Nocapyrones: α- and γ-Pyrones from a Marine-Derived Nocardiopsis sp.
Abstract
:1. Introduction
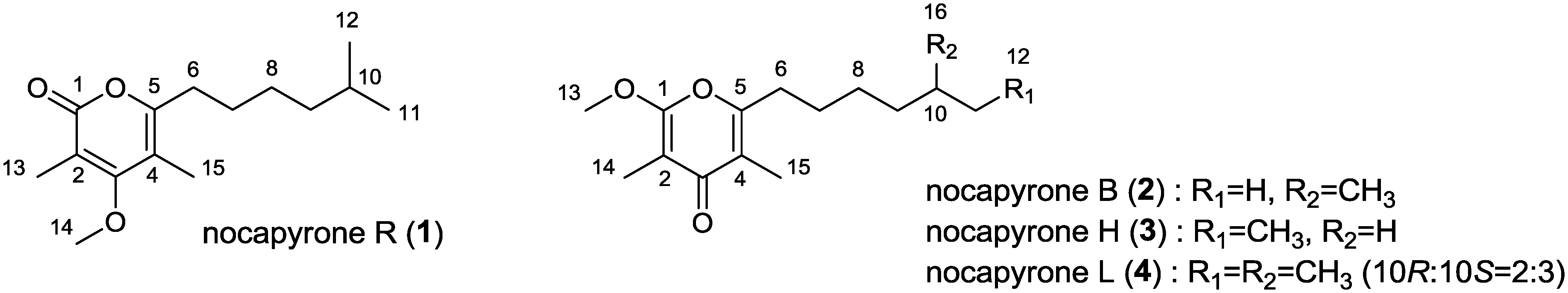
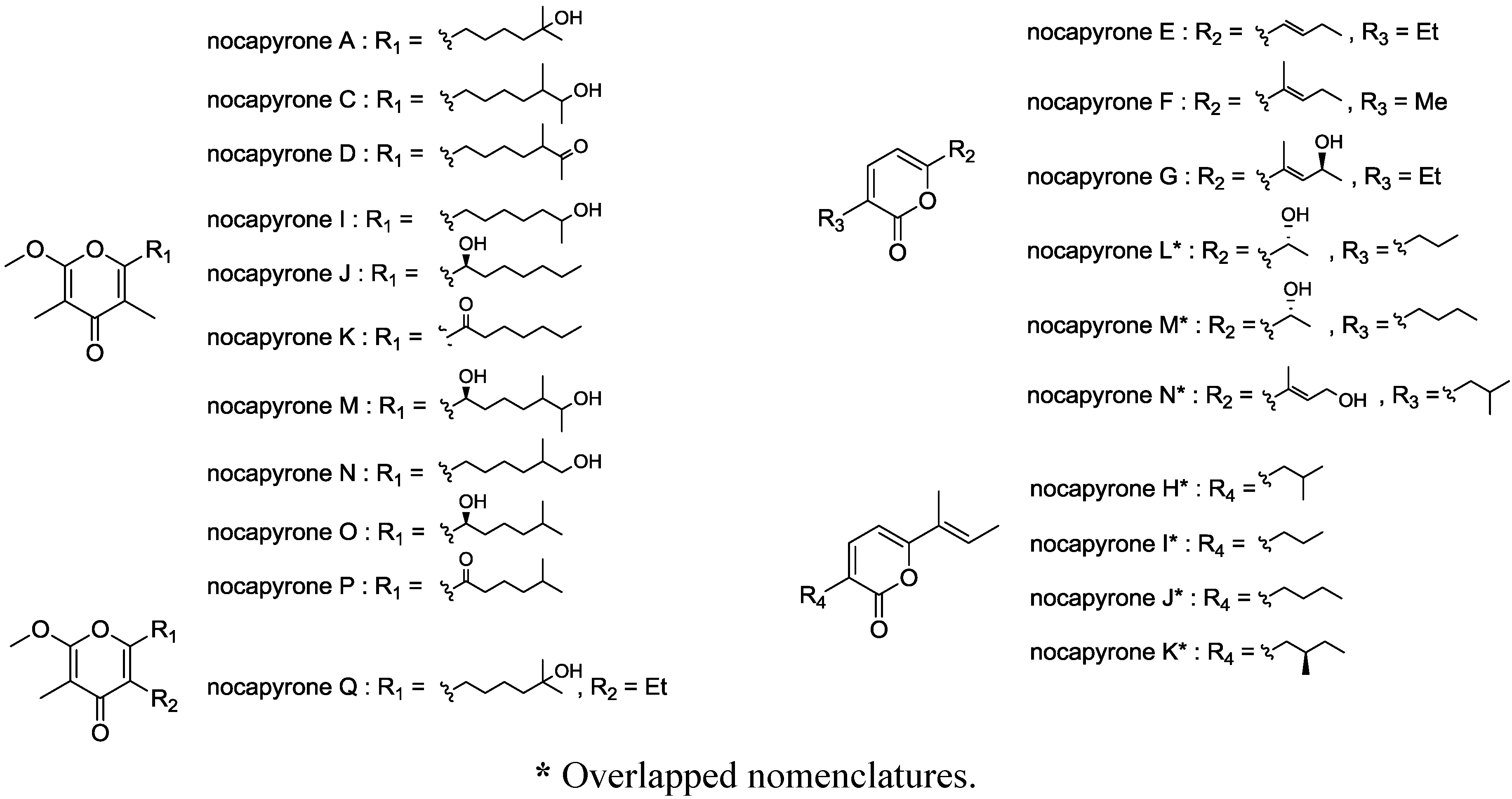
2. Results and Discussion
2.1. Structure Analysis and Characterization
2.1.1. Isolation
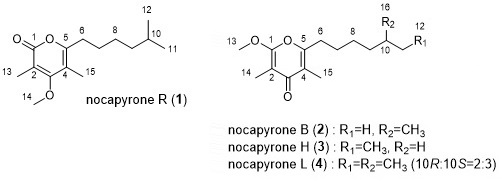
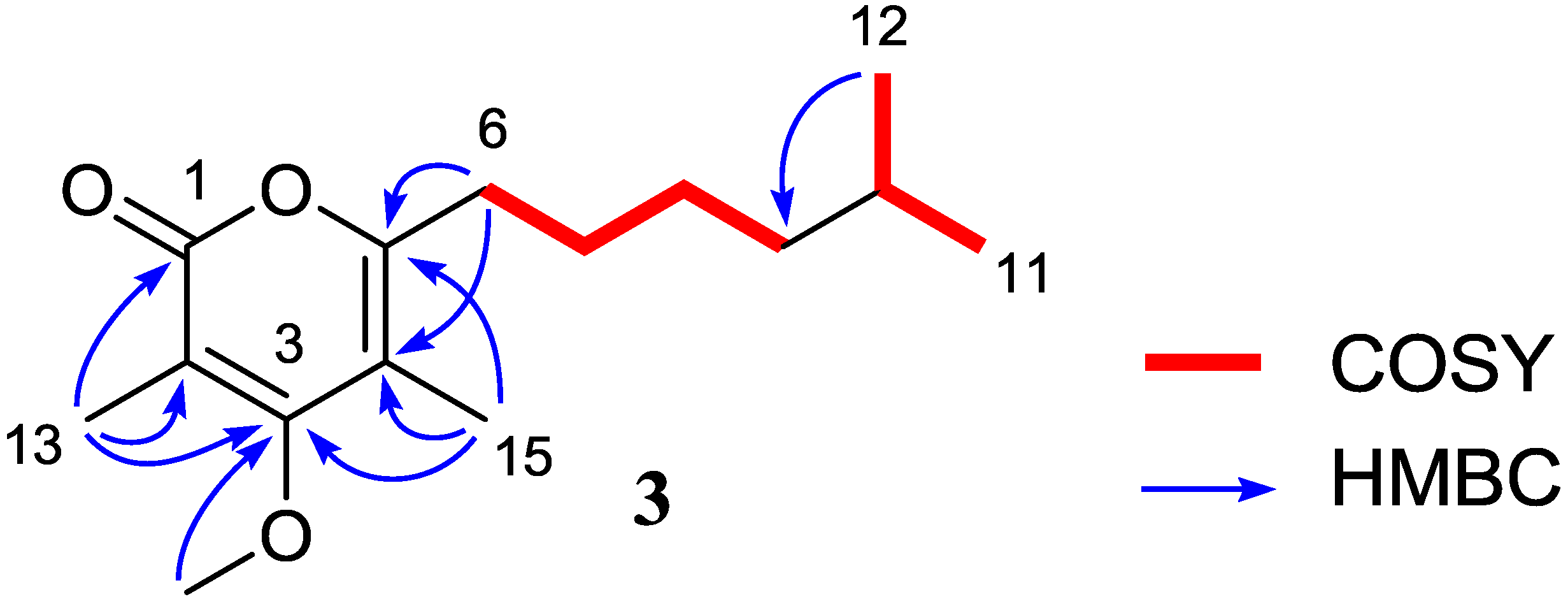
2.1.2. Nocapyrone R (1)
| Position | δC a | δH mult (J in Hz) b | COSY b | HMBC b,c |
|---|---|---|---|---|
| 1 | 166.3, qC | |||
| 2 | 109.3, qC | |||
| 3 | 168.4, qC | |||
| 4 | 109.0, qC | |||
| 5 | 159.3, qC | |||
| 6 | 31.1, CH2 | 2.49, t (7.8) | 7 | 4, 5, 7 |
| 7 | 27.7, CH2 | 1.61, m | 6, 8 | 5 |
| 8 | 27.1, CH2 | 1.31, m | 7, 9 | 10 |
| 9 | 38.7, CH2 | 1.18, m | 8 | 7, 11, 12 |
| 10 | 27.9, CH | 1.52, m | 11, 12 | |
| 11 | 22.6, CH3 | 0.86, d (6.5) | 10, 12 | 9, 10, 12 |
| 12 | 22.6, CH3 | 0.86, d (6.5) | 10, 11 | 9, 10, 11 |
| 13 | 10.2, CH3 | 2.03, s | 1, 2, 3 | |
| 14 | 60.2, CH3 | 3.80, s | 3 | |
| 15 | 10.1, CH3 | 1.92, s | 3, 4, 5 |
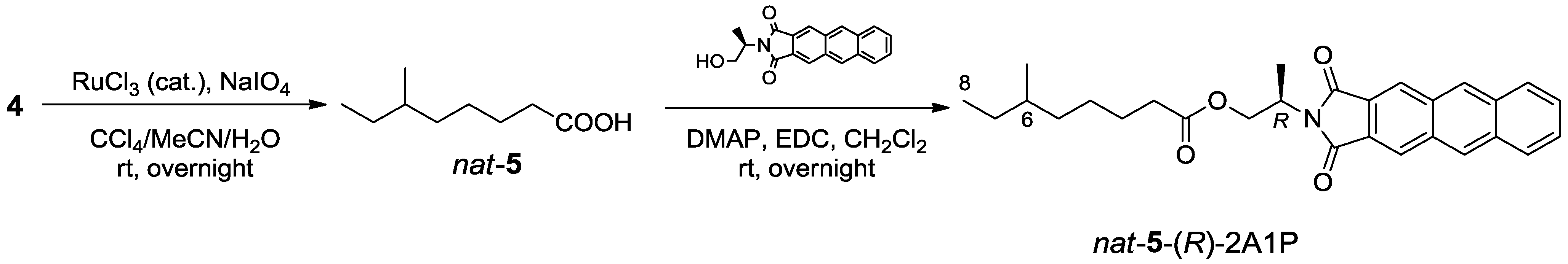
2.1.3. Absolute Configuration of Nocapyrone L (4)
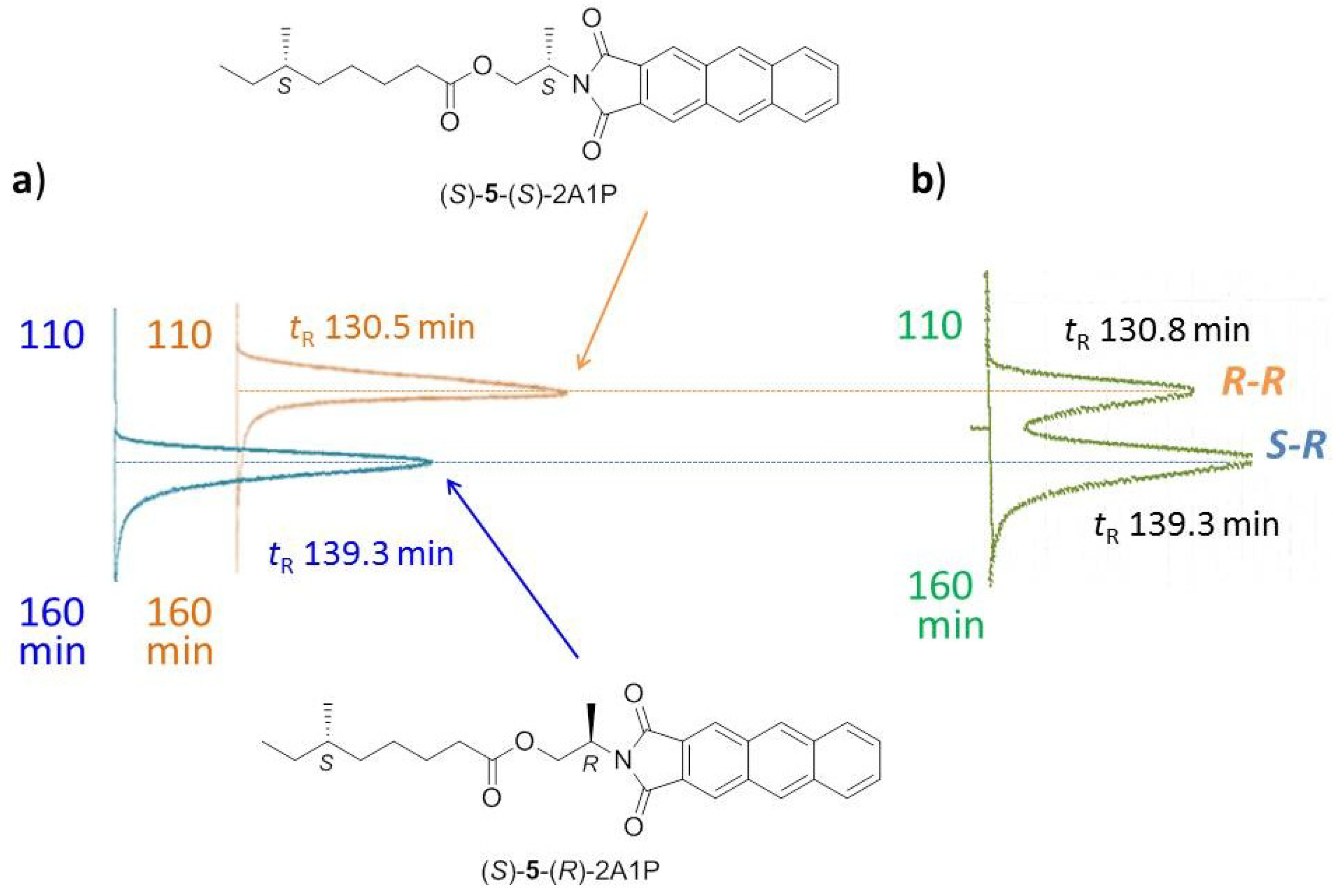
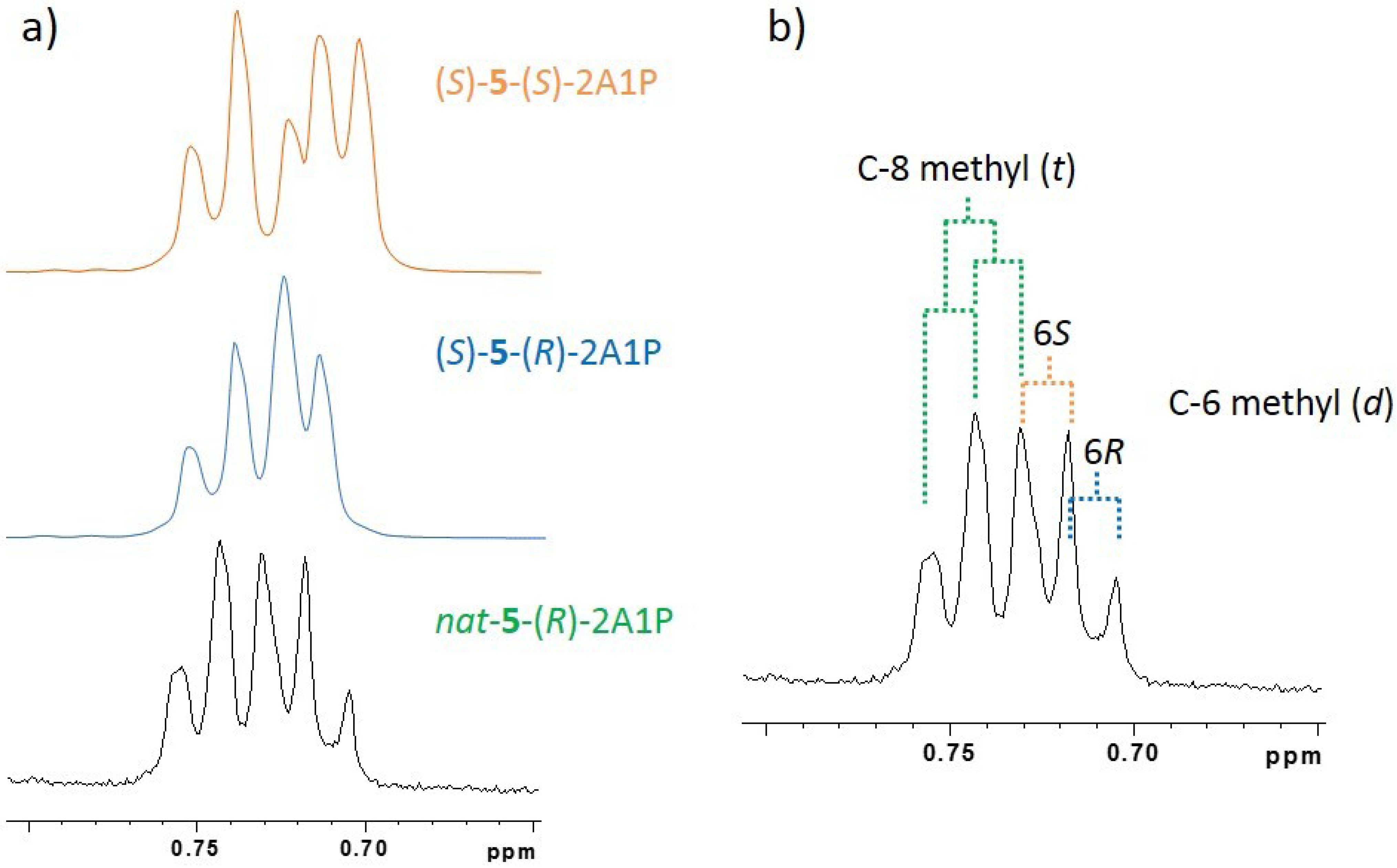
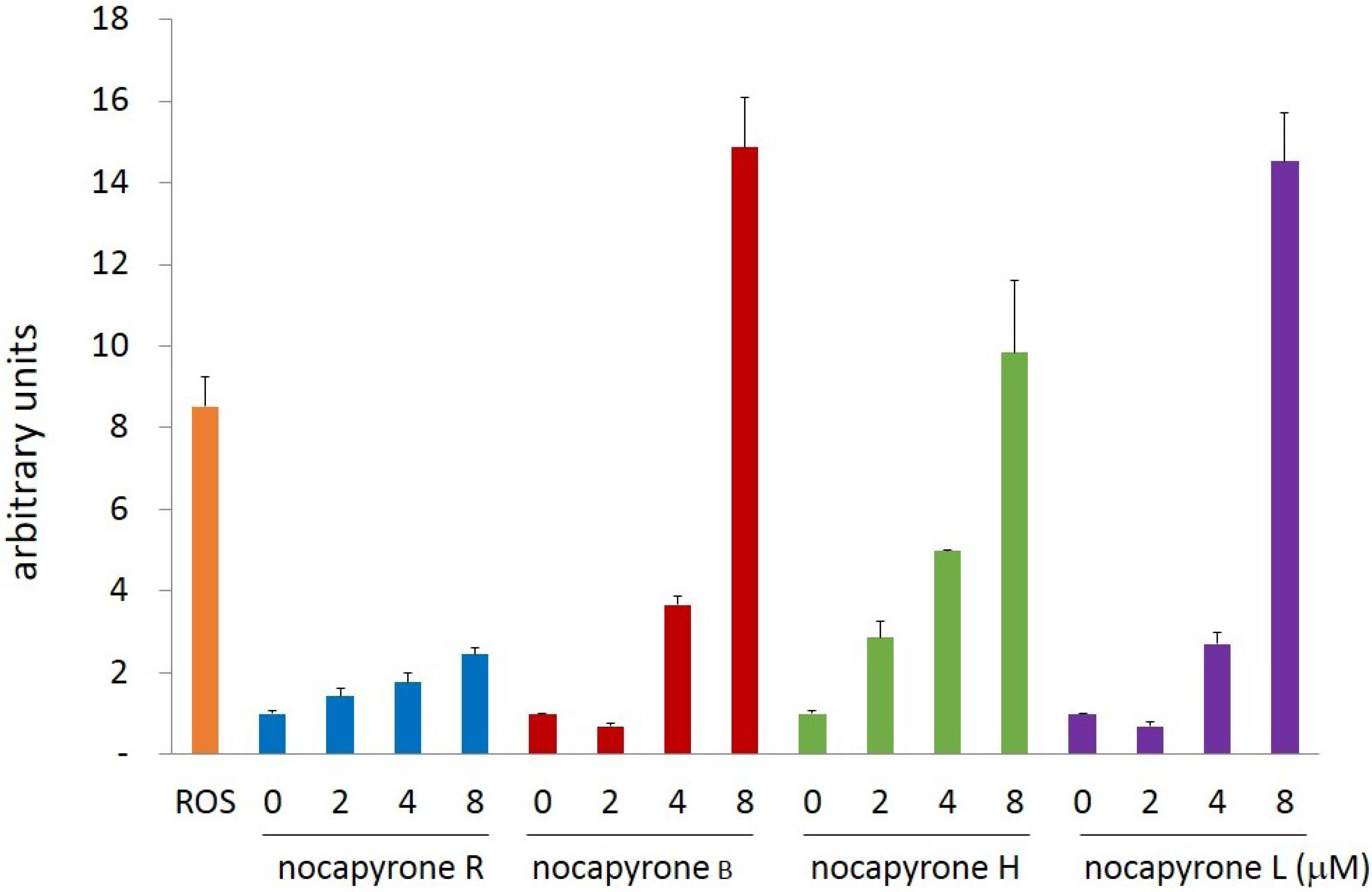
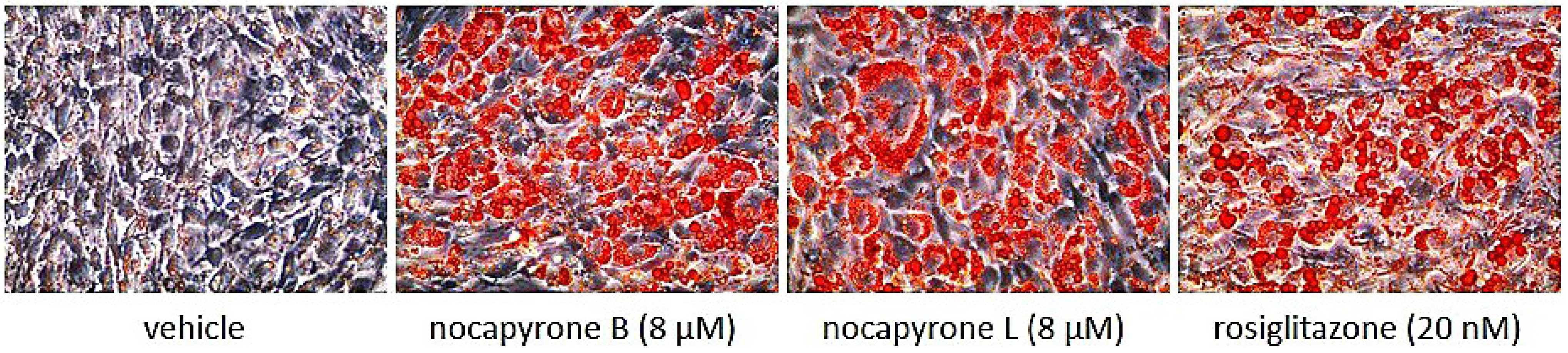
2.2. Biological Activities
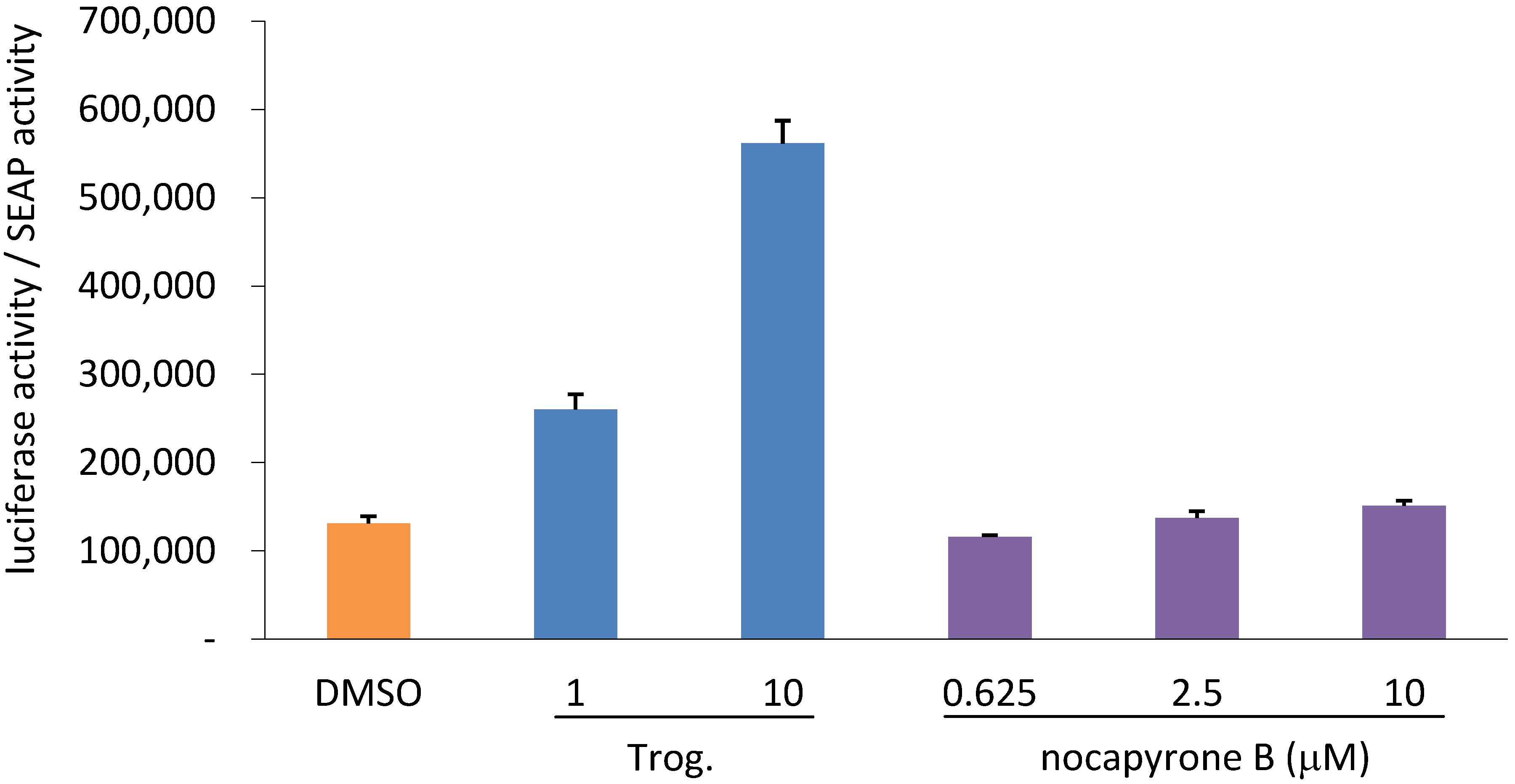
3. Experimental Section
3.1. General Experimental Procedures
3.2. Microorganism
3.3. Fermentation
3.4. Extraction and Isolation
3.5. Determination of the Absolute Configuration of the Anteiso-Methyl Group in 4 by Ohrui-Akasaka Method
3.6. Biological Activity Study
3.6.1. Real-Time Quantitative PCR Analysis
3.6.2. Oil Red O Staining
3.6.3. Luciferase Reporter Assay
4. Conclusions
Supplementary Files
Author Contributions
Conflicts of Interest
References
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Bérdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef]
- Lam, K.S. Discovery of novel metabolites from marine actinomycetes. Curr. Opin. Microbiol. 2006, 9, 245–251. [Google Scholar] [CrossRef]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Candib, L.M. Obesity and diabetes in vulnerable populations: Reflection on proximal and distal causes. Ann. Fam. Med. 2007, 5, 547–556. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I.S. Signalling role of adipose tissue: Adipokines and inflammation in obesity. Biochem. Soc. Trans. 2005, 33, 1078–1081. [Google Scholar] [CrossRef]
- Simons, P.J.; van den Pangaart, P.S.; van Roomen, C.P.; Aerts, J.M.; Boon, L. Cytokine-mediated modulation of leptin and adiponectin secretion during in vitro adipogenesis: Evidence that tumor necrosis factor-alpha- and interleukin-1beta-treated human preadipocytes are potent leptin producers. Cytokine 2005, 32, 94–103. [Google Scholar] [CrossRef]
- Tsao, T.S.; Lodish, H.F.; Fruebis, J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur. J. Pharmacol. 2002, 440, 213–221. [Google Scholar] [CrossRef]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar]
- Hotta, K.; Funahashi, T.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; Maeda, K.; et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1595–1599. [Google Scholar] [CrossRef]
- Lihn, A.S.; Østergard, T.; Nyholm, B.; Pedersen, S.B.; Richelsen, B.; Schmitz, O. Adiponectin expression in adipose tissue is reduced in first-degree relatives of type 2 diabetic patients. Am. J. Physiol. Endocrinol. Metab. 2003, 284, 443–448. [Google Scholar]
- Tiikkainen, M.; Häkkinen, A.M.; Korsheninnikova, E.; Nyman, T.; Mäkimattila, S.; Yki-Järvinen, H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 2004, 53, 2169–2176. [Google Scholar] [CrossRef]
- Yang, W.S.; Jeng, C.Y.; Wu, T.J.; Tanaka, S.; Funahashi, T.; Matsuzawa, Y.; Wang, J.P.; Chen, C.L.; Tai, T.Y.; Chuang, L.M. Synthetic peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care 2002, 25, 376–380. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007, 356, 2457–2471. [Google Scholar] [CrossRef]
- Igarashi, Y.; Tanaka, Y.; Ikeda, M.; Oikawa, T.; Kitani, S.; Nihira, T.; Mongkol, P.; Janhom, M.; Panbangred, W. Prajinamide, a new modified peptide from a soil-derived Streptomyces. J. Antibiot. 2012, 65, 157–159. [Google Scholar] [CrossRef]
- Igarashi, Y.; Yu, L.; Ikeda, M.; Oikawa, T.; Kitani, S.; Nihira, T.; Bayanmunkh, B.; Panbangred, W. Jomthonic acid, a modified amino acid from a soil-derived Streptomyces. J. Nat. Prod. 2012, 75, 986–990. [Google Scholar] [CrossRef]
- Indananda, C.; Igarashi, Y.; Ikeda, M.; Oikawa, T.; Thamchaipenet, A. Linfuranone A, a new polyketide from plant-derived Microbispora sp. GMKU 363. J. Antibiot. 2013, 66, 675–677. [Google Scholar] [CrossRef]
- Schneemann, I.; Ohlendorf, B.; Zinecker, H.; Nagel, K.; Wiese, J.; Imhoff, J.F. Nocapyrones A–D, γ-pyrones from a Nocardiopsis strain isolated from the marine sponge Halichondria panicea. J. Nat. Prod. 2010, 73, 1444–1447. [Google Scholar] [CrossRef]
- Lin, Z.; Torres, J.P.; Ammon, M.A.; Marett, L.; Teichert, R.W.; Reilly, C.A.; Kwan, J.C.; Hughen, R.W.; Flores, M.; Tianero, M.D.; et al. A bacterial source for mollusk pyrone polyketides. Chem. Biol. 2013, 24, 73–81. [Google Scholar]
- Gregory, L.C. Mining microbial genomes for new natural products and biosynthetic pathways. Microbiology 2008, 154, 1555–1569. [Google Scholar] [CrossRef]
- Akasaka, K.; Imizumi, K.; Ohrui, H. Enantiomeric separation of branched fatty acids having chiral centers remote from the carboxyl group by labelling with chiral fluorescent derivatization reagents. Enantiomer 1998, 3, 169–174. [Google Scholar]
- Nunez, M.T.; Martin, V.S. Efficient oxidation of phenyl groups to carboxylic acids with ruthenium tetraoxide. A simple synthesis of (R)-γ-caprolactone, the pheromone of Trogoderma granarium. J. Org. Chem. 1990, 55, 1928–1932. [Google Scholar] [CrossRef]
- Ikeda, M.; Kurotobi, Y.; Namikawa, A.; Kuranuki, S.; Matsuura, N.; Sato, M.; Igarashi, Y.; Nakamura, T.; Oikawa, T. Norlichexanthone isolated from fungus P16 promotes the secretion and expression of adiponectin in cultured ST-13 adipocytes. Med. Chem. 2011, 7, 250–256. [Google Scholar] [CrossRef]
- Singh, M.P.; Kong, F.; Janso, J.E.; Arias, D.A.; Suarez, P.A.; Bernan, V.S.; Petersen, P.J.; Weiss, W.J.; Carter, G.; Greenstein, M. Novel α-pyrones produced by a marine Pseudomonas sp. F92S91: Taxonomy and biological activities. J. Antibiot. 2003, 56, 1033–1044. [Google Scholar] [CrossRef]
- Trisuwan, K.; Rukachaisirikul, V.; Sukpondma, Y.; Preedanon, S.; Phongpaichit, S.; Rungjindamai, N.; Sakayaroj, J. Epoxydons and a pyrone from the marine-derived fungus Nigrospora sp. PSU-F5. J. Nat. Prod. 2008, 71, 1323–1326. [Google Scholar] [CrossRef]
- Yu, K.; Ren, B.; Wei, J.; Chen, C.; Sun, J.; Song, F.; Dai, H.; Zhang, L. Verrucisidinol and Verrucosidinol Acetate, Two pyrone-type polyketides isolated from a marine derived fungus, Penicillium aurantiogriseum. Mar. Drugs 2010, 8, 2744–2754. [Google Scholar]
- Carbone, M.; Ciavatta, M.L.; Wang, J.R.; Cirillo, I.; Mathieu, V.; Kiss, R.; Mollo, E.; Guo, Y.W.; Gavagnin, M. Extending the record of polypropionates from marine pulmonate mollusks. J. Nat. Prod. 2013, 76, 2065–2073. [Google Scholar]
- Fu, P.; Liu, P.; Qu, H.; Wang, Y.; Chen, D.; Wang, H.; Li, J.; Zhu, W. A-pyrones and diketopiperazine derivatives from the marine-derived actinomycete Nocardiopsis dassonvillei HR10–5. J. Nat. Prod. 2011, 74, 2219–2223. [Google Scholar] [CrossRef]
- Kim, M.C.; Kwon, O.W.; Park, J.S.; Kim, S.Y.; Kwon, H.C. Nocapyrones H–J, 3,6-disubstituted α-pyrones from the marine actinomycete Nocardiopsis sp. KMF-001. Chem. Pharm. Bull. 2013, 61, 511–515. [Google Scholar] [CrossRef]
- Fu, P.; Liu, P.; Gong, Q.; Wang, Y.; Wanga, P.; Zhu, W. α-Pyrones from the marine-derived actinomycete Nocardiopsis dassonvillei subsp. dassonvillei XG-8–1. RSC Adv. 2013, 3, 20726–20731. [Google Scholar]
- Shimamura, H.; Sunazuka, T.; Hizoura, T.; Hirose, T.; Shiomi, K.; Omura, S. Total synthesis and biological evaluation of verticipyrone and analogues. Org. Lett. 2007, 9, 65–67. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Amorelli, B. Carboalumination/Ni-catalyzed couplings. A short synthesis of verticipyrone. Tetrahedron Lett. 2009, 50, 2144–2146. [Google Scholar] [CrossRef]
- De Paolis, M.; Rosso, H.; Henrot, M.; Prandi, C.; d’Herouville, F.; Maddaluno, J. A concise route to α′-methoxy-γ-pyrones and verticipyrone based upon the desymmetrization of α,α′-dimethoxy-γ-pyrone. Chem. Eur. J. 2010, 16, 11229–11232. [Google Scholar]
- Rosso, H.; de Paolis, M.; Collin, V.C.; Dey, S.; Hecht, S.M.; Prandi, C.; Richard, V.; Maddaluno, J. One-pot regio- and stereoselective synthesis of α′-methoxy-γ-pyrones: Biological evaluation as mitochondrial respiratory complex inhibitors. J. Org. Chem. 2011, 76, 9429–9437. [Google Scholar] [CrossRef]
- Sharma, P.; Powell, K.J.; Burnley, J.; Awaad, A.S.; Moses, J.E. Total Synthesis of polypropionate-derived γ-pyrone natural products. Synthesis 2011, 2011, 2865–2892. [Google Scholar]
- Wilk, W.; Waldmann, H.; Kaiser, M. Gamma-pyrone natural products—A privileged compound class provided by nature. Bioorg. Med. Chem. 2009, 17, 2304–2309. [Google Scholar] [CrossRef]
- Usami, Y.; Ikura, T.; Amagata, T.; Numata, A. First total synthesesand configurational assignments of cytotoxic trichodenones A–C. Tetrahedron Asymmetry 2000, 11, 3711–3725. [Google Scholar] [CrossRef]
- Usami, Y.; Okada, Y.; Yamada, T. Natural pericosines B and C as enantiomeric mixtures: direct evidence by chiral HPLC analysis. Chirality 2001, 23, E7–E11. [Google Scholar] [CrossRef]
- Akasaka, K.; Tamogami, S.; Beeman, R.W.; Mori, K. Pheromone synthesis. Part 245: Synthesis and chromatographic analysis of the four stereoisomers of 4,8-dimethyldecanal, the male aggregation pheromone of the red flour beetle, Tribolium castaneum. Tetrahedron 2011, 67, 201–209. [Google Scholar] [CrossRef]
- Komaki, H.; Ichikawa, N.; Hosoyama, A.; Fujita, N.; Igarashi, Y. Draft genome sequence of marine-derived actinomycete Nocardiopsis sp. TP-A0876, a producer of polyketide pyrones. Genome Announc. 2014, in press. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, Y.; Ogura, H.; Akasaka, K.; Oikawa, T.; Matsuura, N.; Imada, C.; Yasuda, H.; Igarashi, Y. Nocapyrones: α- and γ-Pyrones from a Marine-Derived Nocardiopsis sp. Mar. Drugs 2014, 12, 4110-4125. https://doi.org/10.3390/md12074110
Kim Y, Ogura H, Akasaka K, Oikawa T, Matsuura N, Imada C, Yasuda H, Igarashi Y. Nocapyrones: α- and γ-Pyrones from a Marine-Derived Nocardiopsis sp. Marine Drugs. 2014; 12(7):4110-4125. https://doi.org/10.3390/md12074110
Chicago/Turabian StyleKim, Youngju, Hiromu Ogura, Kazuaki Akasaka, Tsutomu Oikawa, Nobuyasu Matsuura, Chiaki Imada, Hisato Yasuda, and Yasuhiro Igarashi. 2014. "Nocapyrones: α- and γ-Pyrones from a Marine-Derived Nocardiopsis sp." Marine Drugs 12, no. 7: 4110-4125. https://doi.org/10.3390/md12074110
APA StyleKim, Y., Ogura, H., Akasaka, K., Oikawa, T., Matsuura, N., Imada, C., Yasuda, H., & Igarashi, Y. (2014). Nocapyrones: α- and γ-Pyrones from a Marine-Derived Nocardiopsis sp. Marine Drugs, 12(7), 4110-4125. https://doi.org/10.3390/md12074110