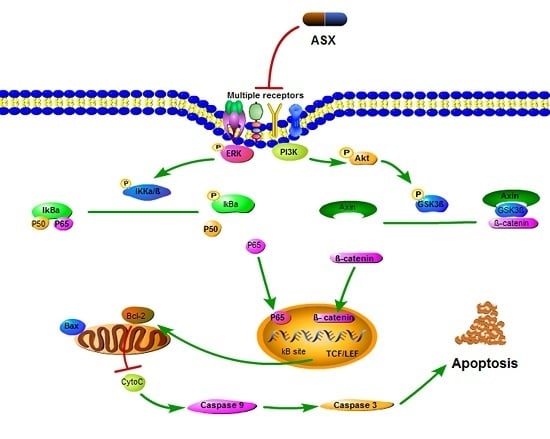
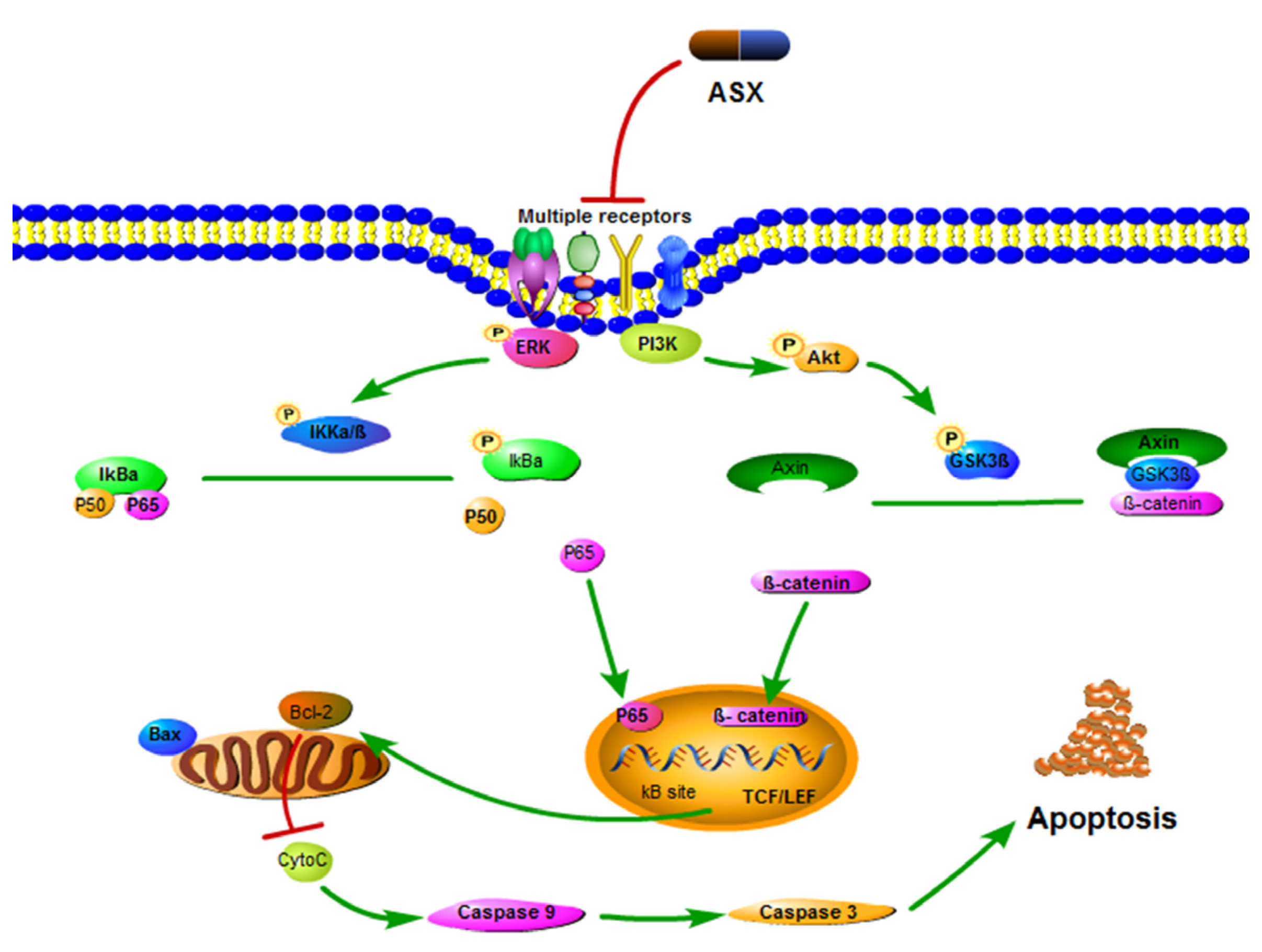
Astaxanthin Inhibits Proliferation and Induces Apoptosis of Human Hepatocellular Carcinoma Cells via Inhibition of Nf-Κb P65 and Wnt/Β-Catenin in Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. ASX Inhibited HCC Cell Proliferation

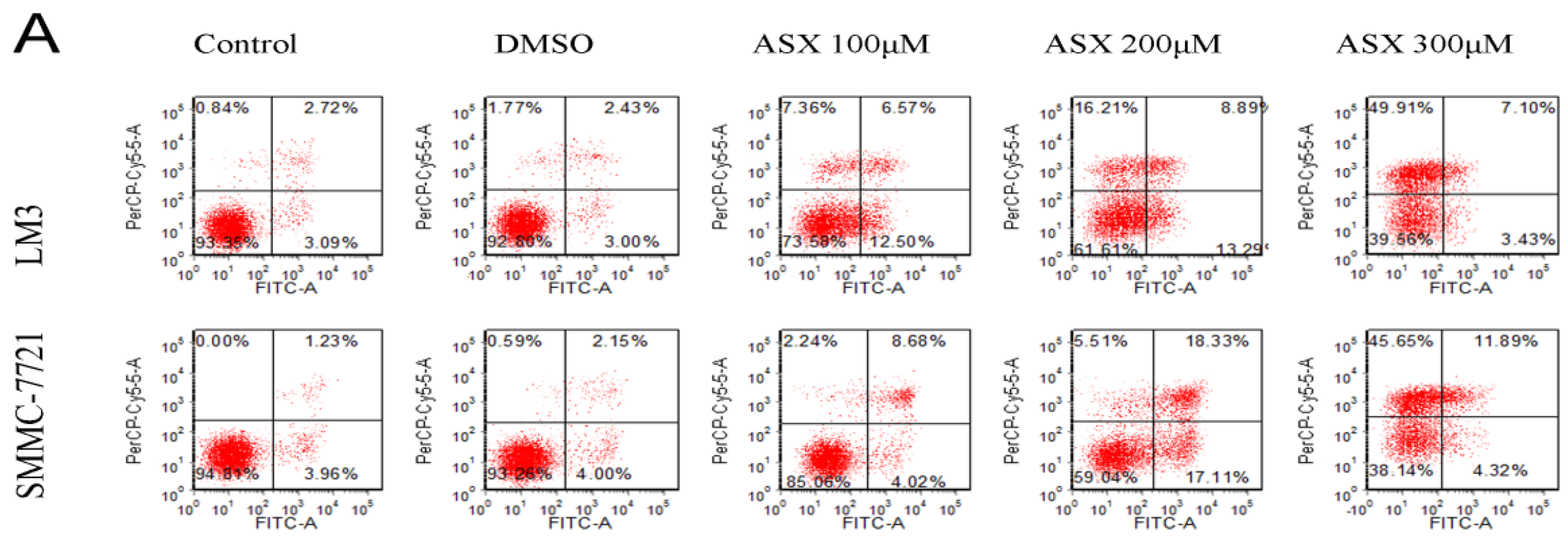
2.2. ASX Induced Apoptosis in HCC Cells
2.3. ASX Suppressed Cell Proliferation and Apoptosis by Blocking the Nuclear Translocation of NF-ΚB P65

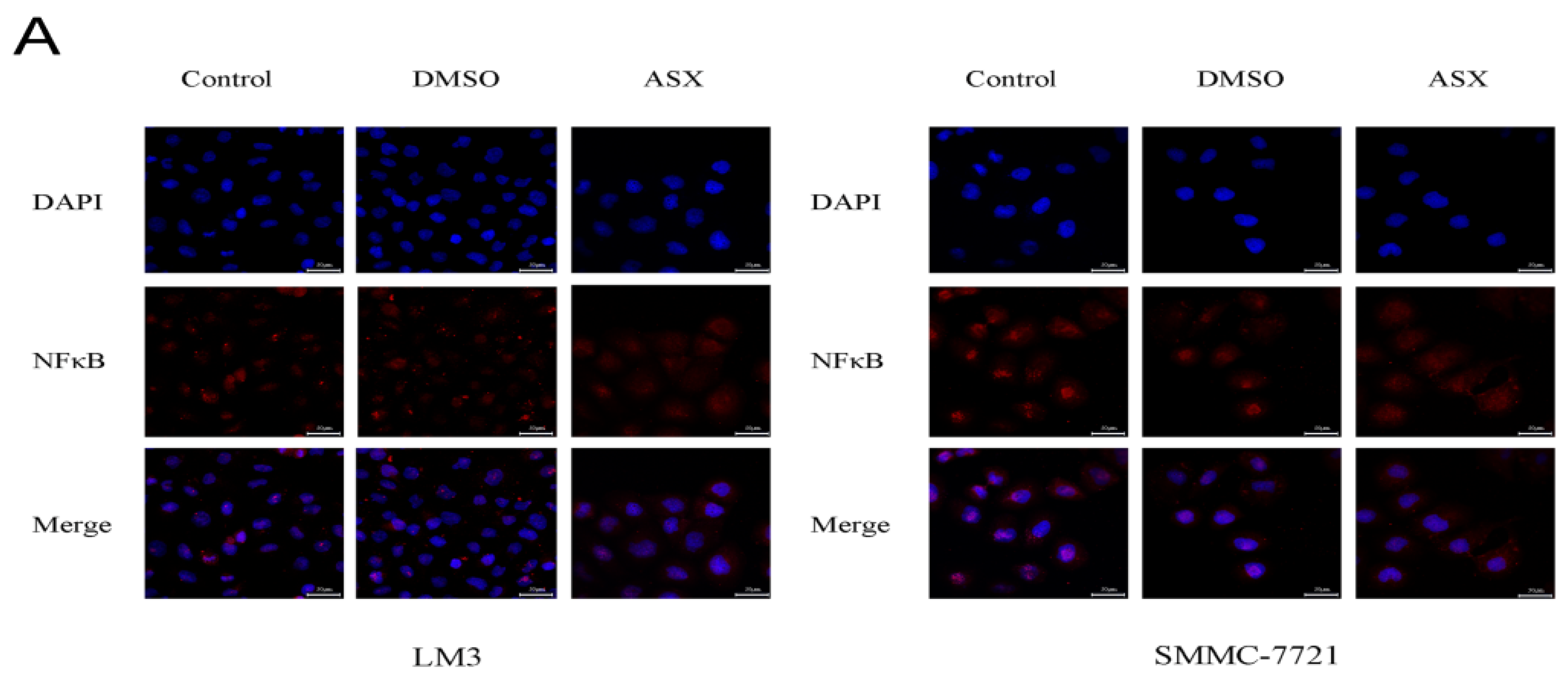

2.4. ASX Suppressed Wnt/β-Catenin Signaling by Blocking the Expression and Phosphorylation of GSK-3β


3. Discussion
4. Experimental Section
4.1. Cell Lines and Culture
4.2. Chemicals
4.3. Cell Proliferation and Viability
4.4. Cell Apoptosis Analyses Using Flow Cytometry
4.5. Hoechst 33342 Staining
| Gene | Primer Sequence (5′-3′) | |
|---|---|---|
| PCNA | Forward | GCTGACATCGGACACTTA |
| Reverse | CTCAGGTACAAACTTGGTG | |
| IκB-α | Forward | TGAAGGACGAGGAGTACGAGC |
| Reverse | TGCAGGAACGAGTCTCCGT | |
| β-catenin | Forward | TACCGTTGGATTGATTCG |
| Reverse | GTCAGAGGTGCTGTGGCT | |
| PI3K | Forward | CCACGACCATCATCAGGTGAA |
| Reverse | CCTCACGGAGGCATTCTAAAGT | |
| GSK-3β | Forward | AGACGCTCCCTGTGATTTATGT |
| Reverse | CCGATGGCAGATTCCAAAGG | |
| β-actin | Forward | CTGGAACGGTGAAGGTGACA |
| Reverse | AAGGGACTTCCTGTAACAATGCA |
4.6. Western Blot Analysis
4.7. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ASX | astaxanthin |
| HCC | hepatocellular carcinoma |
| NF-κB | nuclear factor-κB |
| JAK | janus kinase |
| STAT | signal transducers and activators of transcription |
| MAPK | mitogen-activated protein kinase |
| PI3K | phosphatidylinositol 3-kinase |
| ERK | extracellular signal regulated kinases |
| PCNA | proliferating cell nuclear antigen |
| GSK-3β | glycogen synthase kinase 3β |
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Merle, P.; Mornex, F. Medical therapies for hepatocellular carcinoma. Cancer Radiother. 2011, 15, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Hernandez-Gea, V.; Llovet, J.M. Medical therapies for hepatocellular carcinoma: A critical view of the evidence. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Yamakado, K.; Miyayama, S.; Hirota, S.; Mizunuma, K.; Nakamura, K.; Inaba, Y.; Yamamoto, S.; Matsuo, K.; Nishida, N.; Aramaki, T.; et al. Prognosis of patients with intermediate-stage hepatocellular carcinomas based on the Child-Pugh score: Subclassifying the intermediate stage (Barcelona Clinic Liver Cancer stage B). Jpn. J. Radiol. 2014, 32, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, B.H.; Baek, S.M.; Shin, D.H.; Kim, W.J.; Jeon, Y.K.; Kim, S.S.; Kim, I.J. Incidentally detected inoperable malignant pheochromocytoma with hepatic metastasis treated by transcatheter arterial chemoembolization. Endocrinol. Metab. 2014, 29, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Hilgard, P.; Hamami, M.; Fouly, A.E.; Scherag, A.; Muller, S.; Ertle, J.; Heusner, T.; Cicinnati, V.R.; Paul, A.; Bockisch, A.; et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010, 52, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Xie, P.; Liu, J.; Wu, H.; Xie, Y. Therapeutic value of transcatheter arterial chemoembolization combined with portal vein embolization for primary hepatocellular carcinoma with portal vein tumor thrombus: A pilot study. Asia Pac. J. Clin. Oncol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Dai, W.; Wang, F.; Lu, J.; Shen, M.; Chen, K.; Li, J.; Zhang, Y.; Wang, C.; Yang, J.; et al. Ethyl pyruvate inhibits proliferation and induces apoptosis of hepatocellular carcinoma via regulation of the HMGB1-RAGE and AKT pathways. Biochem. Biophys. Res. Commun. 2014, 443, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Kinzler, K.W. Cancer genes and the pathways they control. Nat. Med. 2004, 10, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, C.; Wang, F.; Wang, Y.; Shen, M.; Chen, K.; Cheng, P.; Zhang, Y.; Yang, J.; Zhu, R.; et al. Anti-miR-197 inhibits migration in HCC cells by targeting KAI 1/CD82. Biochem. Biophys. Res. Commun. 2014, 446, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, F.; Lu, J.; Xia, Y.; He, L.; Chen, K.; Li, J.; Li, S.; Liu, T.; Zheng, Y.; et al. By reducing hexokinase 2, resveratrol induces apoptosis in HCC cells addicted to aerobic glycolysis and inhibits tumor growth in mice. Oncotarget 2015, 6, 13703–13717. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Toffanin, S.; Llovet, J.M. Linking molecular classification of hepatocellular carcinoma and personalized medicine: Preliminary steps. Curr. Opin. Oncol. 2008, 20, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, F.; He, L.; Lin, C.; Wu, S.; Chen, P.; Zhang, Y.; Shen, M.; Wu, D.; Wang, C.; et al. Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial-mesenchymal transition: Partial mediation by the transcription factor NFAT1. Mol. Carcinog. 2015, 54, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.; Kowshik, J.; Kishore, T.K.K.; Baba, A.B.; Nagini, S. Astaxanthin inhibits NF-κB and Wnt/β-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. Biochim. Biophys. Acta. Gen. Subj. 2013, 1830, 4433–4444. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; He, L.; Dai, W.; Xu, Y.; Wu, D.; Lin, C.; Wu, S.; Cheng, P.; Zhang, Y.; Shen, M.; et al. Salinomycin inhibits proliferation and induces apoptosis of human hepatocellular carcinoma cells in vitro and in vivo. PLoS ONE 2012, 7, e50638. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dai, W.; Wang, Y.; Shen, M.; Chen, K.; Cheng, P.; Zhang, Y.; Wang, C.; Li, J.; Zheng, Y.; et al. The Synergistic in vitro and in vivo antitumor effect of combination therapy with salinomycin and 5-fluorouracil against hepatocellular carcinoma. PLoS ONE 2014, 9, e97414. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, F.; Xia, Y.; Dai, W.; Chen, K.; Li, S.; Liu, T.; Zheng, Y.; Wang, J.; Lu, W.; et al. Astaxanthin pretreatment attenuates hepatic ischemia reperfusion-induced apoptosis and autophagy via the ROS/MAPK pathway in mice. Mar. Drugs 2015, 13, 3368–3387. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, Y.; Liu, T.; Wang, J.; Dai, W.; Wang, F.; Zheng, Y.; Chen, K.; Li, S.; Abudumijiti, H.; et al. Protective effects of astaxanthin on ConA-induced autoimmune hepatitis by the JNK/p-JNK pathway-mediated inhibition of autophagy and apoptosis. PLoS ONE 2015, 10, e120440. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Chen, K.; Lu, J.; Cheng, P.; Xu, L.; Dai, W.; Wang, F.; He, L.; Zhang, Y.; Chengfen, W.; et al. Protective effect of astaxanthin on liver fibrosis through modulation of TGF-beta1 expression and autophagy. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Regnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D.; et al. Astaxanthin from Haematococcus pluvialis prevents oxidative stress on human endothelial cells without toxicity. Mar. Drugs 2015, 13, 2857–2874. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol. 2009, 5, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H. Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Mar. Drugs 2015, 13, 4310–4330. [Google Scholar] [CrossRef] [PubMed]
- Chekanov, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Sidorov, R.; Solovchenko, A. Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the white sea coastal rocks (Russia). Mar. Drugs 2014, 12, 4504–4520. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.X.; Zhou, H.L.; Huang, C.L.; You, C.G.; Fang, Q.; Wu, P.; Wang, X.G.; Han, C.M. Astaxanthin attenuates early acute kidney injury following severe burns in rats by ameliorating oxidative stress and mitochondrial-related apoptosis. Mar. Drugs 2015, 13, 2105–2123. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H.; Sun, S.; Iijima, K.; Gross, M.D. Antitumor activity of astaxanthin and its mode of action. Nutr. Cancer 2000, 36, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kowshik, J.; Baba, A.B.; Giri, H.; Deepak Reddy, G.; Dixit, M.; Nagini, S. Astaxanthin inhibits JAK/STAT-3 signaling to abrogate cell proliferation, invasion and angiogenesis in a hamster model of oral cancer. PLoS ONE 2014, 9, e109114. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Nelson, O.L.; Park, J.S.; Mathison, B.D.; Thompson, P.A.; Chew, B.P. Effect of dietary astaxanthin at different stages of mammary tumor initiation in BALB/c mice. Anticancer Res. 2010, 30, 2171–2175. [Google Scholar] [PubMed]
- Ikeda, Y.; Tsuji, S.; Satoh, A.; Ishikura, M.; Shirasawa, T.; Shimizu, T. Protective effects of astaxanthin on 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J. Neurochem. 2008, 107, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Nagendraprabhu, P.; Sudhandiran, G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFkB and COX-2. Investig. New Drugs 2011, 29, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.N.; Jena, G.B. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: Role of Nrf2, p53, p38 and phase-II enzymes. Mutat. Res. 2010, 696, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Song, X.D.; Zhang, J.J.; Wang, M.R.; Liu, W.B.; Gu, X.B.; Lv, C.J. Astaxanthin induces mitochondria-mediated apoptosis in rat hepatocellular carcinoma CBRH-7919 cells. Biol. Pharm. Bull. 2011, 34, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, M.; Zhang, L.; Zhang, J.; Wang, X.; Liu, W.; Gu, X.; Lv, C. Changes in cell ultrastructure and inhibition of JAK1/STAT3 signaling pathway in CBRH-7919 cells with astaxanthin. Toxicol. Mech. Methods 2012, 22, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yin, X.; Ying, J.; Zhang, B. Golgi protein 73 versus alpha-fetoprotein as a biomarker for hepatocellular carcinoma: A diagnostic meta-analysis. BMC Cancer 2012, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Yang, J.; Xu, L.; Dai, W.; Wang, F.; Shen, M.; Zhang, Y.; Zhang, H.; Chen, K.; Cheng, P.; et al. Diagnostic performance of des-gamma-carboxy prothrombin for hepatocellular carcinoma: A meta-analysis. Gastroenterol. Res. Pract. 2014. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, A.G.; Redon, C.E.; Ferguson, N.F.; Kryston, T.B.; Parekh, P.; Dickey, J.S.; Nakamura, A.J.; Mitchell, J.B.; Bonner, W.M.; Martin, O.A. Systemic DNA damage accumulation under in vivo tumor growth can be inhibited by the antioxidant Tempol. Cancer Lett. 2014, 353, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Chen, S.; Ye, X.; Zhong, J.; Wu, N.; Dong, S.; Yang, B.; Liu, D. Antioxidant and anti-tumor activity of a polysaccharide from freshwater clam, Corbicula fluminea. Food Funct. 2013, 4, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Selvam, T.N.; Venkatakrishnan, V.; Damodar, K.S.; Elumalai, P. Antioxidant and tumor cell suppression potential of premna serratifolia linn leaf. Toxicol. Int. 2012, 19, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Maga, G.; Hubscher, U. Proliferating cell nuclear antigen (PCNA): A dancer with many partners. J. Cell Sci. 2003, 116, 3051–3060. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, C. Tracking down the origin of cancer: Metabolic reprogramming as a driver of stemness and tumorigenesis. Crit. Rev. Oncog. 2014, 19, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.; Esposti, M.D.; Gilmore, A.P. Bcl-2 proteins and mitochondria—Specificity in membrane targeting for death. Biochim. Biophys. Acta 2011, 1813, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Farhana, L.; Dawson, M.I.; Fontana, J.A. Apoptosis induction by a novel retinoid-related molecule requires nuclear factor-kappaB activation. Cancer Res. 2005, 65, 4909–4917. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, E.J.; Madigan, J.P.; Boardman, L.A.; Rosenberg, D.W. Ibuprofen inhibits activation of nuclear β-catenin in human colon adenomas and induces the phosphorylation of GSK-3β. Cancer Prev. Res. 2011, 4, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, M.M.; Sung, B.; Yadav, V.R.; Kannappan, R.; Aggarwal, B.B. NF-kappaB addiction and its role in cancer: “One size does not fit allˮ. Oncogene 2011, 30, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, C.; Sharma, M.; Henderson, B.R. Wnt signaling from membrane to nucleus: Beta-catenin caught in a loop. Int. J. Biochem. Cell Biol. 2012, 44, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Zhang, H.; Yu, J.; Francisco, R.; Dent, P.; Ebert, M.P.; Rocken, C.; Farrell, G. Constitutive activation of NF-kappaB in human hepatocellular carcinoma: Evidence of a cytoprotective role. Hum. Gene Ther. 2006, 17, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S.; Tang, Y.; Liu, Q.; Yao, Y. MPT64 protein from Mycobacterium tuberculosis inhibits apoptosis of macrophages through NF-kB-miRNA21-Bcl-2 pathway. PLoS ONE 2014, 9, e100949. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Yamamoto, H.; Sato, A.; Matsumoto, S. New insights into the mechanism of Wnt signaling pathway activation. Int. Rev. Cell Mol. Biol. 2011, 291, 21–71. [Google Scholar] [PubMed]
- Anitha, P.; Priyadarsini, R.V.; Kavitha, K.; Thiyagarajan, P.; Nagini, S. Ellagic acid coordinately attenuates Wnt/beta-catenin and NF-kappaB signaling pathways to induce intrinsic apoptosis in an animal model of oral oncogenesis. Eur. J. Nutr. 2013, 52, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.I.; Uribe, D.; Jaramillo, C.M.; Osorio, G.; Perez, J.C.; Lopez, R.; Hoyos, S.; Hainaut, P.; Pineau, P.; Navas, M.C. Wnt/beta-catenin signaling pathway in hepatocellular carcinomas cases from Colombia. Ann. Hepatol. 2015, 14, 64–74. [Google Scholar] [PubMed]
- Yothaisong, S.; Thanee, M.; Namwat, N.; Yongvanit, P.; Boonmars, T.; Puapairoj, A.; Loilome, W. Opisthorchis viverrini Infection Activates the PI3K/ AKT/PTEN and Wnt/beta-catenin Signaling Pathways in a Cholangiocarcinogenesis Model. Asian Pac. J. Cancer Prev. 2014, 15, 10463–10468. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R. Glycogen synthase kinase 3 beta: Can it be a target for oral cancer. Mol. Cancer 2010, 9, 144. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Dai, W.; Xia, Y.; Chen, K.; Li, S.; Liu, T.; Zhang, R.; Wang, J.; Lu, W.; Zhou, Y.; et al. Astaxanthin Inhibits Proliferation and Induces Apoptosis of Human Hepatocellular Carcinoma Cells via Inhibition of Nf-Κb P65 and Wnt/Β-Catenin in Vitro. Mar. Drugs 2015, 13, 6064-6081. https://doi.org/10.3390/md13106064
Li J, Dai W, Xia Y, Chen K, Li S, Liu T, Zhang R, Wang J, Lu W, Zhou Y, et al. Astaxanthin Inhibits Proliferation and Induces Apoptosis of Human Hepatocellular Carcinoma Cells via Inhibition of Nf-Κb P65 and Wnt/Β-Catenin in Vitro. Marine Drugs. 2015; 13(10):6064-6081. https://doi.org/10.3390/md13106064
Chicago/Turabian StyleLi, Jingjing, Weiqi Dai, Yujing Xia, Kan Chen, Sainan Li, Tong Liu, Rong Zhang, Jianrong Wang, Wenxia Lu, Yuqing Zhou, and et al. 2015. "Astaxanthin Inhibits Proliferation and Induces Apoptosis of Human Hepatocellular Carcinoma Cells via Inhibition of Nf-Κb P65 and Wnt/Β-Catenin in Vitro" Marine Drugs 13, no. 10: 6064-6081. https://doi.org/10.3390/md13106064
APA StyleLi, J., Dai, W., Xia, Y., Chen, K., Li, S., Liu, T., Zhang, R., Wang, J., Lu, W., Zhou, Y., Yin, Q., Abudumijiti, H., Chen, R., Zheng, Y., Wang, F., Lu, J., Zhou, Y., & Guo, C. (2015). Astaxanthin Inhibits Proliferation and Induces Apoptosis of Human Hepatocellular Carcinoma Cells via Inhibition of Nf-Κb P65 and Wnt/Β-Catenin in Vitro. Marine Drugs, 13(10), 6064-6081. https://doi.org/10.3390/md13106064