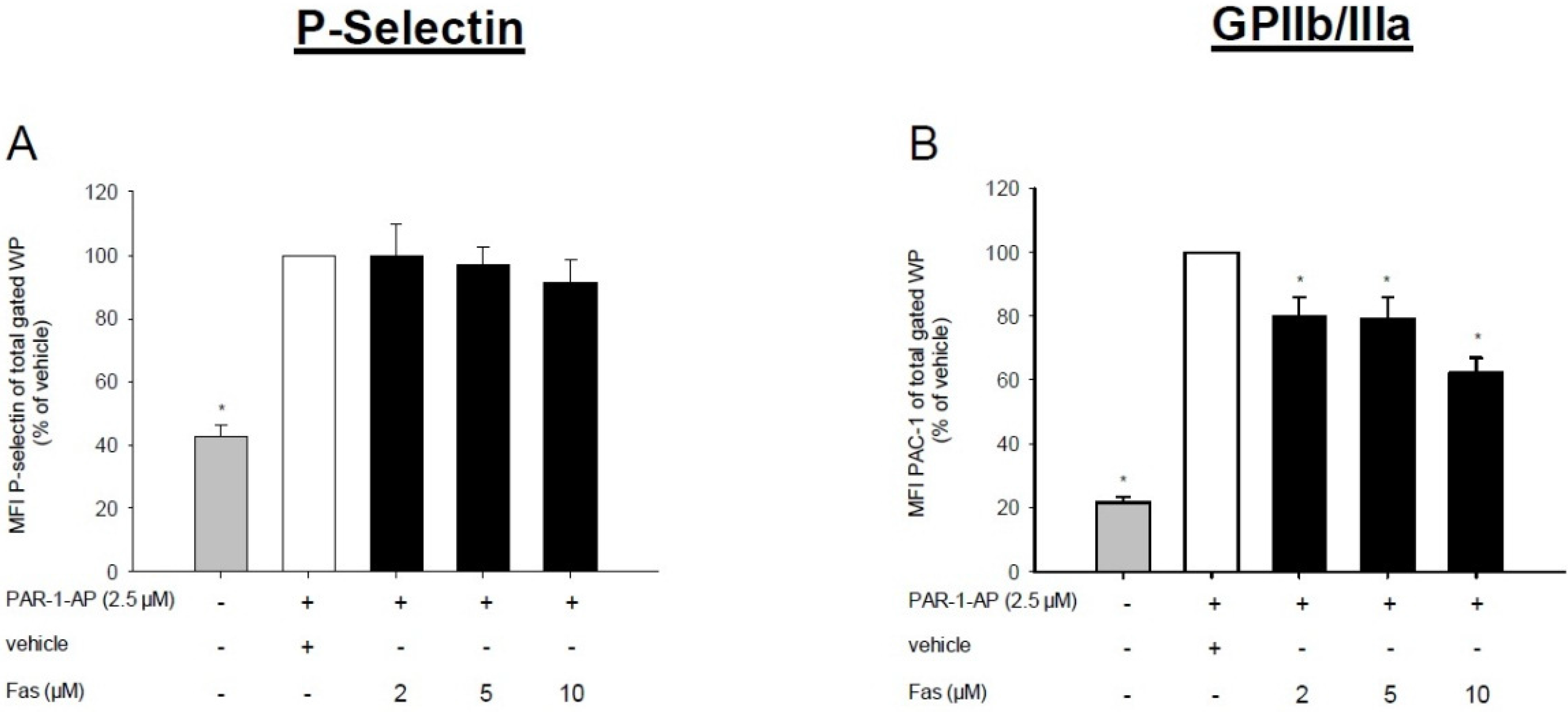
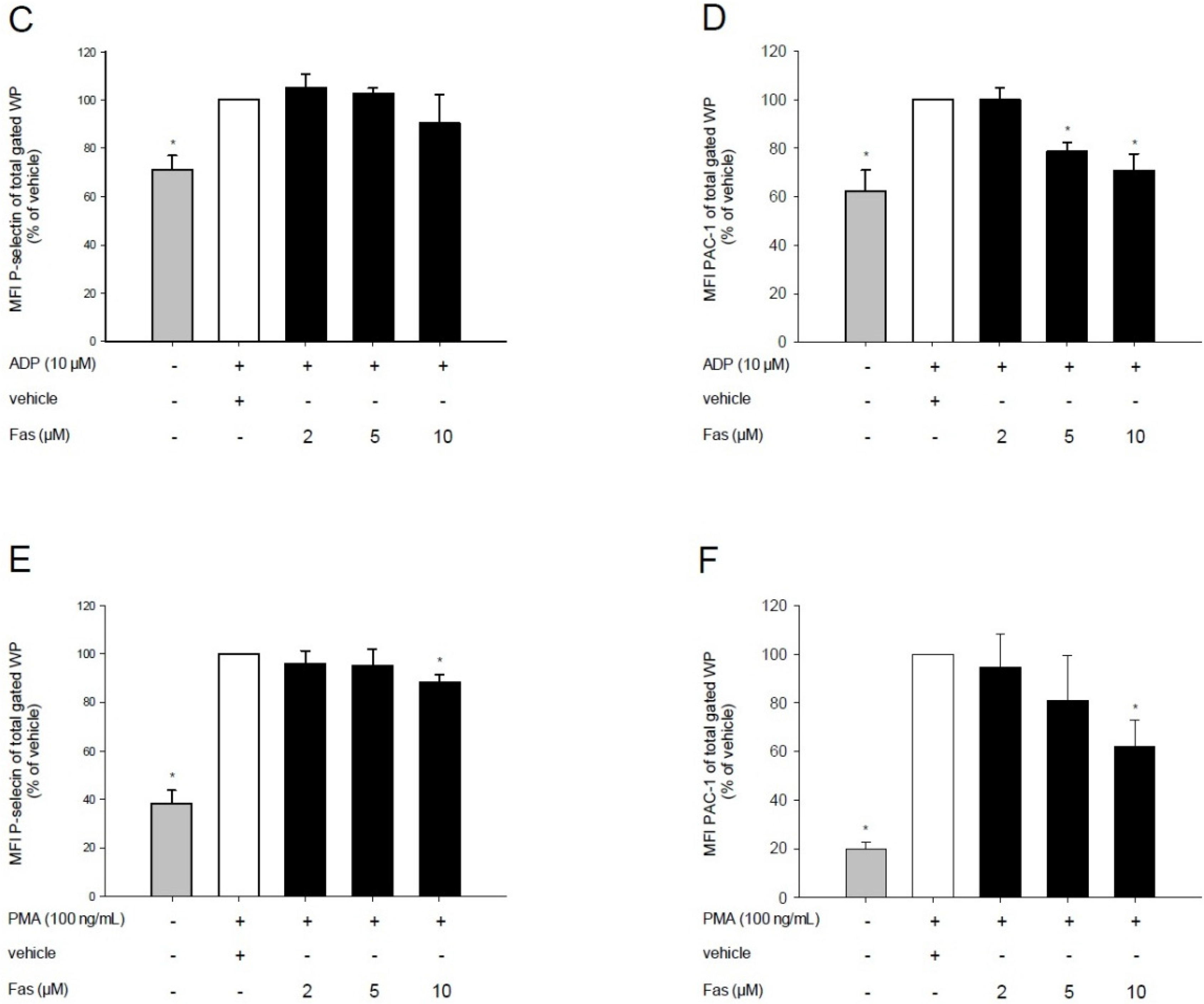
2.2. Effect of Fascaplysin on Platelet Activation
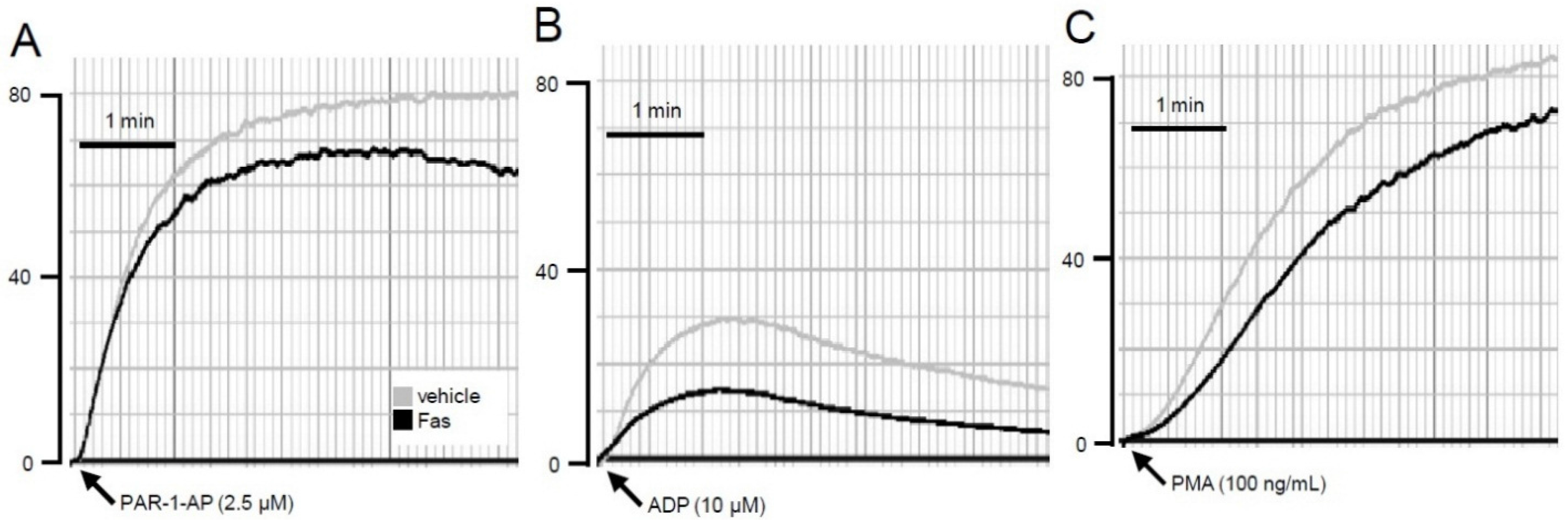
2.3. Effect of Fascaplysin on Platelet Aggregation
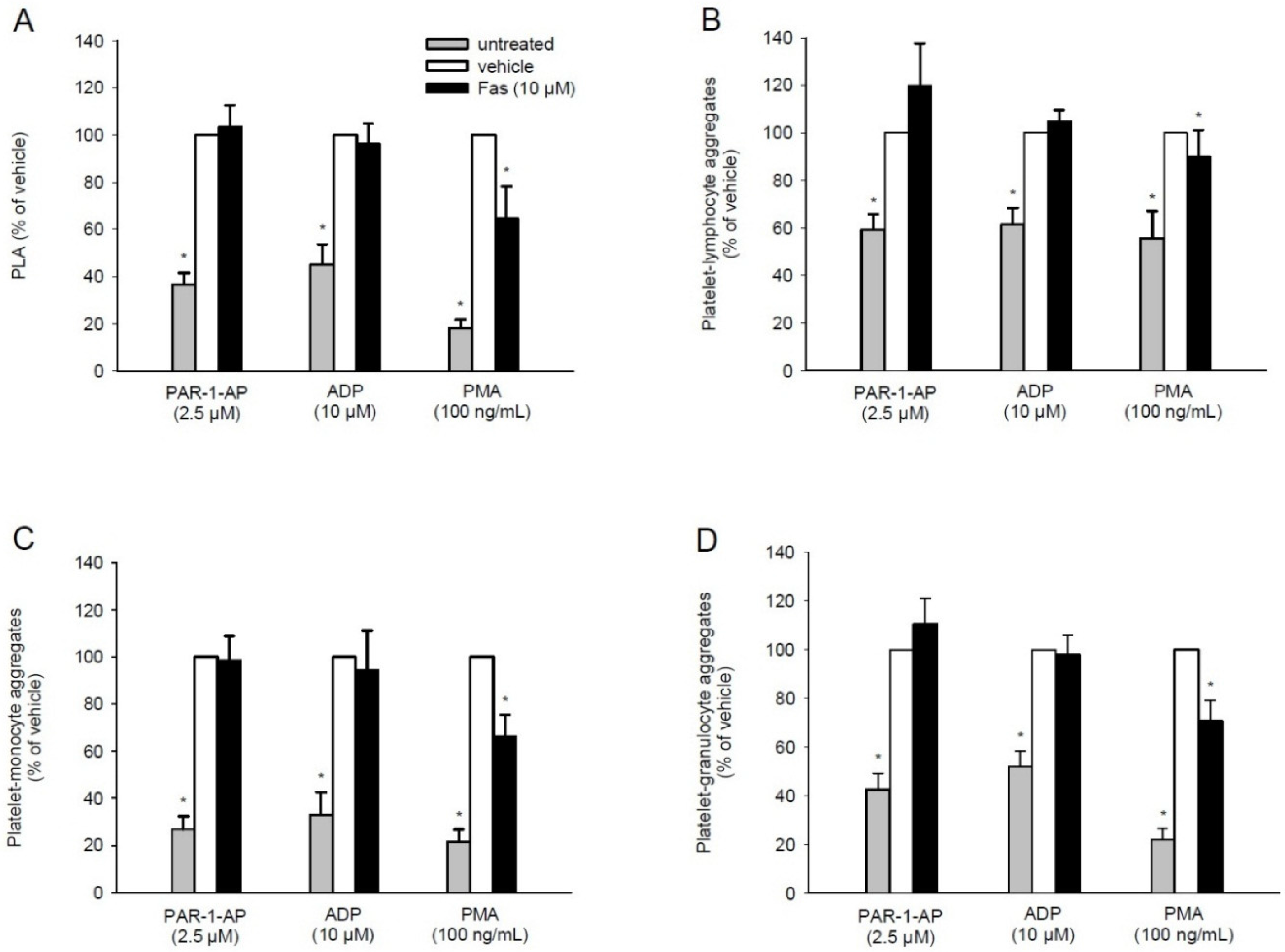
2.4. Effect of Fascaplysin on PLA Formation
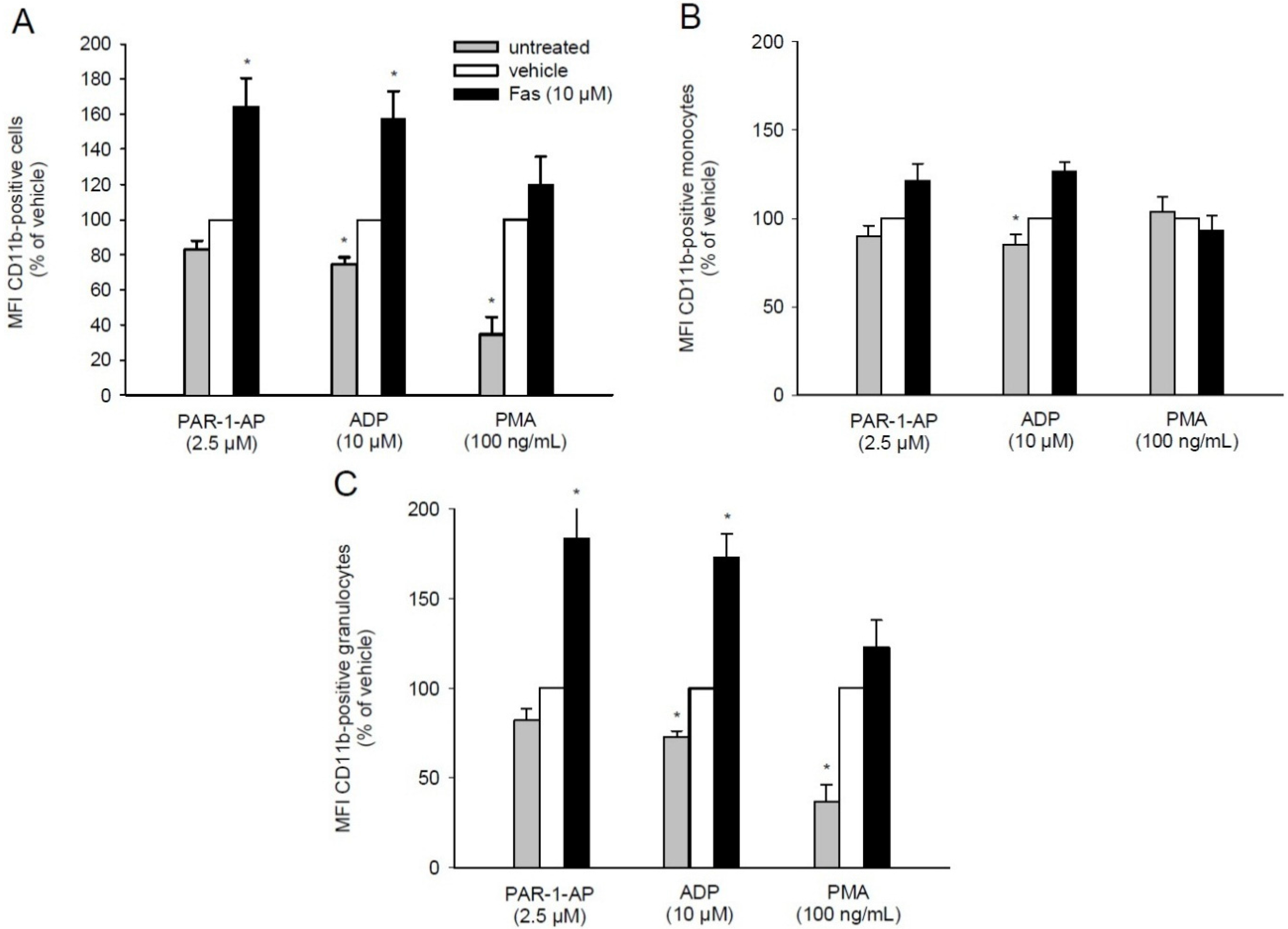
2.5. Effect of Fascaplysin on CD11b Expression on Leukocytes
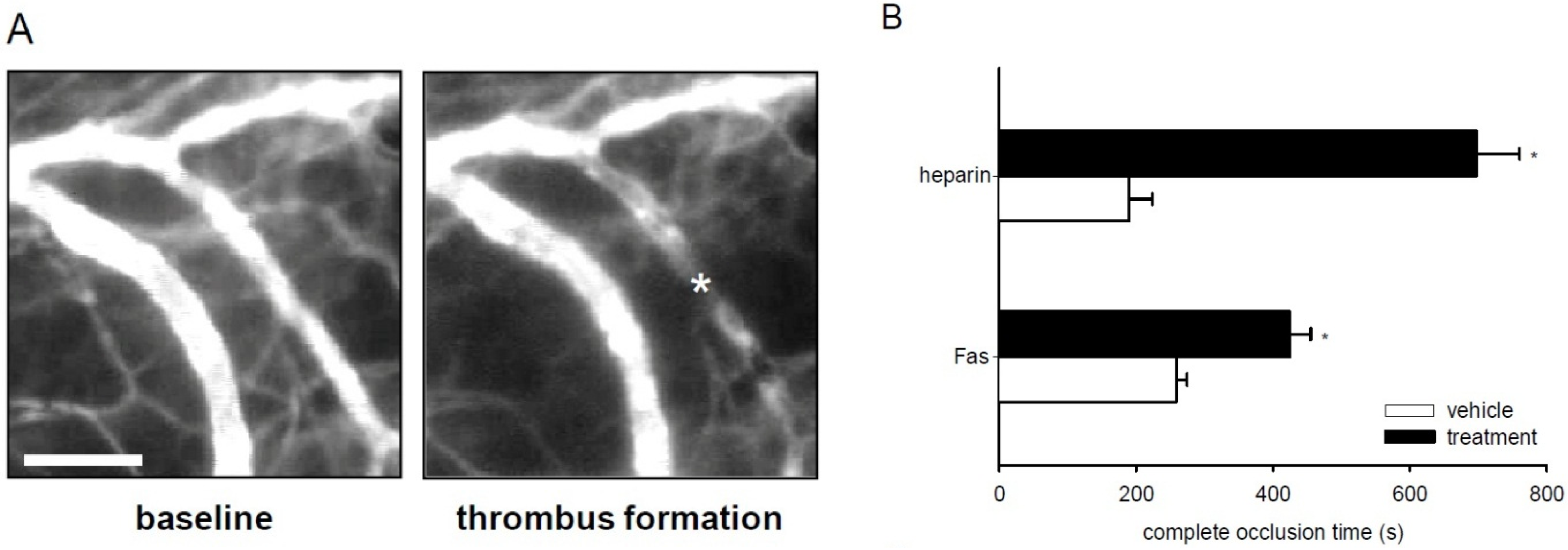
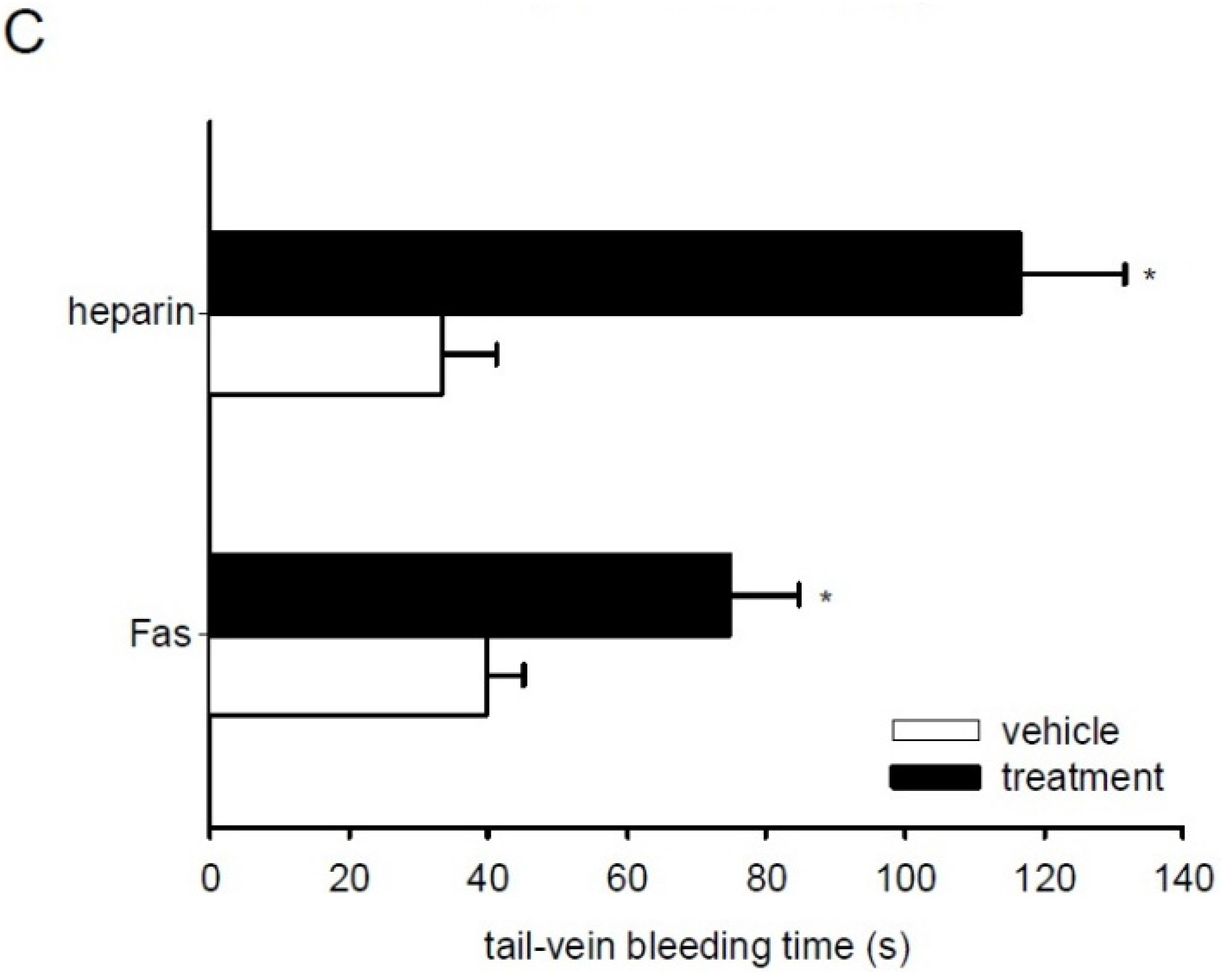
2.6. Effect of Fascaplysin on Thrombus Formation and Tail-Vein Bleeding Time
| Vehicle (DMSO) | Fas | Vehicle (Saline) | Heparin | |
|---|---|---|---|---|
| diameter (μm) | 16.7 ± 0.6 | 18.9 ± 0.7 | 20.9 ± 1.3 | 18.9 ± 1.6 |
| centerline RBC velocity (μm/s) | 205.1 ± 62.2 | 230.9 ± 50.3 | 267.9 ± 45.9 | 219.3 ± 70.3 |
| wall shear rate (s−1) | 98.3 ± 25.9 | 97.7 ± 24.5 | 102.54 ± 32.8 | 92.8 ± 21.7 |
3. Discussion
4. Experimental Section
4.1. Chemical and Biological Reagents
4.2. Antibodies
4.3. Endothelial Cell Culture
4.4. Ethics Statement
4.5. Preparation of Washed Platelets
4.6. Western Blot Analysis
4.7. Platelet Aggregation
4.8. PLA
4.9. Flow Cytometry
4.10. Animals
4.11. Photochemically Induced Thrombus Formation
4.12. Tail-Vein Bleeding Time
4.13. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soni, R.; Muller, L.; Furet, P.; Schoepfer, J.; Stephan, C.; Zumstein-Mecker, S.; Fretz, H.; Chaudhuri, B. Inhibition of cyclin-dependent kinase 4 (Cdk4) by fascaplysin, a marine natural product. Biochem. Biophys. Res. Commun. 2000, 275, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Hormann, A.; Chaudhuri, B.; Fretz, H. DNA binding properties of the marine sponge pigment fascaplysin. Bioorg. Med. Chem. 2001, 9, 917–921. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Lu, X.L.; Lin, J.; Chen, H.M.; Yan, X.J.; Wang, F.; Xu, W.F. Direct effects of fascaplysin on human umbilical vein endothelial cells attributing the anti-angiogenesis activity. Biomed. Pharmacother. 2010, 64, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yan, X.J.; Chen, H.M. Fascaplysin, a selective CDK4 inhibitor, exhibit anti-angiogenic activity in vitro and in vivo. Cancer Chemother. Pharmacol. 2007, 59, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Guru, S.K.; Pathania, A.S.; Manda, S.; Kumar, A.; Bharate, S.B.; Vishwakarma, R.A.; Malik, F.; Bhushan, S. Fascaplysin induces caspase mediated crosstalk between apoptosis and autophagy through the inhibition of PI3K/AKT/mTOR signaling cascade in human leukemia HL-60 cells. J. Cell. Biochem. 2015, 116, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Goggs, R.; Poole, A.W. Platelet signaling—A primer. J. Vet. Emerg. Crit. Care (San Antonio) 2012, 22, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Guinebault, C.; Payrastre, B.; Racaud-Sultan, C.; Mazarguil, H.; Breton, M.; Mauco, G.; Plantavid, M.; Chap, H. Integrin-dependent translocation of phosphoinositide 3-kinase to the cytoskeleton of thrombin-activated platelets involves specific interactions of p85α with actin filaments and focal adhesion kinase. J. Cell Biol. 1995, 129, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Wu, S.Y.; Liao, C.Y.; Teng, C.M.; Wu, Y.C.; Kuo, S.C. The roles and mechanisms of PAR4 and P2Y12/phosphatidylinositol 3-kinase pathway in maintaining thrombin-induced platelet aggregation. Br. J. Pharmacol. 2010, 161, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shattil, S.J.; Cunningham, M.C.; Rittenhouse, S.E. Phosphoinositide 3-kinase gamma and p85/phosphoinositide 3-kinase in platelets. Relative activation by thrombin receptor or beta-phorbol myristate acetate and roles in promoting the ligand-binding function of αIIbβ3 integrin. J. Biol. Chem. 1996, 271, 6265–6272. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.T.; Poole, A.W. Diverse functions of protein kinase C isoforms in platelet activation and thrombus formation. J. Thromb. Haemost. 2010, 8, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Toyoda, H.; Tanaka, S.; Yamamoto, H.; Komada, Y.; Gabazza, E.C.; Hayashi, T.; Suzuki, K.; Ido, M. Phosphoinositide 3-kinase induced activation and cytoskeletal translocation of protein kinase CK2 in protease activated receptor 1-stimulated platelets. Thromb. Res. 2010, 126, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Gratacap, M.P.; Guillermet-Guibert, J.; Martin, V.; Chicanne, G.; Tronchere, H.; Gaits-Iacovoni, F.; Payrastre, B. Regulation and roles of PI3Kβ, a major actor in platelet signaling and functions. Adv. Enzyme Regul. 2011, 51, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.F.; van den Bosch, M.T.; Hunter, R.W.; Sakamoto, K.; Poole, A.W.; Hers, I. Dual regulation of glycogen synthase kinase 3 (GSK3)alpha/beta by protein kinase C (PKC)α and Akt promotes thrombin-mediated integrin αIIbβ3 activation and granule secretion in platelets. J. Biol. Chem. 2013, 288, 3918–3928. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.J. Mechanisms of Platelet Activation and Integrin αIIbβ3. Korean Circ. J. 2012, 42, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Plow, E.F.; D’Souza, S.E.; Ginsberg, M.H. Ligand binding to GPIIb-IIIa: A status report. Semin. Thromb. Hemost. 1992, 18, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Davenpeck, K.L.; Brummet, M.E.; Hudson, S.A.; Mayer, R.J.; Bochner, B.S. Activation of human leukocytes reduces surface P-selectin glycoprotein ligand-1 (PSGL-1, CD162) and adhesion to P-selectin in vitro. J. Immunol. 2000, 165, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Sugama, Y.; Tiruppathi, C.; Offakidevi, K.; Andersen, T.T.; Fenton, J.W., 2nd; Malik, A.B. Thrombin-induced expression of endothelial P-selectin and intercellular adhesion molecule-1: A mechanism for stabilizing neutrophil adhesion. J. Cell Biol. 1992, 119, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Nachman, R.L.; Leung, L.L. Complex formation of platelet membrane glycoproteins IIb and IIIa with fibrinogen. J. Clin. Investig. 1982, 69, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Plow, E.F.; McEver, R.P.; Coller, B.S.; Woods, V.L., Jr.; Marguerie, G.A.; Ginsberg, M.H. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood 1985, 66, 724–727. [Google Scholar] [PubMed]
- Li, N.; Goodall, A.H.; Hjemdahl, P. Efficient flow cytometric assay for platelet-leukocyte aggregates in whole blood using fluorescence signal triggering. Cytometry 1999, 35, 154–161. [Google Scholar] [CrossRef]
- McEver, R.P.; Cummings, R.D. Role of PSGL-1 binding to selectins in leukocyte recruitment. J. Clin. Investig. 1997, 100, S97–S103. [Google Scholar] [CrossRef] [PubMed]
- Londin, E.R.; Hatzimichael, E.; Loher, P.; Edelstein, L.; Shaw, C.; Delgrosso, K.; Fortina, P.; Bray, P.F.; McKenzie, S.E.; Rigoutsos, I. The human platelet: Strong transcriptome correlations among individuals associate weakly with the platelet proteome. Biol. Direct 2014, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, L.; Zuo, W.; Luo, H.; Mao, J.; Ye, D.; Li, Y.; Liu, S.; Wei, Y.; Ye, W.; et al. The ClC-3 chloride channel protein is a downstream target of cyclin D1 in nasopharyngeal carcinoma cells. Int. J. Biochem. Cell Biol. 2013, 45, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Kim, S.; Bhavaraju, K.; Schoenwaelder, S.M.; Kunapuli, S.P. Role of phosphoinositide 3-kinase β in platelet aggregation and thromboxane A2 generation mediated by Gi signalling pathways. Biochem. J. 2010, 429, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Spangenberg, P.; Redlich, H.; Bergmann, I.; Losche, W.; Gotzrath, M.; Kehrel, B. The platelet glycoprotein IIb/IIIa complex is involved in the adhesion of activated platelets to leukocytes. Thromb. Haemost. 1993, 70, 514–521. [Google Scholar] [PubMed]
- Rinder, H.M.; Bonan, J.L.; Rinder, C.S.; Ault, K.A.; Smith, B.R. Dynamics of leukocyte-platelet adhesion in whole blood. Blood 1991, 78, 1730–1737. [Google Scholar] [PubMed]
- Hu, H.; Zhang, W.; Li, N. Glycoprotein IIb/IIIa inhibition attenuates platelet-activating factor-induced platelet activation by reducing protein kinase C activity. J. Thromb. Haemost. 2003, 1, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Patko, Z.; Csaszar, A.; Acsady, G.; Peter, K.; Schwarz, M. Roles of Mac-1 and glycoprotein IIb/IIIa integrins in leukocyte-platelet aggregate formation: Stabilization by Mac-1 and inhibition by GpIIb/IIIa blockers. Platelets 2012, 23, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Zohlnhofer, D.; Fakhoury, L.; Ott, I.; Gawaz, M.; Schomig, A. Effect of glycoprotein IIb/IIIa receptor blockade on platelet-leukocyte interaction and surface expression of the leukocyte integrin Mac-1 in acute myocardial infarction. J. Am. Coll. Cardiol. 1999, 34, 1420–1426. [Google Scholar] [CrossRef]
- Lindenblatt, N.; Platz, U.; Hameister, J.; Klar, E.; Menger, M.D.; Vollmar, B. Distinct effects of acute and chronic nicotine application on microvascular thrombus formation and endothelial function in male and female mice. Langenbecks Arch. Surg. 2007, 392, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Li, Q.; Shen, J.; Ren, L.; Liu, X.; Wang, Q.; He, S.; Wu, Q.; Hu, H.; Mao, X.; et al. Modulation of platelet activation and thrombus formation using a Pan-PI3K inhibitor S14161. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Kovacsovics, T.J.; Bachelot, C.; Toker, A.; Vlahos, C.J.; Duckworth, B.; Cantley, L.C.; Hartwig, J.H. Phosphoinositide 3-kinase inhibition spares actin assembly in activating platelets but reverses platelet aggregation. J. Biol. Chem. 1995, 270, 11358–11366. [Google Scholar] [CrossRef] [PubMed]
- Anastassiadis, T.; Deacon, S.W.; Devarajan, K.; Ma, H.; Peterson, J.R. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat. Biotechnol. 2011, 29, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Stults, N.L.; Diaz, S.; Smith, D.F.; Cummings, R.D.; Varki, A.; McEver, R.P. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J. Cell Biol. 1992, 118, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Theroux, P.; Frojmovic, M. Modulation of platelet-neutrophil interaction with pharmacological inhibition of fibrinogen binding to platelet GPIIb/IIIa receptor. Thromb. Haemost. 1999, 81, 281–285. [Google Scholar] [PubMed]
- Li, N.; Wallen, N.H.; Savi, P.; Herault, J.P.; Herbert, J.M. Effects of a new platelet glycoprotein IIb/IIIa antagonist, SR121566, on platelet activation, platelet-leukocyte interaction and thrombin generation. Blood Coagul. Fibrinolysis 1998, 9, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.J.; Hollers, J.C.; Crockett-Torabi, E.; Smith, C.W. Recruitment of CD11b/CD18 to the neutrophil surface and adherence-dependent cell locomotion. J. Clin. Investig. 1992, 90, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hu, H.; Lindqvist, M.; Wikstrom-Jonsson, E.; Goodall, A.H.; Hjemdahl, P. Platelet-leukocyte cross talk in whole blood. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Langlois, A.; Chouinard, F.; Flamand, N.; Ferland, C.; Rola-Pleszczynski, M.; Laviolette, M. Crucial implication of protein kinase C (PKC)-δ, PKC-ζ, ERK-1/2, and p38 MAPK in migration of human asthmatic eosinophils. J. Leukoc. Biol. 2009, 85, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, B.; Schmits, R.; Kunz, D.; Menger, M.D. Lack of in vivo function of CD31 in vascular thrombosis. Thromb. Haemost. 2001, 85, 160–164. [Google Scholar] [PubMed]
- Calvo, E.; Tokumasu, F.; Mizurini, D.M.; McPhie, P.; Narum, D.L.; Ribeiro, J.M.; Monteiro, R.Q.; Francischetti, I.M. Aegyptin displays high-affinity for the von Willebrand factor binding site (RGQOGVMGF) in collagen and inhibits carotid thrombus formation in vivo. FEBS J. 2010, 277, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Mizurini, D.M.; Aslan, J.S.; Gomes, T.; Ma, D.; Francischetti, I.M.; Monteiro, R.Q. Salivary Thromboxane A2-Binding Proteins from Triatomine Vectors of Chagas Disease Inhibit Platelet-Mediated Neutrophil Extracellular Traps (NETs) Formation and Arterial Thrombosis. PLoS Negl. Trop. Dis. 2015, 9, e0003869. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Salomon, D.R.; Ikeda, Y.; Ruggeri, Z.M. Characterization of the unique mechanism mediating the shear-dependent binding of soluble von Willebrand factor to platelets. J. Biol. Chem. 1995, 270, 23352–23361. [Google Scholar] [CrossRef] [PubMed]
- Hartley, P.S.; Savill, J.; Brown, S.B. The death of human platelets during incubation in citrated plasma involves shedding of CD42b and aggregation of dead platelets. Thromb. Haemost. 2006, 95, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Vollmar, B.; Menger, M.D. The dorsal skinfold chamber: Window into the dynamic interaction of biomaterials with their surrounding host tissue. Eur. Cell Mater. 2011, 22, 147–164. [Google Scholar] [PubMed]
- Subramanian, B.; Nakeff, A.; Tenney, K.; Crews, P.; Gunatilaka, L.; Valeriote, F. A new paradigm for the development of anticancer agents from natural products. J. Exp. Ther. Oncol. 2006, 5, 195–204. [Google Scholar] [PubMed]
- Yan, X.; Chen, H.; Lu, X.; Wang, F.; Xu, W.; Jin, H.; Zhu, P. Fascaplysin exert anti-tumor effects through apoptotic and anti-angiogenesis pathways in sarcoma mice model. Eur. J. Pharm. Sci. 2011, 43, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Roesken, F.; Ruecker, M.; Vollmar, B.; Boeckel, N.; Morgenstern, E.; Menger, M.D. A new model for quantitative in vivo microscopic analysis of thrombus formation and vascular recanalisation: The ear of the hairless (hr/hr) mouse. Thromb. Haemost. 1997, 78, 1408–1414. [Google Scholar] [PubMed]
- De Vriese, A.S.; Verbeuren, T.J.; Vallez, M.O.; Lameire, N.H.; de Buyzere, M.; Vanhoutte, P.M. Off-line analysis of red blood cell velocity in renal arterioles. J. Vasc. Res. 2000, 37, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Broze, G.J., Jr.; Yin, Z.F.; Lasky, N. A tail vein bleeding time model and delayed bleeding in hemophiliac mice. Thromb. Haemost. 2001, 85, 747–748. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ampofo, E.; Später, T.; Müller, I.; Eichler, H.; Menger, M.D.; Laschke, M.W. The Marine-Derived Kinase Inhibitor Fascaplysin Exerts Anti-Thrombotic Activity. Mar. Drugs 2015, 13, 6774-6791. https://doi.org/10.3390/md13116774
Ampofo E, Später T, Müller I, Eichler H, Menger MD, Laschke MW. The Marine-Derived Kinase Inhibitor Fascaplysin Exerts Anti-Thrombotic Activity. Marine Drugs. 2015; 13(11):6774-6791. https://doi.org/10.3390/md13116774
Chicago/Turabian StyleAmpofo, Emmanuel, Thomas Später, Isabelle Müller, Hermann Eichler, Michael D. Menger, and Matthias W. Laschke. 2015. "The Marine-Derived Kinase Inhibitor Fascaplysin Exerts Anti-Thrombotic Activity" Marine Drugs 13, no. 11: 6774-6791. https://doi.org/10.3390/md13116774
APA StyleAmpofo, E., Später, T., Müller, I., Eichler, H., Menger, M. D., & Laschke, M. W. (2015). The Marine-Derived Kinase Inhibitor Fascaplysin Exerts Anti-Thrombotic Activity. Marine Drugs, 13(11), 6774-6791. https://doi.org/10.3390/md13116774





