A Feedback Mechanism to Control Apoptosis Occurs in the Digestive Gland of the Oyster Crassostrea gigas Exposed to the Paralytic Shellfish Toxins Producer Alexandrium catenella
Abstract
:1. Introduction
2. Results and Discussion
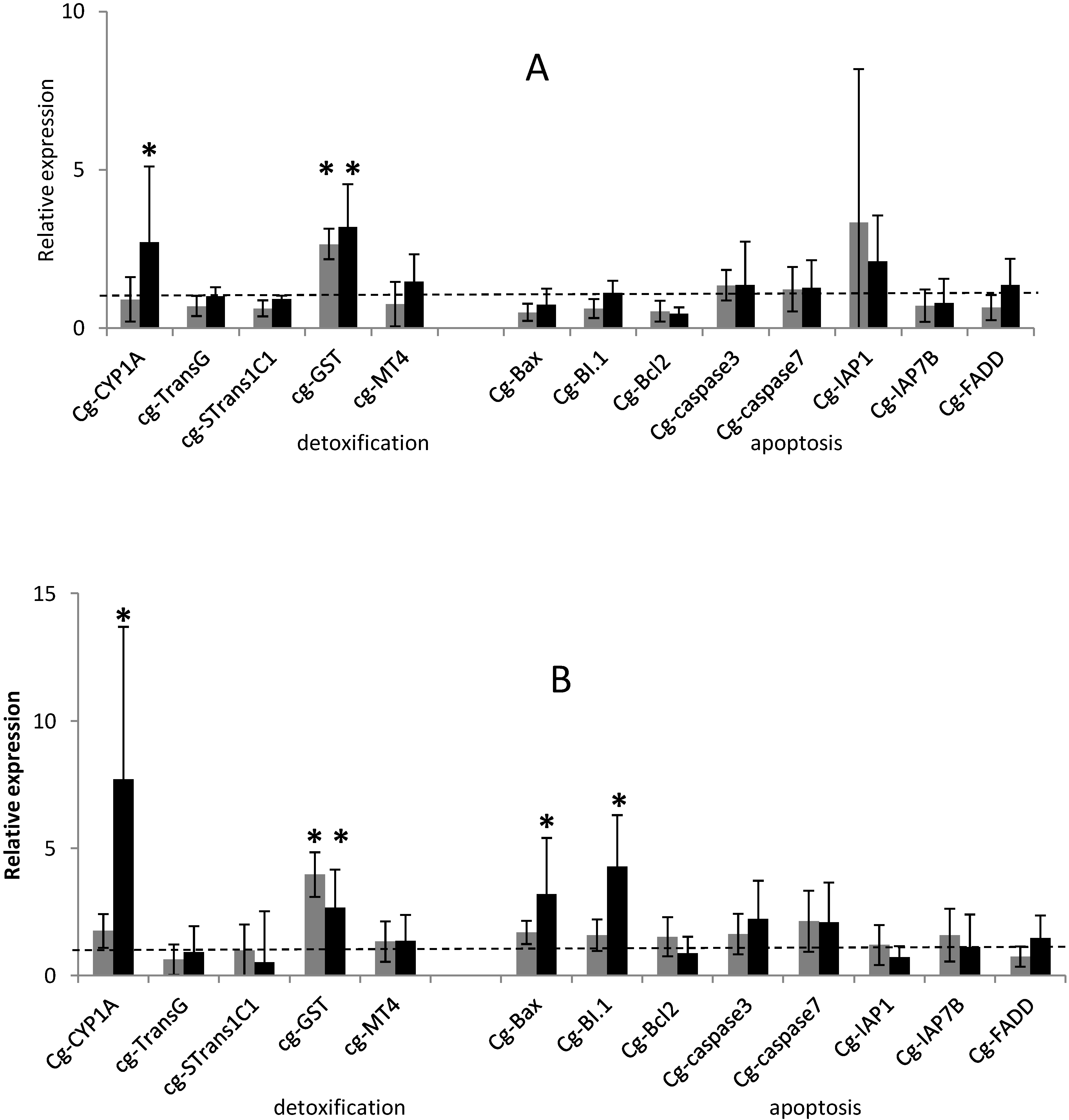
2.1. Temporal Expression of the Genes Related to Detoxification and Apoptotic Processes
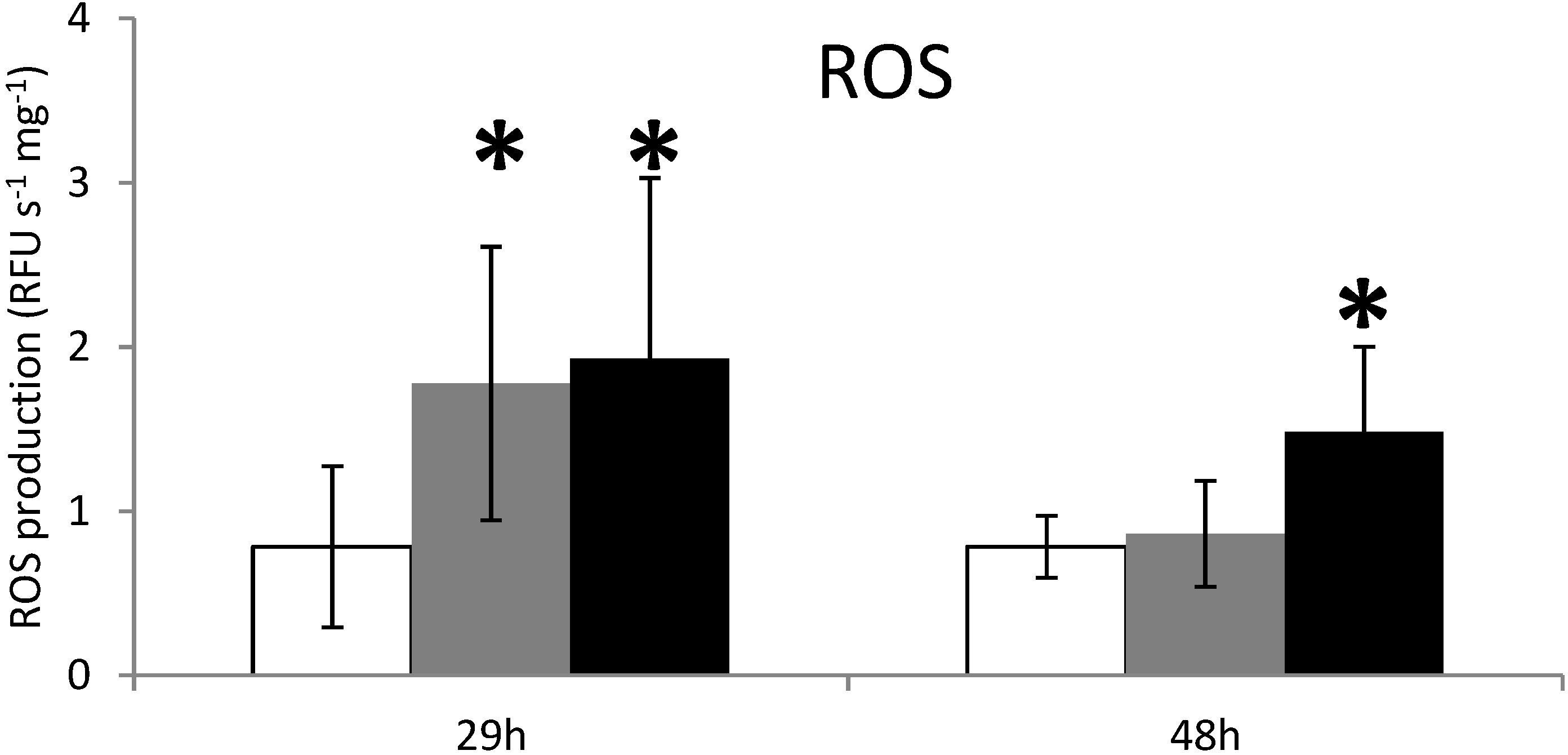
2.2. ROS Production
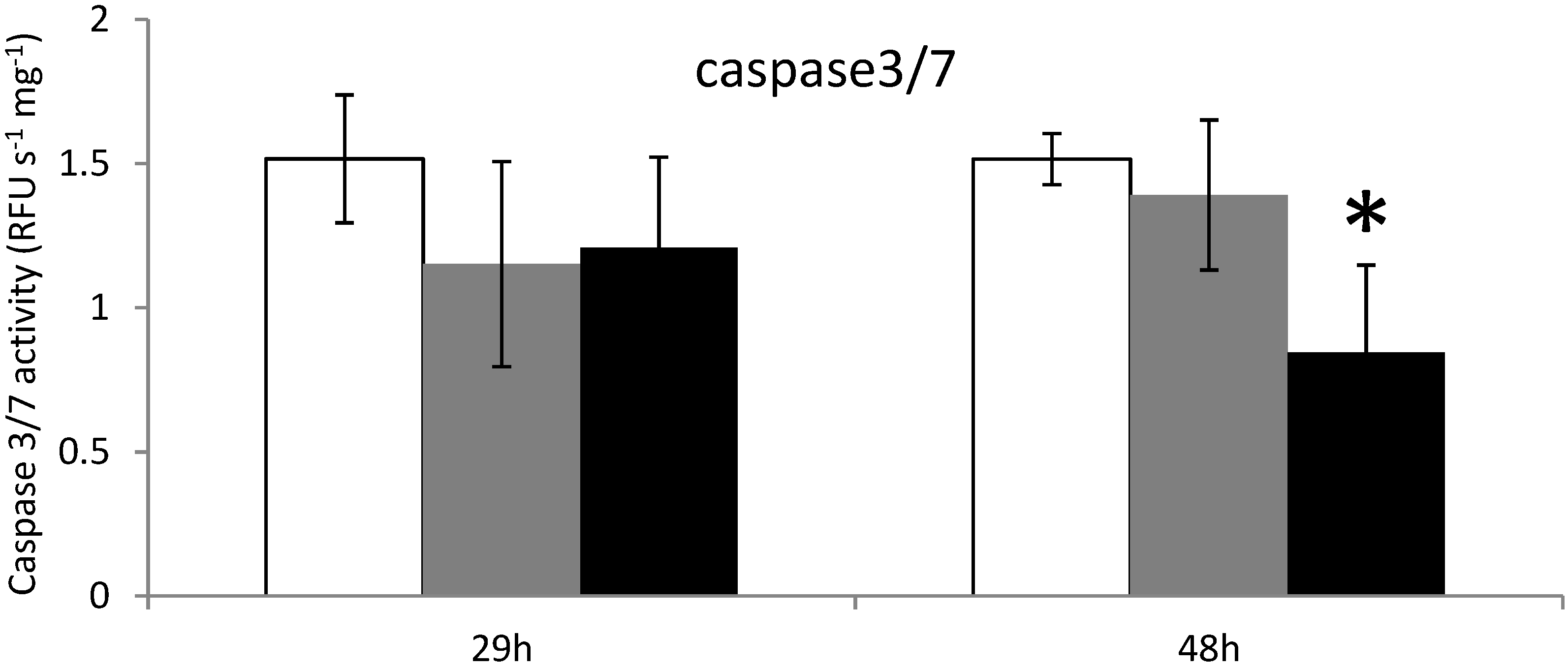
2.3. Apoptosis in Digestive Gland
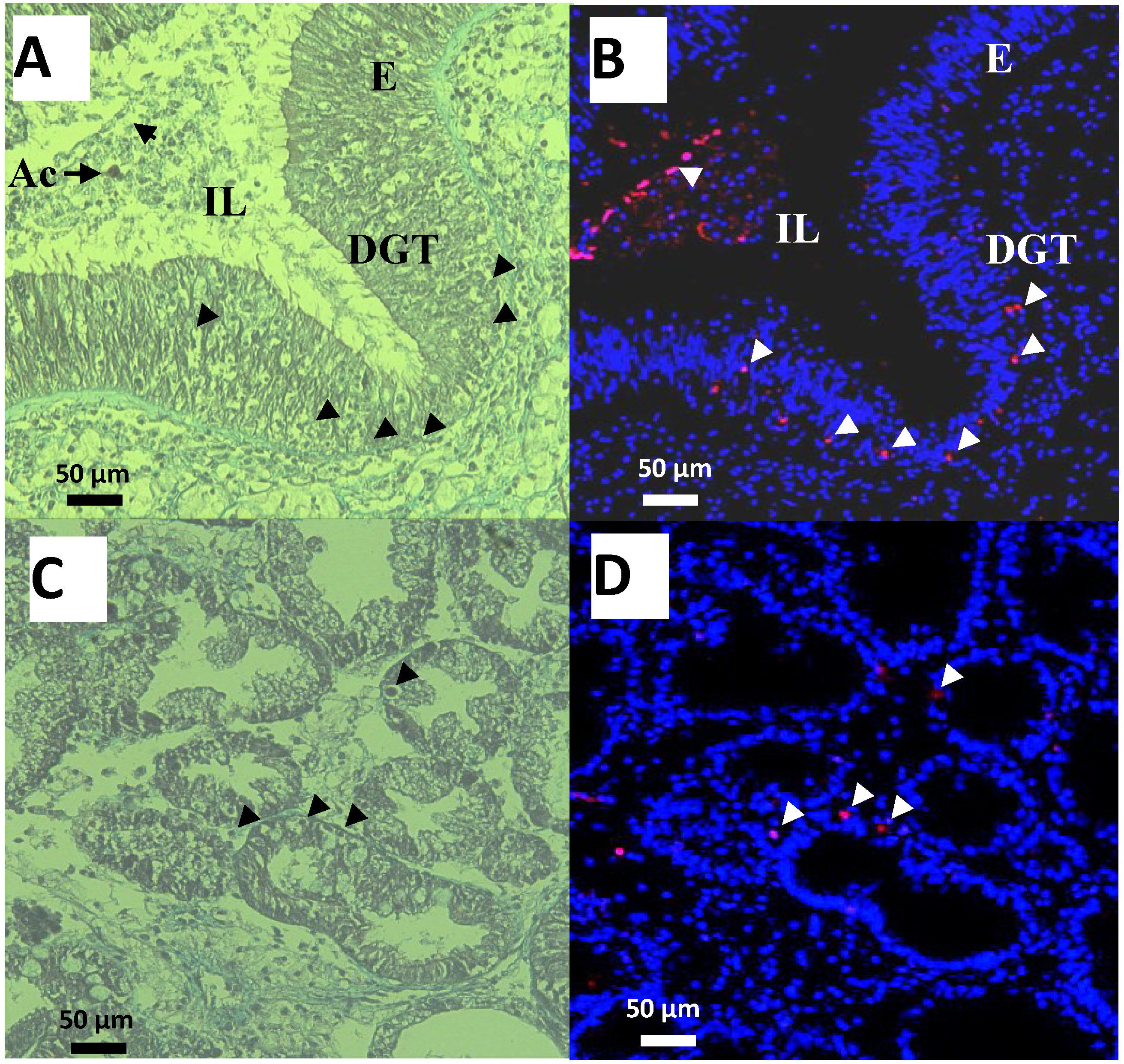
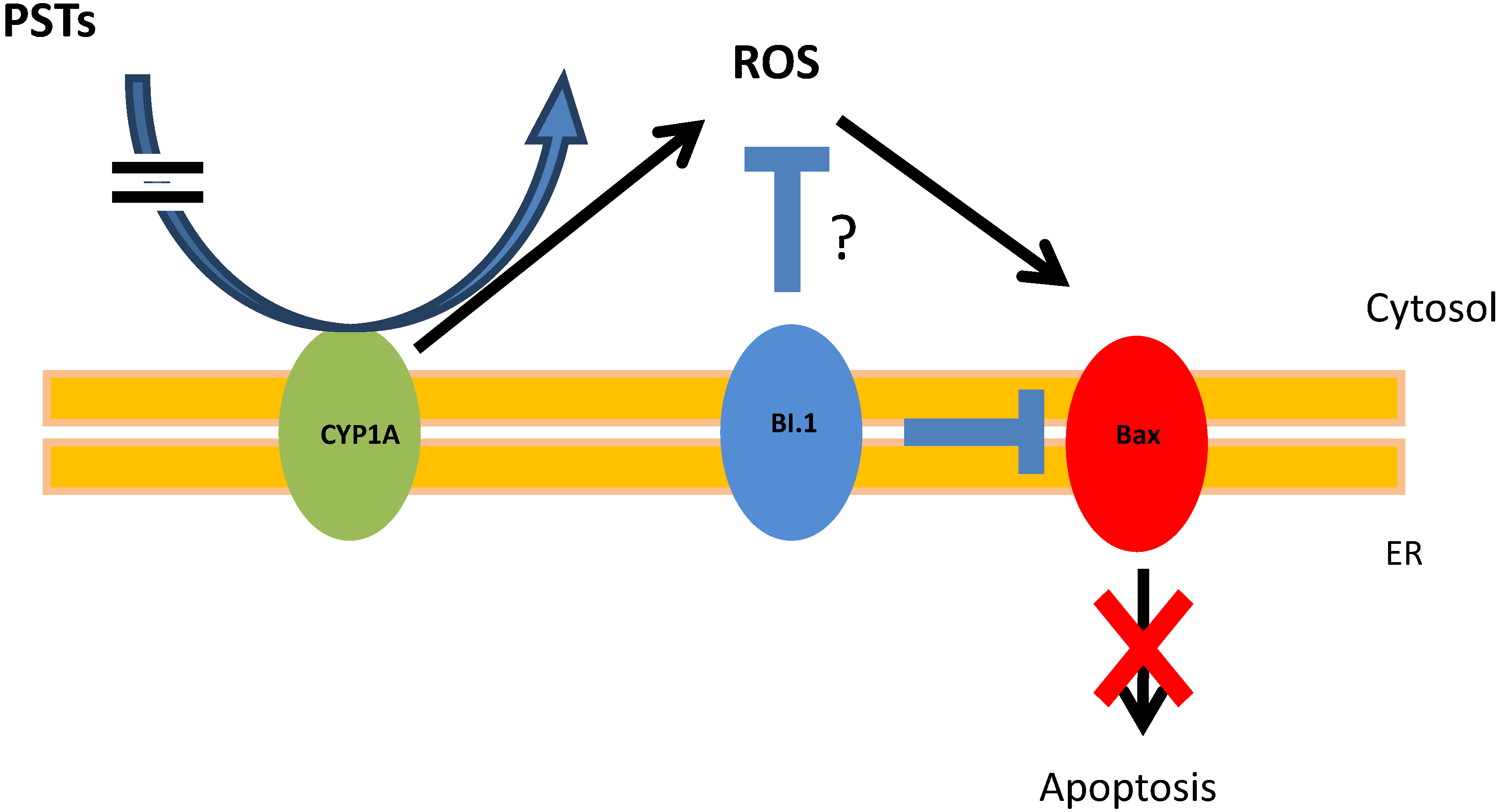
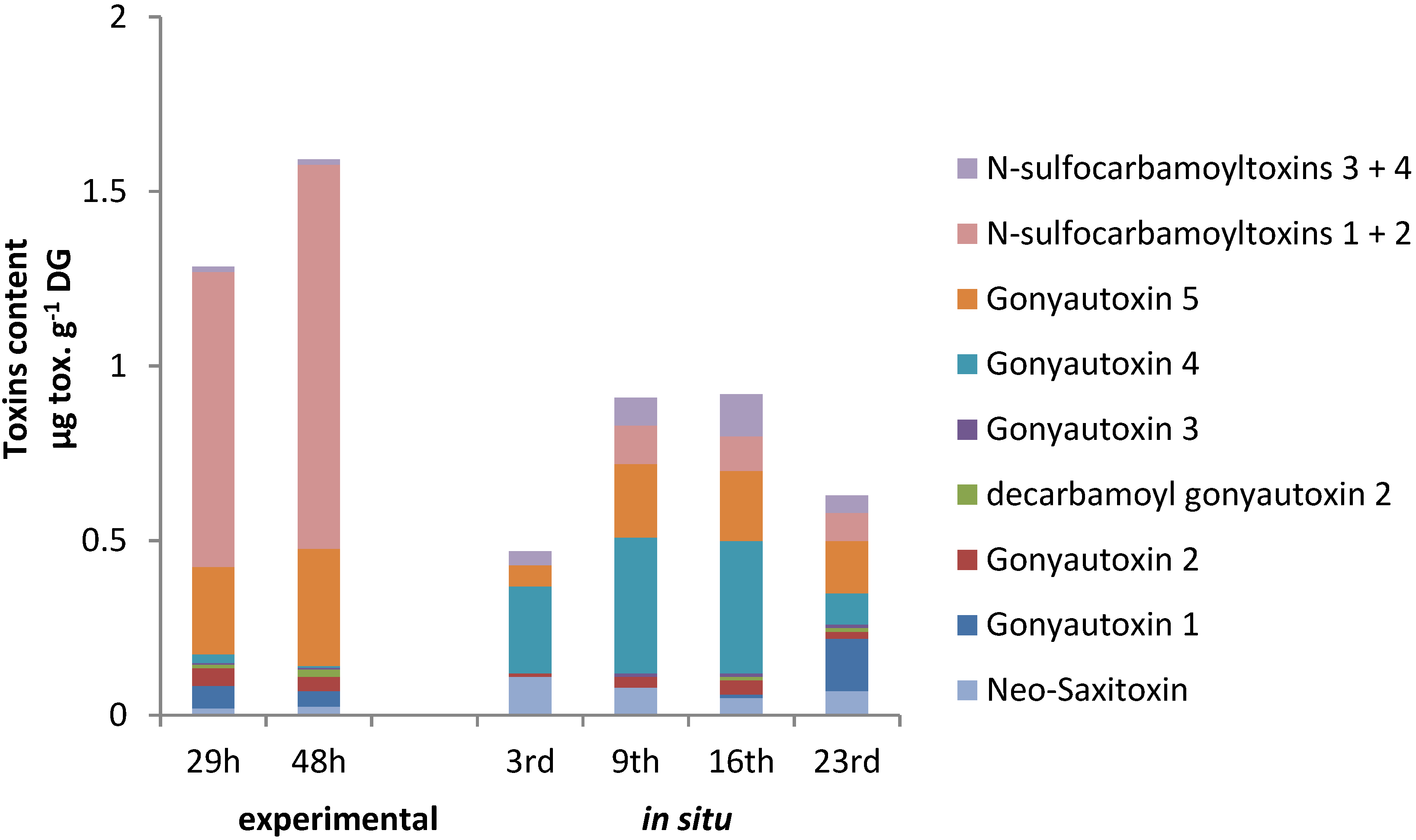
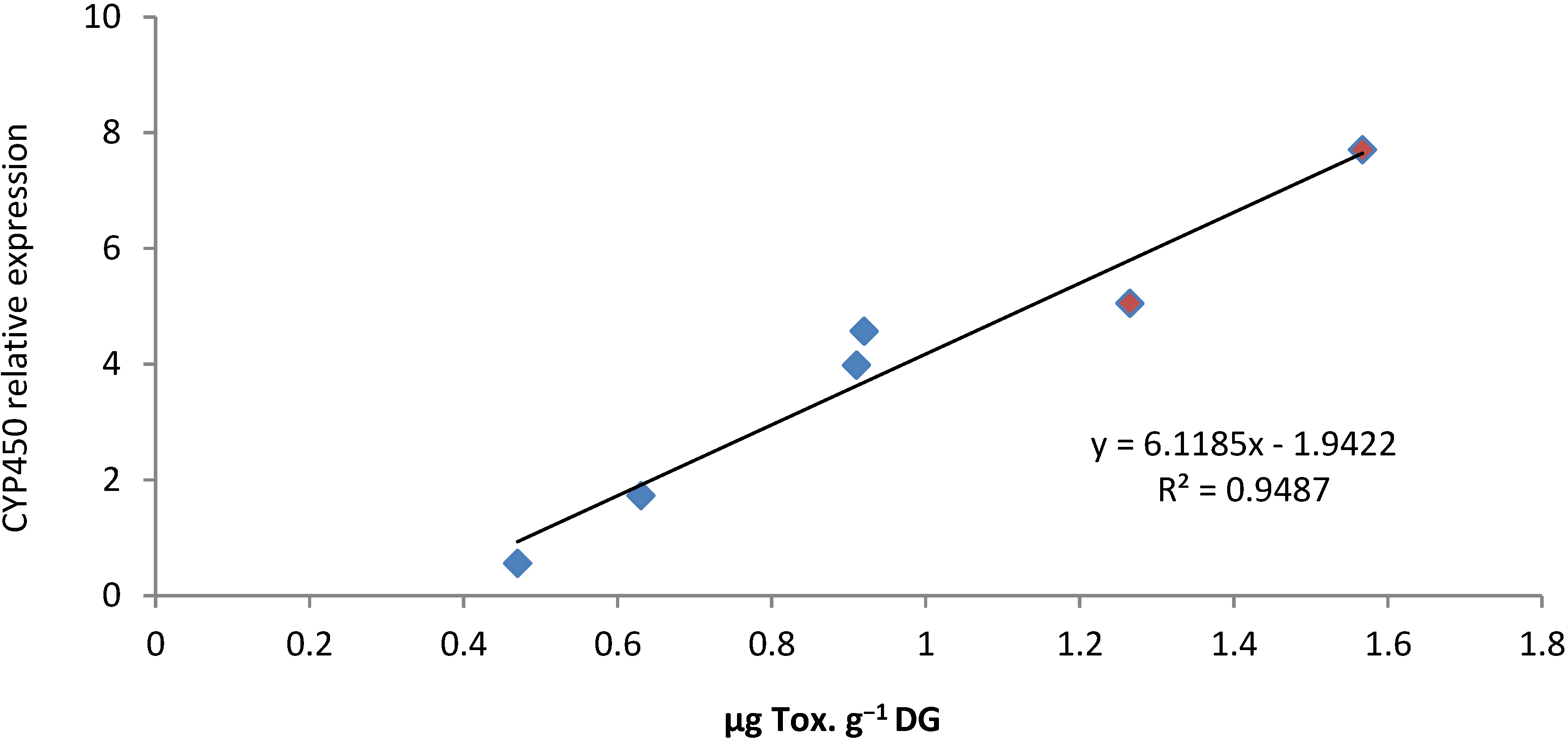
2.4. PSTs Accumulation and Correlations with Natural Exposure to Alexandrium catenella

3. Experimental Section
3.1. In Situ Contamination
3.2. Experimental Exposure to the Neurotoxic Alexandrium catenella
3.2.1. Oysters and Microalgae
3.2.2. Experiments
3.2.3. Tissue Sampling
3.3. Genes Expression Analysis
| Gene | Primers Sequences 5′→3′ | Product Size (bp) | GenBank ID |
|---|---|---|---|
| Cg-CYP1A | GACCGGAATCCAAGACTC GCAGTGTTTCCATGACGAC | 70 | CB 617404 |
| Cg-TransG | AATCTACATCAAGAGTGACG AGGCTGCATTTCGTAAGAGG | 144 | EE 677859 |
| Cg-STrans1C1 | GGATGTCGAGTGTTCAGATG TTCTGTAGTGCTGGACTTTAG | 119 | CB 617550 |
| Cg-GSTO | CTGTCCGTATGCTCAACGAG CTGCTGTGACTATTTGGACC | 188 | CB 617512 |
| Cg-MT4 | CAGCTCACACAGTCCCTTC CATGTACAGTTACACGATGC | 143 | AM 265551 |
3.4. Apoptotic Cells Detection Assay
3.5. Caspase-3/7 and ROS Activities Assays
3.6. Chemical Analysis of PSPs by Liquid Chromatography/Fluorescence Detection (LC/FD)
3.7. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clark, R.F.; Williams, S.R.; Nordt, S.P.; Manoguerra, A.S. A review of selected seafood poisonings. Undersea Hyperb. Med. 1999, 26, 175–184. [Google Scholar] [PubMed]
- Laabir, M.; Jauzein, C.; Genovesi, B.; Masseret, E.; Grzebyk, D.; Cecchi, P.; Vaquer, A.; Perrin, Y.; Colos, Y. Influence of temperature, salinity and irradiance on the growth and cell yield of the harmful red tide dinoflagellate Alexandrium catenella colonizing Mediterranean waters. J. Plankton Res. 2011, 33, 1550–1563. [Google Scholar] [CrossRef]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Catterall, W.A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Zingone, A.; Enevoldsen, H.O. The diversity of harmful algal blooms: A challenge for science and management. Ocean Coast. Manag. 2000, 43, 725–748. [Google Scholar] [CrossRef]
- Shumway, S.E. A review of the effects of algal blooms on shellfish and aquaculture. J. World Aquacul. Soc. 1990, 21, 65–104. [Google Scholar] [CrossRef]
- Bricelj, V.M.; Shumway, S.E. Paralytic shellfish toxins in bivalve mollusks: Occurrence, transfer kinetics and biotransformation. Rev. Fish Sci. 1998, 6, 315–383. [Google Scholar] [CrossRef]
- Gainey, L.F.; Shumway, S.E. A compendium of the responses of bivalve mollusks to toxic dinoflagellates. J. Shellfish Res. 1988, 7, 623–628. [Google Scholar]
- Alexander, J.A.; Stoecker, D.K.; Meritt, D.W.; Alexander, S.T.; Johns, D.; van Heukelem, L.; Glibert, P. Differential production of feces and pseudo-feces by the oyster Crassostrea ariakensis when exposed to diets containing harmful dinoflagellate and raphidophyte species. J. Shellfish Res. 2008, 27, 567–579. [Google Scholar] [CrossRef]
- Li, S.C.; Wang, W.X.; Hsieh, D.P.F. Effects of toxic dinoflagellate Alexandrium tamarense on the energy budgets and growth of two marine bivalves. Mar. Environ. Res. 2002, 53, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Haberkorn, H.; Lambert, C.; Le Goic, N.; Moal, J.; Suquet, M.; Gueguen, M.; Sunila, I.; Soudant, P. Effects of Alexandrium minutum exposure on nutrition-related processes and reproductive output in oysters Crassostrea gigas. Harmful Algae 2010, 9, 427–439. [Google Scholar]
- Bardouil, M.; Bohec, M.; Cormerais, M.; Bougrier, S.; Lassus, P. Experimental study of the effects of a toxic microalgal diet on feeding of the oyster Crassostrea gigas Thunberg. J. Shellfish Res. 1993, 12, 417–422. [Google Scholar]
- Wildish, D.; Lassus, P.; Martin, J.; Saulnier, A.; Bardouil, M. Effect of the PSP-causing dinoflagellate, Alexandrium sp. on the initial feeding response of Crassostrea gigas. Aquat. Living Resour. 1998, 11, 35–43. [Google Scholar]
- Lassus, P.; Baron, R.; Garen, P.; Truquet, P.; Masselin, P.; Bardouil, M.; Leguay, D.; Amzil, Z. Paralytic shellfish poison outbreaks in the Penze estuary: Environmental factors affecting toxin uptake in the oyster, Crassostrea gigas. Aquat. Living Resour. 2004, 17, 207–214. [Google Scholar] [CrossRef]
- Medhioub, W.; Lassus, P.; Truquet, P.; Bardouil, M.; Amzil, Z.; Séchet, V.; Sibat, M.; Soudant, P. Spirolide uptake and detoxification by Crassostrea gigas exposed to the toxic dinoflagellate Alexandrium ostenfeldii. Aquaculture 2012, 358–359, 108–115. [Google Scholar] [CrossRef]
- Rolland, J.L.; Pelletier, K.; Masseret, E.; Rieuvilleneuve, F.; Savar, V.; Santini, A.; Amzil, Z.; Laabir, M. paralytic toxins accumulation and tissue Expression of α-amylase and lipase genes in the pacific oyster Crassostrea gigas fed with the neurotoxic dinoflagellate Alexandrium catenella. Mar. Drugs 2012, 10, 2519–2534. [Google Scholar] [CrossRef]
- Garcia-Lagunas, N.; Romero-Geraldo, R.; Hernandez-Saavedra, N.Y. Genomics Study of the Exposure Effect of Gymnodinium catenatum, a Paralyzing Toxin Producer, on Crassostrea gigas Defense System and Detoxification Genes. PLoS One 2013, 8, e72323. [Google Scholar] [CrossRef] [PubMed]
- Hégaret, H.; Wikfors, G.H.; Soudant, P.; Lambert, C.; Shumway, S.E.; Berard, J.B.; Lassus, P. Toxic dinoflagellates (Alexandrium fundyense and A. catenella) have minimal apparent effect on oyster hemocytes. Mar. Biol. 2007, 152, 441–447. [Google Scholar]
- Hégaret, H.; da Silva, P.M.; Wikfors, G.H.; Haberkorn, H.; Shumway, S.E.; Soudant, P. In vitro interactions between several species of harmful algae and haemocytes of bivalve mollusks. Cell Biol. Toxicol. 2011, 27, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Medhioub, W.; Ramondenc, S.; Vanhove, A.; Vergnes, A.; Masseret, E.; Savar, V.; Amzil, Z.; Laabir, M.; Rolland, J.L. Exposure to the Neurotoxic Dinoflagellate Alexandrium catenella induces apoptosis of the oyster Crassostrea gigas hemocytes. Mar. Drugs 2013, 11, 4799–4814. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, M.; Bardouil, M.; Baron, R.; Lassus, P.; Truquet, P.; Massardier, J.; Amzil, Z. Detoxification of Pacific oyster Crassostrea gigas fed on diets of Skeletonema costatum with and without silt, following PSP contamination by Alexandrium minutum. Aquat. Living Resour. 2008, 1, 13–20. [Google Scholar] [CrossRef]
- Vrolijk, N.H.; Targett, N.M. Biotransformation enzymes in Cyphoma gibbosum (Gastropoda: Ovulidae): Implications for detoxification of gorgonian allelochernicals. Mar. Environ. Res. 1992, 88, 237–246. [Google Scholar]
- Agianian, B.; Tucker, P.A.; Schouten, A.; Leonard, K.; Bullard, B.; Gros, P. Structure of a Drosophila sigma class glutathione S-transferase reveals a novel active site topography suited for lipid peroxidation products. J. Biol. Chem. 2003, 326, 151–165. [Google Scholar]
- Manduzio, H.; Rocher, B.; Durand, F.; Galap, C.; Leboulenger, F. The point about oxidative stress in mollusks. Invertebr. Surviv. J. 2005, 2, 91–104. [Google Scholar]
- Lesser, P.M. Oxidative stress in marine environments: Biochemistry and Physiological Ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Estrada, N.; Campa-Cόrdova, A.; Luna, A.; Ascencio, F. Effects of the toxic dinoflagellate, Gymnodinium catenatum on hydrolytic and antioxidant enzymes, in tissues of the giant lions-paw scallop Nodipecten subnodosus. Comp. Biochem. Phys. C 2007, 146, 502–510. [Google Scholar]
- Whalen, E.K.; Morin, D.; Lin, Y.C.; Tjeerdema, S.R.; Goldstone, V.J. Proteomic identification, cDNA cloning and enzymatic activity of glutathione S-transferases from the generalist marine gastropod, Cyphoma gibbosum. Arch. Biochem. Biophys. 2008, 478, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Pulford, D.J. The glutathione S-Transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 445–600. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, J.J. Benzo[a]pyrene oxidation and microsomal enzyme activity in the mussel (Mytilus edulis) and other bivalve mollusc species from the Western North Atlantic. Mar. Biol. 1985, 89, 21–30. [Google Scholar] [CrossRef]
- Livingstone, D.R. Cytochrome P450 and oxidative metabolism in invertebrates. Biochem. Soc. Trans. 1990, 18, 15–19. [Google Scholar] [PubMed]
- Jo, P.G.; Min, T.S.; An, K.W.; Choi, C.Y. Molecular Cloning and mRNA Expression of Cytochrome P450 (CYP450)-related Protein in the Pacific Oyster, Crassostrea gigas: A Water Temperature and Time Study. Anim. Cells Syst. 2009, 13, 447–452. [Google Scholar]
- Boutet, I.; Tanguy, A.; Moraga, D. Response of the Pacific oyster Crassostrea gigas to hydrocarbon contamination under experimental conditions. Gene 2004, 329, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Mello, D.F.; de Oliveira, E.S.; Vieira, R.C.; Simoes, E.; Trevisan, R.; Dafre, A.L.; Barracco, M.A. Cellular and transcriptional responses of Crassostrea gigas hemocytes exposed in vitro to brevetoxin (PbTx-2). Mar. Drugs 2012, 10, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Enari, M.; Talanian, R.V.; Wrong, W.W.; Nagata, S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature 1996, 380, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.T.; Wolter, K.G.; Youle, R.J. Cytosol-to-membrane redistribution of Bax and Bcl-X-L during apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 3668–3672. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the cytochrome c-initiated caspase cascade: Hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell. Biol. 1999, 144, 281–292. [Google Scholar]
- Antonsson, B.; Montessuit, S.; Sanchez, B.; Martinou, J.C. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 2001, 276, 11615–11623. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, G.S.; Abrams, J.M. Caspase activation–stepping on the gas or releasing the brakes? Lessons from humans and flies. Oncogene 2004, 23, 2774–2784. [Google Scholar] [CrossRef] [PubMed]
- Mello, D.F.; da Silva, P.M.; Barracco, M.A.; Soudant, P.; Hegaret, H. Effects of the dinoflagellate Alexandrium minutum and its toxin (saxitoxin) on the functional activity and gene expression of Crassostrea gigas hemocytes. Harmful Algae 2013, 26, 45–51. [Google Scholar] [CrossRef]
- Puntarulo, S.; Cederbaum, A.I. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic. Biol. Med. 1998, 24, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Shertzer, H.G.; Clay, C.D.; Genter, M.B.; Schneider, S.N.; Nebert, D.W.; Dalton, T.P. CYP1A2 protects against reactive oxygen production in mouse liver microsomes. Free Radic. Biol. Med. 2004, 36, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Kopf, P.G.; Walker, M.K. 2,3,7,8-Tetrachlorodibenzo-p-dioxin Increases Reactive Oxygen Species Production in Human Endothelial Cells Via Induction of Cytochrome P4501A1. Toxicol. Appl. Pharmacol. 2010, 245, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zangar, R.C.; Davydov, D.R.; Verma, S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol. Appl. Pharmacol. 2004, 199, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Reed, J.C. Bax Inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol. Cell 1988, 1, 337–346. [Google Scholar] [CrossRef]
- Lindsay, J.; Esposti, M.D.; Gilmore, A.P. Bcl-2 proteins and mitochondria-Specificity in membrane targeting for death. Biochim. Biophys. Acta 2011, 1813, 532–539. [Google Scholar] [CrossRef]
- Robinson, K.S.; Clements, A.; Williams, A.C.; Berger, C.N.; Frankel, G. Bax Inhibitor 1 in apoptosis and disease. Oncogene 2011, 30, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Park, H.; Park, J.G.; Jun, J.G.; Park, J.H.Y. The Grape Component Piceatannol Induces Apoptosis in DU145 Human Prostate Cancer Cells via the Activation of Extrinsic and Intrinsic Pathways. J. Med. Food 2009, 12, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Kim, H.K.; Chae, S.W.; Kim, D.S.; Ha, K.C.; Cuddy, M.; Kress, C.; Reed John, C.; Kim, H.R.; Chae, H.J. Bax inhibitor-1 regulates endoplasmic reticulum stress-associated reactive oxygen species and heme oxygenase-1 expression. J. Biol. Chem. 2007, 282, 21618–21628. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Gallo, G.; Gavioli, M.; Pruzzo, C. Bacteria-hemocyte interactions and phagocytosis in marine bivalves. Microsc. Res. Tech. 2002, 57, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Lewis, A.M.; Hatfield, R.G.; Galloway, A.W.; Higman, W.A. Transformation of paralytic shellfish poisoning toxins in Crassostrea gigas and Pecten maximus reference materials. Toxicon 2012, 60, 1117–1134. [Google Scholar]
- Fast, M.; Cembella, A.; Ross, N. In vitro transformation of paralytic shellfish toxins in the clams Mya arenaria and Protothaca staminea. Harmful Algae 2006, 5, 79–90. [Google Scholar] [CrossRef]
- Lassus, P.; Amzil, Z.; Baron, R.; Séchet, V.; Barillé, L.; Abadie, E.; Bardouil, M.; Sibat, M.; Truquet, P.; Bereard, J.; et al. Modelling the accumulation of PSP toxins in Thau Lagoon oysters (Crassostrea gigas) from trials using mixed cultures of Alexandrium catenella and Thalassiosira weissflogii. Aquat. Living Resour. 2007, 20, 59–67. [Google Scholar] [CrossRef]
- Contreras, A.M.; Marsden, I.D.; Munro, M.H.G. Effects of short-term exposure to paralytic shellfish toxins on clearance rates and toxin uptake in five species of New Zealand bivalve. Mar. Freshw. Res. 2011, 63, 166–174. [Google Scholar] [CrossRef]
- Lopez-Barea, J.; Pueyo, C. Mutagen content and metabolic activation of promutagens by molluscs as biomarkers of marine pollution. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 1998, 399, 3–15. [Google Scholar]
- Hahn, M.E.; Stegeman, J.J. Regulation of Cytochrome P4501A1 in Teleosts: Sustained Induction of CYP1A1 mRNA, Protein, and Catalytic Activity by 2,3,7,8-Tetrachlorodibenzofuran in the Marine Fish Stenotomus chrysops. Toxicol. Appl. Pharmacol. 1994, 127, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, U.; Stein, J.E.; Nishimoto, M.; Reichert, W.L.; Collier, T.K. Chemical carcinogenesis in feral fish: Uptake, activation, and detoxication of organic xenobiotics. Environ. Health Perspect. 1987, 71, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC) Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Washington, DC, USA, 2005; pp. 79–80.
- Van de Riet, J.; Gibbs, R.S.; Muggah, P.M.; Rourke, W.A.; MacNeil, J.D.; Quilliam, M.A. Liquid Chromatography Post-Column Oxidation (PCOX) Method for the Determination of Paralytic Shellfish Toxins in Mussels, Clams, Oysters, and Scallops: Collaborative Study. J. AOAC Int. 2011, 94, 1154–1176. [Google Scholar]
- Garet, E.; Gonzalez-Fernandez, A.; Lago, J.; Vieites, J.M.; Cabado, A.G. Comparative Evaluation of Enzyme-Linked Immunoassay and Reference Methods for the Detection of Shellfish Hydrophilic Toxins in Several Presentations of Seafood. J. Agric. Food. Chem. 2010, 58, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Dong, J.; Ueda, U. Open-sandwich immunoassay for sensitive and broad-range detection of a shellfish toxin gonyautoxin. Anal. Chim. Acta 2013, 793, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J.; Waters, R.E.; Taylor, F.J.R. A broad-spectrum artificial seawater medium for coastal and open ocean phytoplankton. J. Phycol. 1980, 16, 28–35. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Show, B.I.; Battle, H.I. The gross and microscopic anatomy of the digestive tract of the oyster, Crassostrea virginica (Gmelin). Can. J. Zool. 1957, 35, 325–347. [Google Scholar]
- Bancroft, J.D.; Stevens, A. Theory and Practice of Histological Techniques, 4th ed.; Churchill Livingstone: Edinburgh, UK, 1996. [Google Scholar]
- Ravn, H.; Anthoni, U.; Christophersen, C.; Nielsen, P.H.; Oshima, Y. Standardized extraction method for paralytic shellfish toxins in phytoplankton. J. Appl. Phycol. 1995, 7, 589–594. [Google Scholar] [CrossRef]
- Masselin, P.; Amzil, Z.; Abadie, E.; Nézan, E.; Le Bec, C.; Chiantella, C.; Truquet, P. Paralytic shellfish poisoning on the French Mediterranean coast in the autumn 1998: Alexandrium tamarense complex (Dinophyceae) as causative agent. In Harmful Algal Blooms 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; JOC of UNESCO Publish: Paris, France, 2001; pp. 407–410. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rolland, J.-L.; Medhioub, W.; Vergnes, A.; Abi-khalil, C.; Savar, V.; Abadie, E.; Masseret, E.; Amzil, Z.; Laabir, M. A Feedback Mechanism to Control Apoptosis Occurs in the Digestive Gland of the Oyster Crassostrea gigas Exposed to the Paralytic Shellfish Toxins Producer Alexandrium catenella. Mar. Drugs 2014, 12, 5035-5054. https://doi.org/10.3390/md12095035
Rolland J-L, Medhioub W, Vergnes A, Abi-khalil C, Savar V, Abadie E, Masseret E, Amzil Z, Laabir M. A Feedback Mechanism to Control Apoptosis Occurs in the Digestive Gland of the Oyster Crassostrea gigas Exposed to the Paralytic Shellfish Toxins Producer Alexandrium catenella. Marine Drugs. 2014; 12(9):5035-5054. https://doi.org/10.3390/md12095035
Chicago/Turabian StyleRolland, Jean-Luc, Walid Medhioub, Agnes Vergnes, Celina Abi-khalil, Véronique Savar, Eric Abadie, Estelle Masseret, Zouher Amzil, and Mohamed Laabir. 2014. "A Feedback Mechanism to Control Apoptosis Occurs in the Digestive Gland of the Oyster Crassostrea gigas Exposed to the Paralytic Shellfish Toxins Producer Alexandrium catenella" Marine Drugs 12, no. 9: 5035-5054. https://doi.org/10.3390/md12095035
APA StyleRolland, J.-L., Medhioub, W., Vergnes, A., Abi-khalil, C., Savar, V., Abadie, E., Masseret, E., Amzil, Z., & Laabir, M. (2014). A Feedback Mechanism to Control Apoptosis Occurs in the Digestive Gland of the Oyster Crassostrea gigas Exposed to the Paralytic Shellfish Toxins Producer Alexandrium catenella. Marine Drugs, 12(9), 5035-5054. https://doi.org/10.3390/md12095035






