A New Cytotoxicity Assay for Brevetoxins Using Fluorescence Microscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cell Line Survey for Brevetoxin Cytotoxicity
| Cell Line | Cell Type | Seeded Density | 24 h EC50 | 48 h EC50 | 72 h EC50 |
|---|---|---|---|---|---|
| B35 | Rat neuronal neuroblastoma | 5000 | 3.39 × 10−6 M | 6.40 × 10−7 M | 4.57 × 10−7 M |
| B50 | Rat neuronal neuroblastoma | 5000 | 5.24 × 10−6 M | 2.22 × 10−6 M | 2.70 × 10−6 M |
| BE(2)-M17 | Human neuroblastoma | 5000 | 2.60 × 10−6 M | 9.40 × 10−7 M | 8.77 × 10−7 M |
| Neuro-2A | Mouse neuronal neuroblastoma | 5000 | 3.66 × 10−6 M | 1.94 × 10−6 M | 2.18 × 10−6 M |
| PC-12 | Rat adrenal pheochromocytoma | 10,000 | 3.60 × 10−6 M | 2.92 × 10−6 M | 2.99 × 10−6 M |
| SH-SY5Y | Human bone marrow neuroblastoma | 5000 | 2.26 × 10−6 M | 1.14 × 10−6 M | 8.11 × 10−7 M |
| SJCRH30 | Human muscle rhabdomyosarcoma | 5000 | 1.70 × 10−6 M | 8.61 × 10−7 M | 7.69 × 10−7 M |

| Cell Line | Time Point | Cell Count without Ouabain/Veratridine | Cell Count with Ouabain/Veratridine | Percentage Knockdown (of 24 h) |
|---|---|---|---|---|
| B35 | 24 h | 199.2 | 105.4 | 47% |
| B35 | 48 h | 420.9 | 120.3 | 40% |
| B35 | 72 h | 1020.3 | 153.2 | 23% |
| B50 | 24 h | 201.6 | 106.9 | 47% |
| B50 | 48 h | 541.1 | 122.8 | 39% |
| B50 | 72 h | 820.6 | 158.4 | 21% |
| BE(2)-M17 | 24 h | 164.3 | 5.5 | 97% |
| BE(2)-M17 | 48 h | 296.0 | 3.9 | 98% |
| BE(2)-M17 | 72 h | 696.2 | 7.8 | 95% |
| Neuro-2A | 24 h | 532.0 | 270.8 | 49% |
| Neuro-2A | 48 h | 722.8 | 105.2 | 80% |
| Neuro-2A | 72 h | 766.8 | 48.3 | 91% |
| PC-12 | 24 h | 334.2 | 275.3 | 18% |
| PC-12 | 48 h | 540.1 | 317.4 | 5% |
| PC-12 | 72 h | 927.5 | 602.2 | −80% |
| SH-SY5Y | 24 h | 73.5 | 8.2 | 89% |
| SH-SY5Y | 48 h | 71.0 | 9.7 | 87% |
| SH-SY5Y | 72 h | 62.4 | 2.7 | 96% |
| SJCRH30 | 24 h | 208.0 | 1.8 | 99% |
| SJCRH30 | 48 h | 306.0 | 0.4 | 100% |
| SJCRH30 | 72 h | 570.1 | 0.1 | 100% |
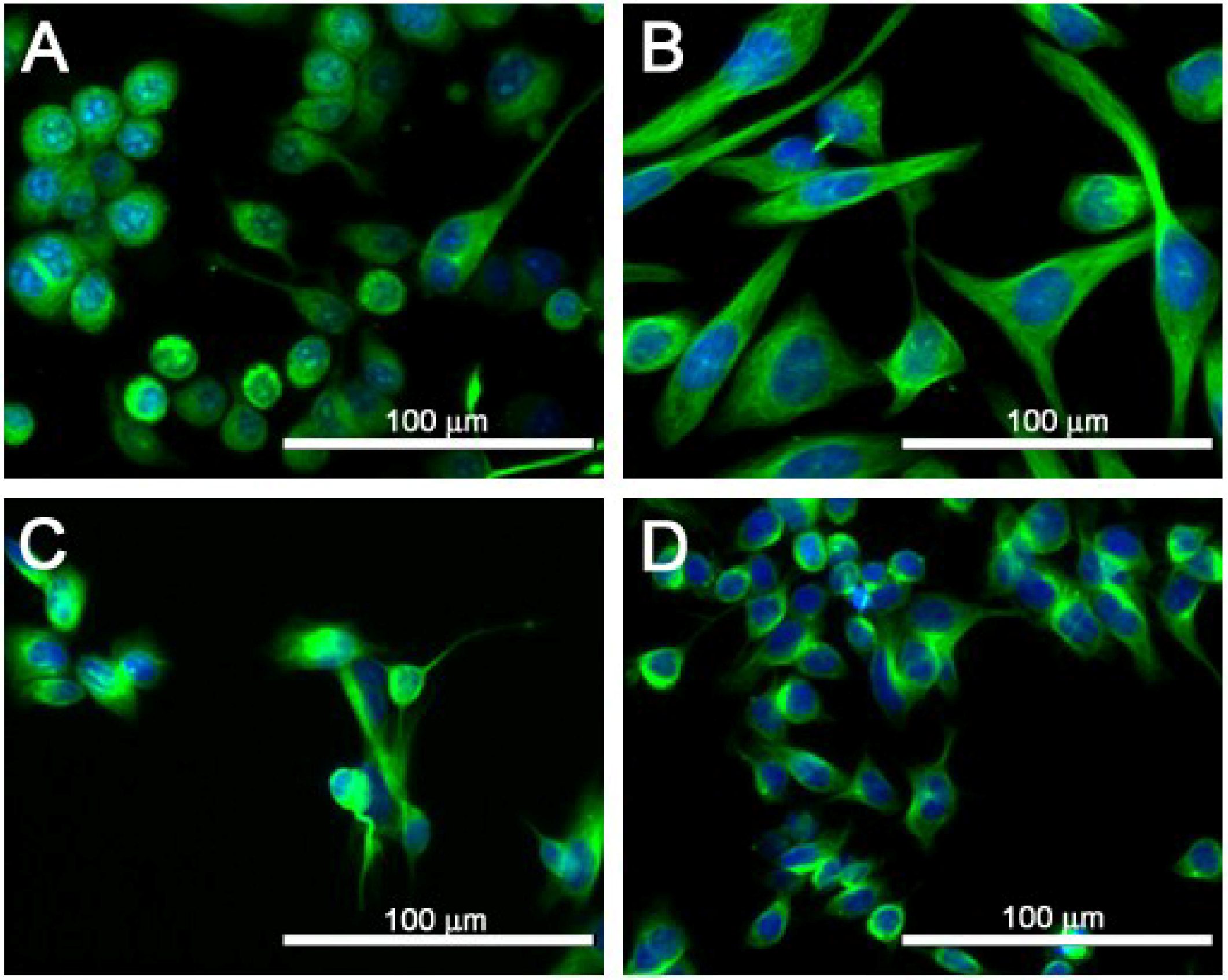
2.2. Cytotoxicity of PbTx-1 and PbTx-2 to SJCRH30 and Neuro-2A Cell Lines
| Cell Line | H-Dye: 24 h | H-Dye: 48 h | XTT: 24 h | XTT: 48 h |
|---|---|---|---|---|
| SJCRH30 | 2.38 ± 0.69 μM | 1.79 ± 0.45 μM | 3.23 ± 0.90 μM | 2.04 ± 0.07 μM |
| Neuro-2A | 2.80 ± 0.91 μM | 2.51 ± 0.25 μM * | 3.75 ± 1.16 μM | 3.93 ± 1.07 μM * |
| Neuro-2A (25 μM Veratridine, 500 μM ouabain) | 6.69 ± 3.12 nM *,# | 6.61 ± 1.29 nM *,# | 10.2 ± 6.57 μM | 2.85 ± 1.18 μM |
| Neuro-2A (50 μM Veratridine, 500 μM ouabain) | 2.79 ± 1.24 nM *,# | 4.74 ± 2.18 nM *,# | 0.73 ± 1.20 μM *,# | 0.67 ± 1.06 μM # |
| Cell Line | H-Dye: 24 h | H-Dye: 48 h | XTT: 24 h | XTT: 48 h |
|---|---|---|---|---|
| SJCRH30 | 1.76 ± 0.37 μM | 0.52 ± 0.09 μM | 2.60 ± 1.33 μM | 1.10 ± 0.97 μM |
| Neuro-2A | 2.09 ± 0.65 μM | 1.94 ± 0.17 μM * | 2.60 ± 0.31 μM | 2.83 ± 0.69 μM |
| Neuro-2A (25 μM veratridine, 500 μM ouabain) | 2.40 ± 1.80 μM | 0.43 ± 0.68 μM # | 6.78 ± 3.30 μM | 5.49 ± 0.90 μM * |
| Neuro-2A (50 μM veratridine, 500 μM ouabain) | 20.6 ± 6.23 nM *,# | 17.9 ± 8.87 nM *,# | 1.59 ± 1.41 μM | 3.99 ± 3.61 μM |
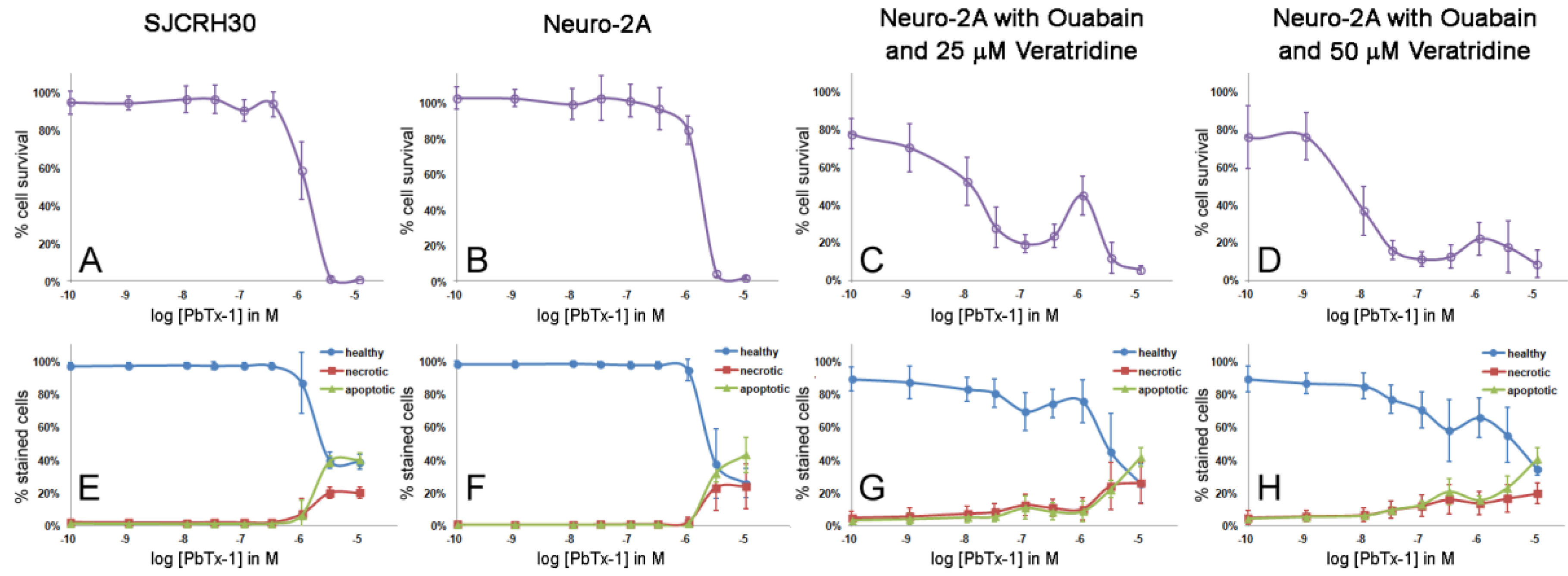
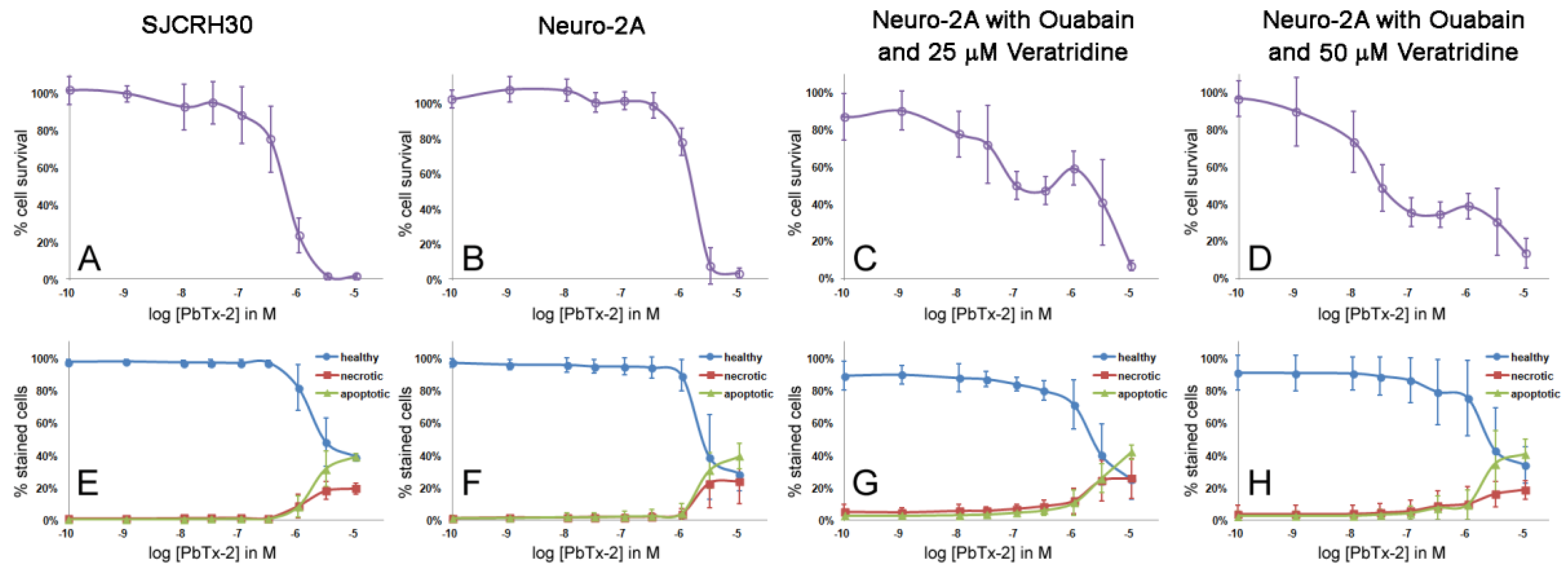
2.3. Apoptosis/Necrosis Effects of PbTx and/or Ouabain and Veratridine
2.4. Cell Morphology of SJCRH30 and Neuro-2A Cell Lines
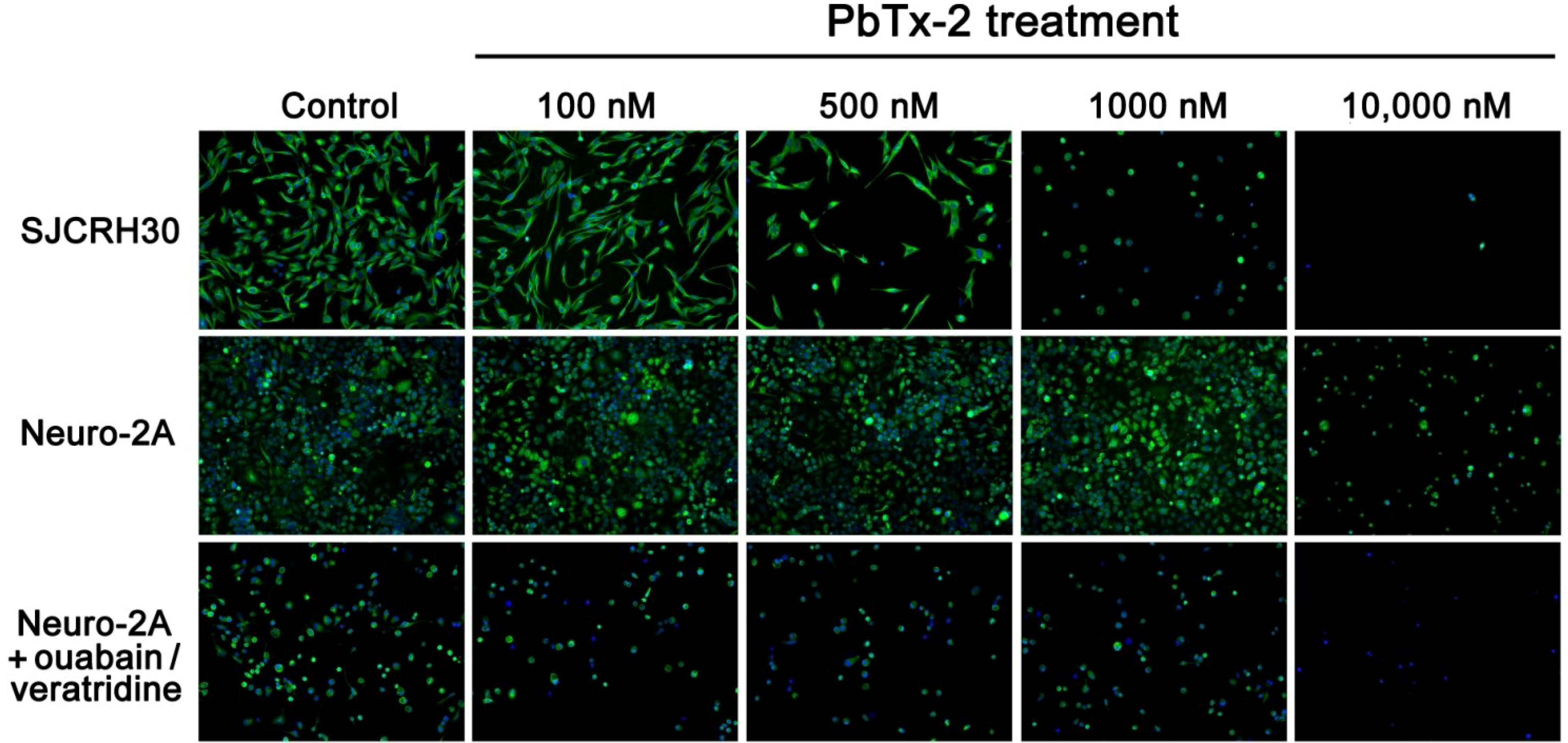
| [PbTx-2] | Cell Count | Cell Area | Nuclear Area | % Cells with Tubulin | Tubulin Area |
|---|---|---|---|---|---|
| 0 | 289 | 637.8 | 203.0 | 95.1% | 621.1 |
| 100 nM | 234 | 681.2 | 209.7 | 98.3% | 674.6 |
| 500 nM | 74 | 908.6 | 258.7 | 94.6% | 899.2 |
| 1000 nM | 50 | 367.1 | 222.4 | 74.0% | 331.5 |
| 10,000 nM | 3 | 352.5 | 251.7 | 66.7% | 290.1 |
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baden, D.G.; Tomas, C.R. Variations in major toxin composition for six clones of Ptychodiscus brevis. Toxicon 1988, 26, 961–963. [Google Scholar] [PubMed]
- Bossart, G.D.; Baden, D.G.; Ewing, R.Y.; Roberts, B.; Wright, S.D. Brevetoxicosis in manatees (Trichechus manatus latirostris) from the 1996 epizootic: Gross, histologic, and immunohistochemical features. Toxicol. Pathol. 1998, 26, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Kreuder, C.; Mazet, J.A.; Bossart, G.D.; Carpender, T.E.; Holyoak, M.; Elie, M.S.; Wright, S.D. Clinicopathologic features of suspected brevetoxicosis in double-crested cormorants (Phalacrocorax auritus) along the Florida gulf coast. J. Zool. Wildl. Med. 2002, 33, 8–15. [Google Scholar]
- Flewelling, L.J.; Naar, J.P.; Abbot, J.P.; Baden, D.G.; Barros, N.B.; Bossart, G.D.; Bottein, M.D.; Hammond, D.G.; Haubold, E.M.; Heil, C.A.; et al. Red tides and marine mammal mortalities: Unexpected brevetoxin vectors may account for deaths long after or remote from an algal bloom. Nature 2005, 435, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.J.; Leggett, S.R.; Carter, B.J.; Colle, C. Effects of brevetoxin exposure on the immune system of loggerhead sea turtles. Aquat. Toxicol. 2010, 97, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Jeglitsch, G.; Rein, K.; Baden, D.G.; Adams, D.G. Brevetoxin-3 (PbTx-3) and its derivatives modulate single tetrodotoxin-sensitive sodium channels in rat sensory neurons. J. Pharmacol. Exp. Ther. 1998, 284, 516–525. [Google Scholar] [PubMed]
- Purkerson, S.L.; Baden, D.G.; Fieber, L.A. Brevetoxin modulates neuronal sodium cannels in two cell lines derived from rat brain. Neurotoxicology 1999, 6, 909–920. [Google Scholar]
- Baden, D.G.; Bourdelais, A.J.; Jacocks, H.; Michelliza, S.; Naar, J. Natural and derivative brevetoxins: Historical background, multiplicity, and effects. Environ. Health Perspect. 2005, 113, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Poli, M.A.; Musser, S.M.; Dickey, R.W.; Eilers, P.P.; Hall, S. Neurotoxic shellfish poisoning and brevetoxin metabolites: A case study from Florida. Toxicon 2000, 38, 981–993. [Google Scholar] [PubMed]
- Watkins, S.; Reich, A.; Fleming, L.; Hammond, R. Neurotoxic shellfish poisoning. Mar. Drugs 2008, 6, 431–455. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; Fleming, L.E.; Rowan, A.; Cheng, Y.S.; Benson, J.; Pierce, R.H.; Zaias, J.; Bean, J.; Bossard, G.D.; Johnson, D.; et al. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae 2003, 2, 19–28. [Google Scholar]
- Kirkpatrick, B.; Fleming, L.E.; Backer, L.C.; Bean, J.A.; Tamer, R.; Kirkpatrick, G.; Kane, T.; Wanner, A.; Dalpra, D.; Reich, A.; et al. Environmental exposures to Florida red tides: Effects on emergency room respiratory diagnoses admissions. Harmful Algae 2006, 5, 526–533. [Google Scholar] [PubMed]
- Fleming, L.E.; Kirkpatrick, B.; Backer, L.C.; Bean, J.A.; Wanner, A.; Reich, A.; Zaias, J.; Cheng, Y.S.; Pierce, R.; Naar, J.; et al. Aerosolized red-tide toxins (brevetoxins) and asthma. Chest 2007, 131, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Dickey, R.; Jester, E.; Granade, R.; Mowdy, D.; Moncreiff, C.; Rebarchik, D.; Robl, M.; Musser, S.; Poli, M. Monitoring Brevetoxins During a Gymnodinium Breve Red Tide: Comparison of Sodium Channel Specific Cytotoxicity Assay and Mouse Bioassay for Determination of Neurotoxic Shellfish Toxins in Shellfish Extracts. Nat. Toxins 1999, 7, 157–165. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Fish and Fisheries Products Hazards and Control Guide, 4th ed.; Center for Food Safety and Applied Nutrition, US Food and Drug Administration: Rockville, MD, USA, 2011. [Google Scholar]
- FAO Food and Nutrition Paper 80. Marine Biotoxins; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Poli, M.A.; Mende, T.J.; Baden, D.G. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol. Pharmacol. 1986, 30, 129–135. [Google Scholar] [PubMed]
- Worrell, C.; Xiao, N.; Vidal, J.E.; Chen, L.; Zhong, B.; Remais, R. Field detection of Schistosoma japonicum cercariae in environmental water samples by quantitative PCR. Appl. Environ. Microbiol. 2011, 77, 2192–2195. [Google Scholar] [CrossRef] [PubMed]
- Kogure, K.; Tamplin, M.L.; Simidu, U.; Colwell, R.R. A tissue culture assay for tetrodotoxin, saxitoxin and related toxins. Toxicon 1988, 26, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Jellett, J.F.; Marks, L.J.; Stewart, J.E.; Dorey, M.L.; Watson-Wright, W.; Lawrence, J.F. Paralytic shellfish poison (saxitoxin family) bioassays: Automated endpoint determination and standardization of the in vitro tissue culture bioassay, and comparison with the standard mouse bioassay. Toxicon 1992, 30, 1146–1156. [Google Scholar] [CrossRef]
- Manger, R.L.; Leja, L.S.; Lee, S.Y.; Hungerford, J.M.; Wekell, M.M. Tetrazolium-based cell bioassay for neurotoxins active on voltage-sensitive sodium channels: Semiautomated assay for saxitoxins, brevetoxins, and ciguatoxins. Anal. Biochem. 1993, 214, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Manger, R.L.; Leja, L.S.; Lee, S.Y.; Hungerford, J.M.; Hokama, Y.; Dickey, R.W.; Granade, H.R.; Lewins, R.; Yasumoto, T.; Wekell, M.M. Detection of sodium channel toxins: Directed cytotoxicity assay of purified ciguatoxins, brevetoxins, saxitoxins, and seafood extracts. J. AOAC Int. 1995, 78, 521–527. [Google Scholar] [PubMed]
- Abraham, A.; Plakas, S.M.; Wang, Z.; Jester, E.L.E.; El Said, K.R.; Granade, H.R.; Henry, M.S.; Blum, P.C.; Pierce, R.H.; Dickey, R.W. Characterization of polar brevetoxin derivatives isolated from Karenia brevis cultures and natural blooms. Toxicon 2006, 48, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Twiner, M.J.; Bottein Dechraoui, M.Y.; Wang, Z.; Mikulski, C.M.; Henry, M.S.; Pierce, R.H.; Doucette, G.J. Extraction and analysis of lipophilic brevetoxins from the red tide dinoflagellate Karenia brevis. Anal. Biochem. 2007, 369, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Truman, P. A cellular target for the lipophilic toxins from Karenia brevisulcata. Toxicon 2007, 50, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Roth, P.B.; Twiner, M.J.; Wang, Z.; Bottein Dechraoui, M.Y.; Doucette, G.J. Fate and distribution of brevetoxin (PbTx) following lysis of Karenia brevis by algicidal bacteria, including analysis of open A-ring derivatives. Toxicon 2007, 50, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Bottein Dechraoui, M.Y.; Fuquay, J.M.; Munday, R.; Selwood, A.I.; van Ginkel, R.; Miles, C.O.; Loader, J.I.; Wilkins, A.L.; Ramsdell, J.S. Bioassay methods for detection of N-palmitoylbrevetoxin-B2 (BTX-B4). Toxicon 2010, 55, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Bottein Dechraoui, M.Y.; Tiedeken, J.A.; Persad, R.; Wang, Z.; Granade, H.R.; Dickey, R.W.; Ramsdell, J.S. Use of two detection methods to discriminate ciguatoxins from brevetoxins: Application to great barracuda from Florida Keys. Toxicon 2005, 46, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Fairey, E.R.; Edmunds, J.S.G.; Ramsdell, J.S. A cell-based assay for brevetoxins, saxitoxins, and ciguatoxins usaing a stably expressed c-fos—luciferase reporter gene. Anal. Biochem. 1997, 251, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Dechraoui, M.Y.; Naar, J.; Pauillac, S.; Legrand, A.M. Ciguatoxins and brevetoxins, neurotoxic polyether compounds active on sodium channels. Toxicon 1999, 37, 125–143. [Google Scholar] [CrossRef]
- Fairey, E.R.; Bottein Dechraoui, M.Y.; Sheets, M.F.; Ramsdell, J.S. Modification of the cell based assay for brevetoxins using human cardiac voltage dependent sodium channels expressed in HEK-293 cells. Biosen. Bioelectron. 2001, 16, 579–586. [Google Scholar] [CrossRef]
- Koopman, G.; Reutelingsperger, C.P.; Kuijten, G.A.; Keehnen, R.M.; Pals, S.T.; van Oers, M.H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994, 84, 1415–1420. [Google Scholar] [PubMed]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar]
- Choi, V.W.Y.; Yum, E.H.W.; Konishi, T.; Oikawa, M.; Cheng, S.H.; Yu, K.N. Triphasic Low-dose response in zebrafish embryos irradiated by microbean protons. J. Radiat. Res. 2012, 53, 475–481. [Google Scholar] [PubMed]
- Calabrese, E.J. Hormesis: A revolution in toxicology, risk assessment and medicine. EMBO Rep. 2004, 5, S37–S40. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Blain, R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: An overview. Toxicol. Appl. Pharmacol. 2005, 202, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Bourdelais, A.J.; Campbell, S.; Jacocks, H.; Naar, J.; Wright, J.L.C.; Carsi, J.; Baden, D.G. Brevenal is a natural inhibitor of brevetoxin action in sodium channel receptor binding assays. Cell. Mol. Neurobiol. 2004, 24, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Truxal, L.T.; Bourdelais, A.J.; Jacocks, H.; Abraham, W.M.; Baden, D.G. Characterization of Tamulamides A and B, polyethers isolated from the marine dinoflagellate Karenia brevis. J. Nat. Prod. 2010, 73, 536–540. [Google Scholar] [CrossRef]
- McCall, J.R.; Jacocks, H.M.; Baden, D.G.; Bourdelais, A.J. Development of a competitive fluorescence-based synaptosome binding assay for brevetoxins. Harmful Algae 2012, 19, 85–91. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
McCall, J.R.; Elliott, E.A.; Bourdelais, A.J. A New Cytotoxicity Assay for Brevetoxins Using Fluorescence Microscopy. Mar. Drugs 2014, 12, 4868-4882. https://doi.org/10.3390/md12094868
McCall JR, Elliott EA, Bourdelais AJ. A New Cytotoxicity Assay for Brevetoxins Using Fluorescence Microscopy. Marine Drugs. 2014; 12(9):4868-4882. https://doi.org/10.3390/md12094868
Chicago/Turabian StyleMcCall, Jennifer R., Elizabeth A. Elliott, and Andrea J. Bourdelais. 2014. "A New Cytotoxicity Assay for Brevetoxins Using Fluorescence Microscopy" Marine Drugs 12, no. 9: 4868-4882. https://doi.org/10.3390/md12094868
APA StyleMcCall, J. R., Elliott, E. A., & Bourdelais, A. J. (2014). A New Cytotoxicity Assay for Brevetoxins Using Fluorescence Microscopy. Marine Drugs, 12(9), 4868-4882. https://doi.org/10.3390/md12094868






