Link between Domoic Acid Production and Cell Physiology after Exchange of Bacterial Communities between Toxic Pseudo-nitzschia multiseries and Non-Toxic Pseudo-nitzschia delicatissima
Abstract
:1. Introduction
2. Results
2.1. Algal Growth and Death
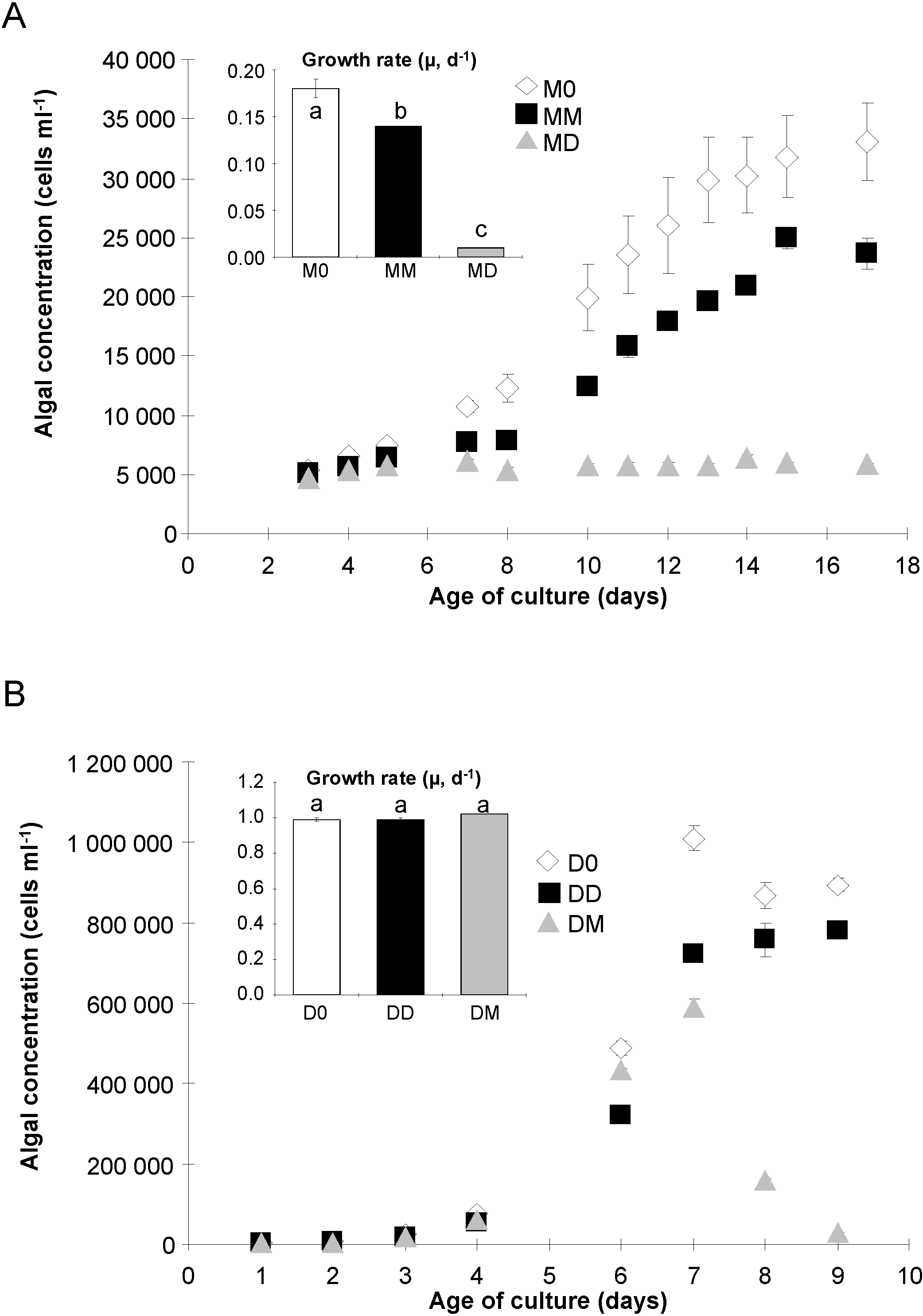
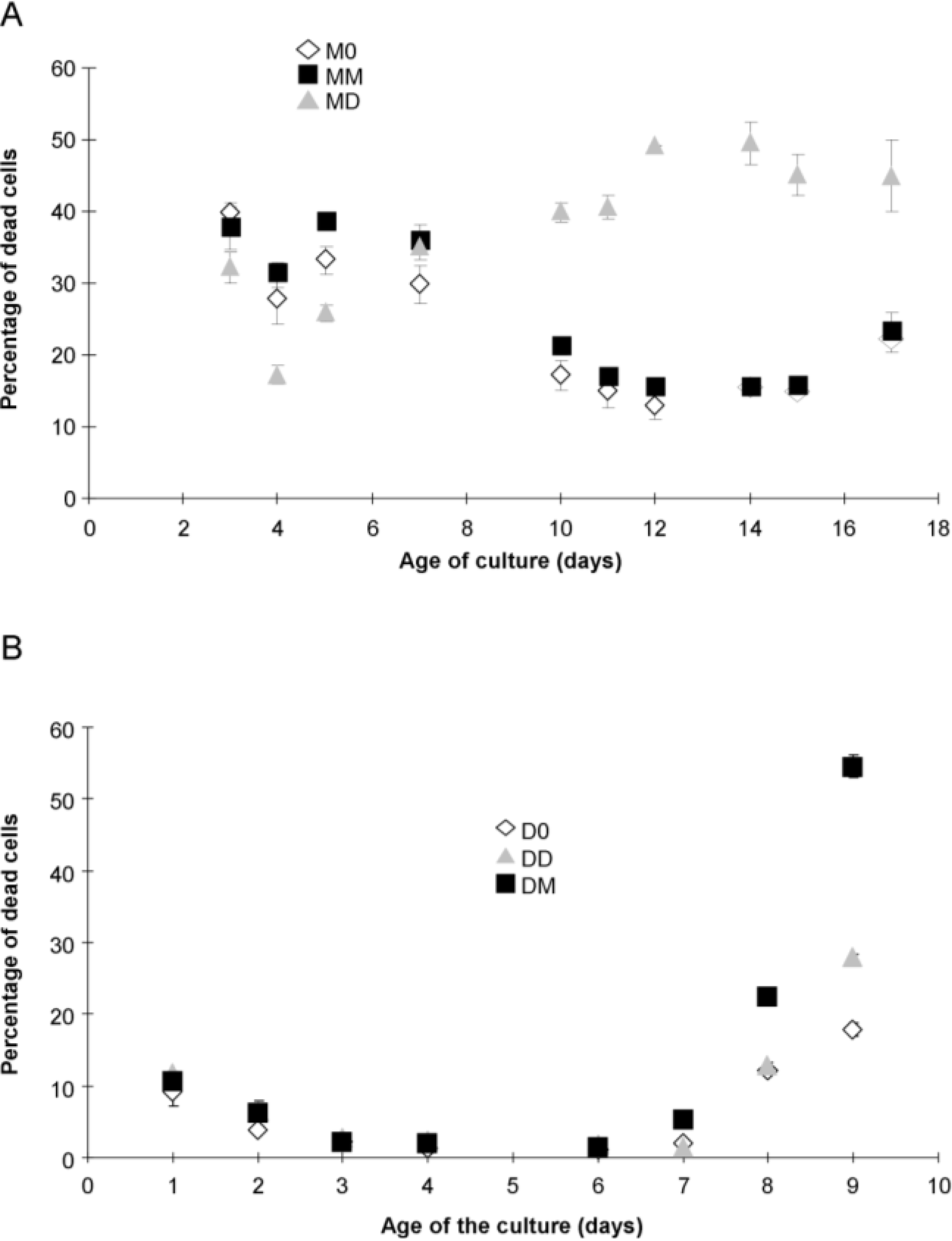
| Physiological Parameter | Age of Culture (Days) | Mean ± SE | Mean ± SE | Mean ± SE |
| P. multiseries | ||||
| M0 | MM | MD | ||
| Percentage of Active Cells | 3 | 76.9 ± 2.0 | 73.9 ± 1.2 | 81.1 ± 0.8 |
| 4 | 97.6 ± 1.4 | 95.5 ± 1.1 | 93.6 ± 1.5 | |
| 5 | 97.2 ± 0.8 | 98.3 ± 0.4 | 95.8 ± 1.0 | |
| 7 | 97.3 ± 0.2 | 96.6 ± 0.5 | 95.2 ± 0.4 | |
| 8 | 96.7 ± 0.4 | 95.7 ± 0.2 | 93.2 ± 0.8 | |
| 10 | 97.3 ± 0.4 | 97.7 ± 0.1 | 95.0 ± 0.7 | |
| 11 | 97.6 ± 0.4 | 98.0 ± 0.2 | 92.8 ± 1.5 | |
| 12 | 97.5 ± 0.2 | 97.0 ± 0.5 | 92.1 ± 1.2 | |
| 14 | 97.3 ± 0.3 | 98.1 ± 0.2 | 90.7 ± 1.5 | |
| 15 | 96.5 ± 0.2 | 98.1 ± 0.7 | 92.8 ± 0.7 | |
| 17 | 94.4 ± 0.4 | 96.2 ± 1.2 | 85.9 ± 2.9 | |
| M0 | MM | MD | ||
| Esterase Activity | 3 | 130.0 ± 7.8 | 149.3 ± 13.2 | 138.7 ± 20.8 |
| 4 | 157.3 ± 37.3 | 132.7 ± 11.4 | 167.2 ± 27.7 | |
| 5 | 76.8 ± 45.5 | 114.2 ± 8.5 | 187.1 ± 15.3 | |
| 7 | 122.5 ± 8.3 | 139.5 ± 6.4 | 198.5 ± 21.5 | |
| 8 | 116.3 ± 3.6 | 129.4 ± 7.9 | 159.9 ± 7.4 | |
| 10 | 121.6 ± 1.6 | 148.9 ± 9.0 | 145.2 ± 15.1 | |
| 11 | 112.4 ± 5.1 | 137.3 ± 8.7 | 148.5 ± 9.0 | |
| 12 | 111.1 ± 1.5 | 128.7 ± 9.7 | 141.9 ± 9.9 | |
| 14 | 126.6 ± 9.2 | 161.6 ± 7.6 | 128.5 ± 15.0 | |
| 15 | 106.2 ± 7.4 | 140.7 ± 11.5 | 114.1 ± 7.7 | |
| 17 | 131.9 ± 15.6 | 174.0 ± 13.8 | 111.9 ± 15.6 | |
| M0 | MM | MD | ||
| Lipid Content | 3 | 1466.4 ± 65.6 | 1443.7 ± 42.7 | 1303.8 ± 48.4 |
| 4 | 1516.1 ± 123.7 | 1572.0 ± 212.6 | 1493.2 ± 293.8 | |
| 5 | 1530.4 ± 83.2 | 1336.8 ± 171.1 | 1353.7 ± 23.5 | |
| 7 | 1099.5 ± 123.6 | 1099.4 ± 255.4 | 1147.7 ± 38.0 | |
| 8 | 1121.6 ± 63.7 | 1193.9 ± 67.1 | 1050.7 ± 17.1 | |
| 10 | 1013.9 ± 41.5 | 1092.6 ± 30.8 | 961.0 ± 19.2 | |
| 11 | 907.0 ± 39.5 | 1019.8 ± 11.4 | 878.9 ± 45.9 | |
| 12 | 826.9 ± 33.0 | 999.3 ± 11.6 | 912.5 ± 141.7 | |
| 14 | 913.2 ± 81.8 | 1240.1 ± 20.7 | 982.0 ± 70.3 | |
| 15 | 822.7 ± 16.3 | 984.0 ± 37.2 | 794.7 ± 30.1 | |
| 17 | 785.7 ± 28.7 | 989.5 ± 3.7 | 730.0 ± 43.5 | |
| Physiological Parameter | Age of Culture (Days) | Mean ± SE | Mean ± SE | Mean ± SE |
| P. delicatissima | ||||
| D0 | DD | DM | ||
| Percentage of Active Cells | 1 | 96.0 ± 0.5 | 95.4 ± 1.3 | 95.3 ± 1.5 |
| 2 | 98.9 ± 0.3 | 98.4 ± 0.4 | 98.7 ± 0.3 | |
| 3 | 99.6 ± 0.2 | 99.2 ± 0.2 | 99.1 ± 0.2 | |
| 4 | 99.5 ± 0.1 | 99.0 ± 0.4 | 99.2 ± 0.0 | |
| 6 | 99.4 ± 0.2 | 98.6 ± 0.1 | 98.9 ± 0.1 | |
| 7 | 99.3 ± 0.1 | 99.0 ± 0.1 | 99.0 ± 0.0 | |
| 8 | 98.9 ± 0.1 | 98.4 ± 0.0 | 98.5 ± 0.1 | |
| 9 | 97.5 ± 0.1 | 94.9 ± 0.0 | 86.7 ± 0.5 | |
| D0 | DD | DM | ||
| Esterase Activity | 1 | 33.6 ± 3.0 | 37.9 ± 2.4 | 35.1 ± 2.3 |
| 2 | 46.5 ± 0.4 | 51.1 ± 4.6 | 52.0 ± 3.4 | |
| 3 | 33.9 ± 0.2 | 40.9 ± 1.0 | 38.8 ± 2.0 | |
| 4 | 34.9 ± 1.8 | 37.8 ± 4.5 | 40.5 ± 0.8 | |
| 6 | 40.1 ± 4.4 | 33.1 ± 0.8 | 45.6 ± 5.4 | |
| 7 | 47.4 ± 0.2 | 38.2 ± 1.7 | 60.2 ± 2.0 | |
| 8 | 33.4 ± 1.6 | 38.9 ± 1.4 | 73.0 ± 15.7 | |
| 9 | 34.2 ± 1.6 | 45.4 ± 1.0 | 59.5 ± 9.5 | |
| D0 | DD | DM | ||
| Lipid Content | 1 | 108.5 ± 20.9 | 102.7 ± 5.3 | 105.6 ± 4.2 |
| 2 | 124.9 ± 13.6 | 138.4 ± 27.3 | 128.8 ± 23.9 | |
| 3 | 116.3 ± 3.7 | 151.9 ± 7.4 | 135.8 ± 4.4 | |
| 4 | 124.9 ± 4.3 | 161.8 ± 4.6 | 152.0 ± 15.3 | |
| 6 | 150.3 ± 11.6 | 227.6 ± 10.7 | 184.2 ± 9.6 | |
| 7 | 95.5 ± 13.2 | 158.6 ± 8.3 | 148.8 ± 5.5 | |
| 8 | 118.9 ± 17.3 | 143.7 ± 1.6 | 171.8 ± 3.5 | |
| 9 | 207.7 ± 10.6 | 199.8 ± 5.6 | 242.9 ± 4.7 | |
2.2. Bacterial Growth
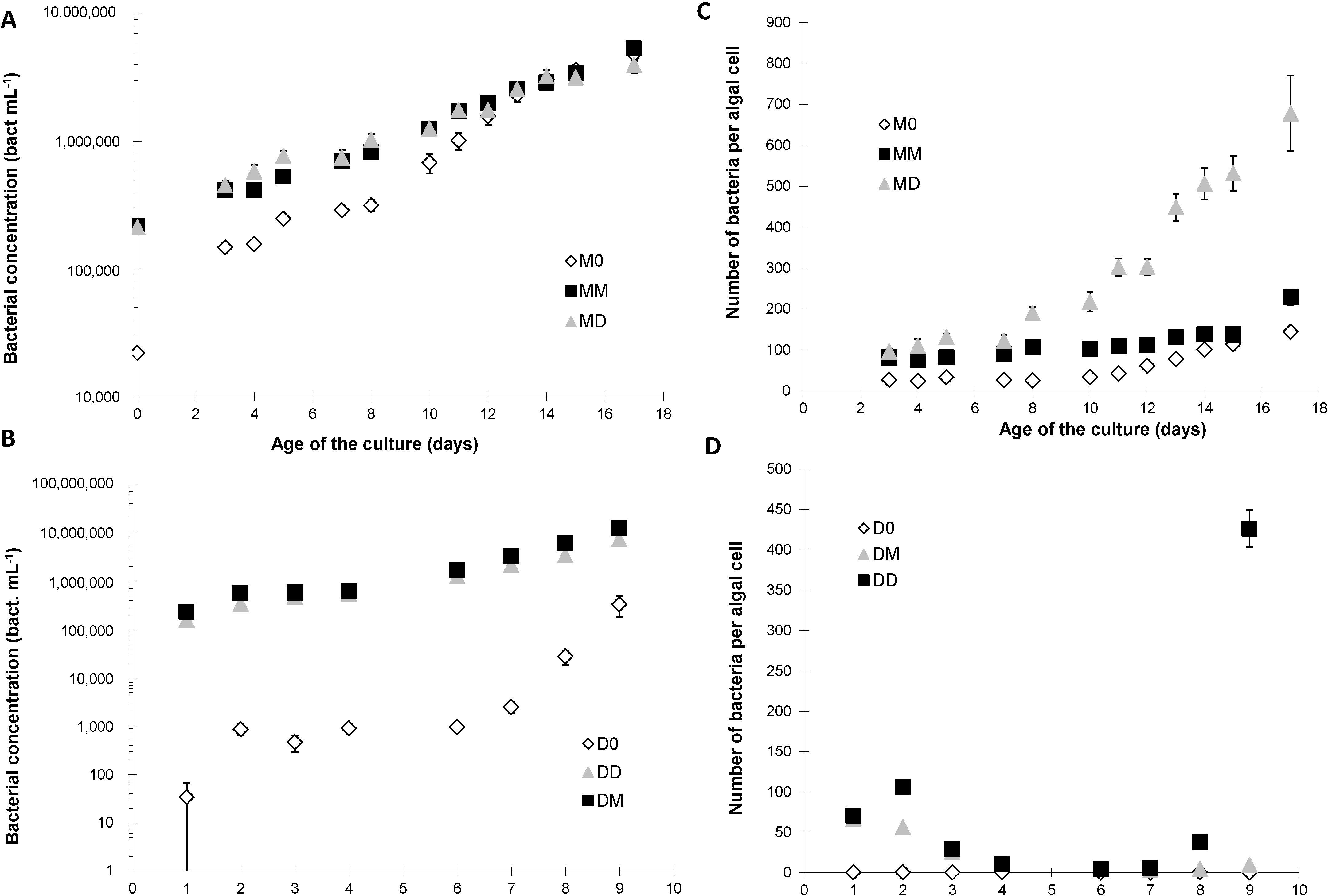
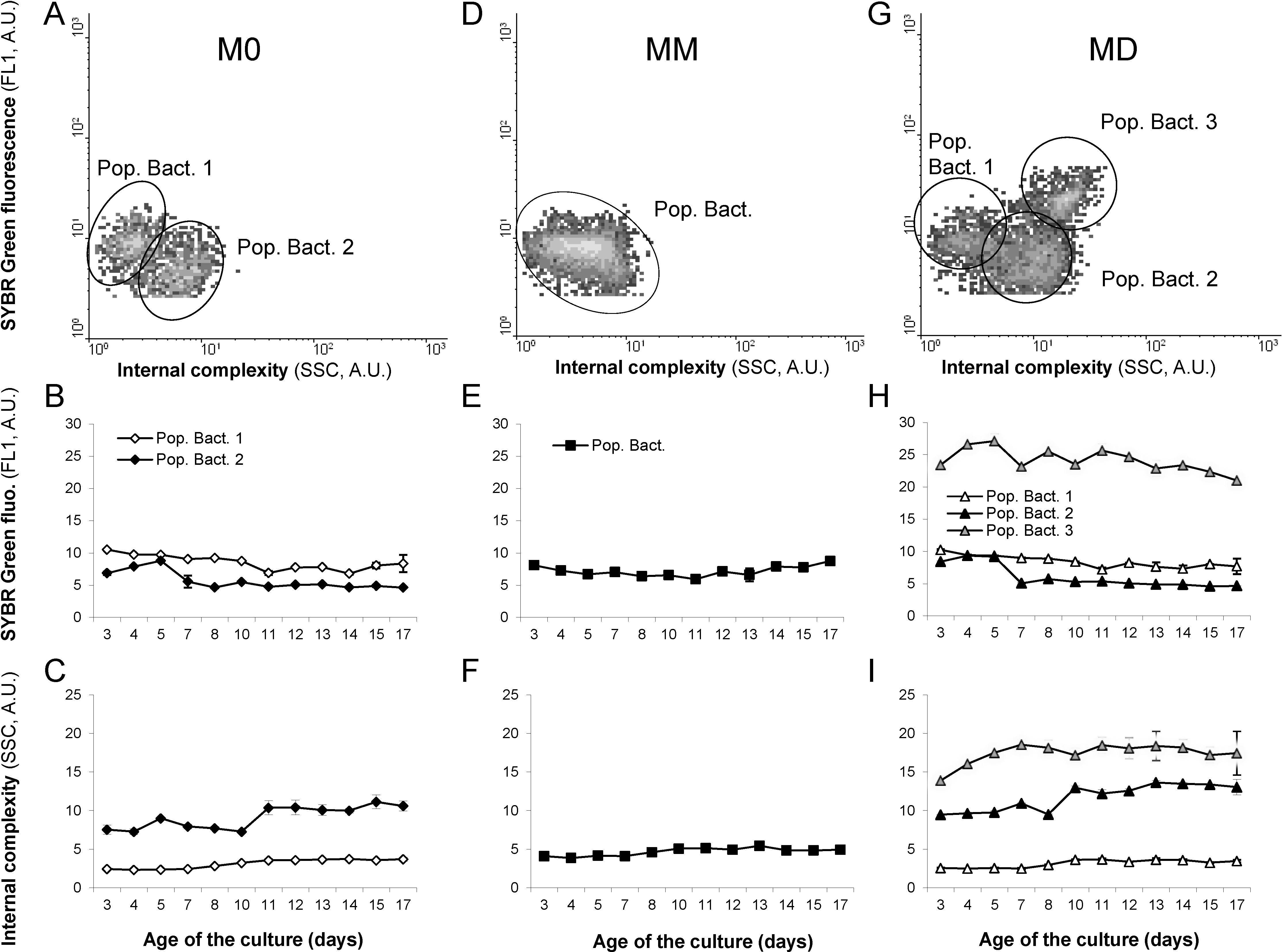
2.3. Domoic Acid
2.4. Photosynthetic Parameters
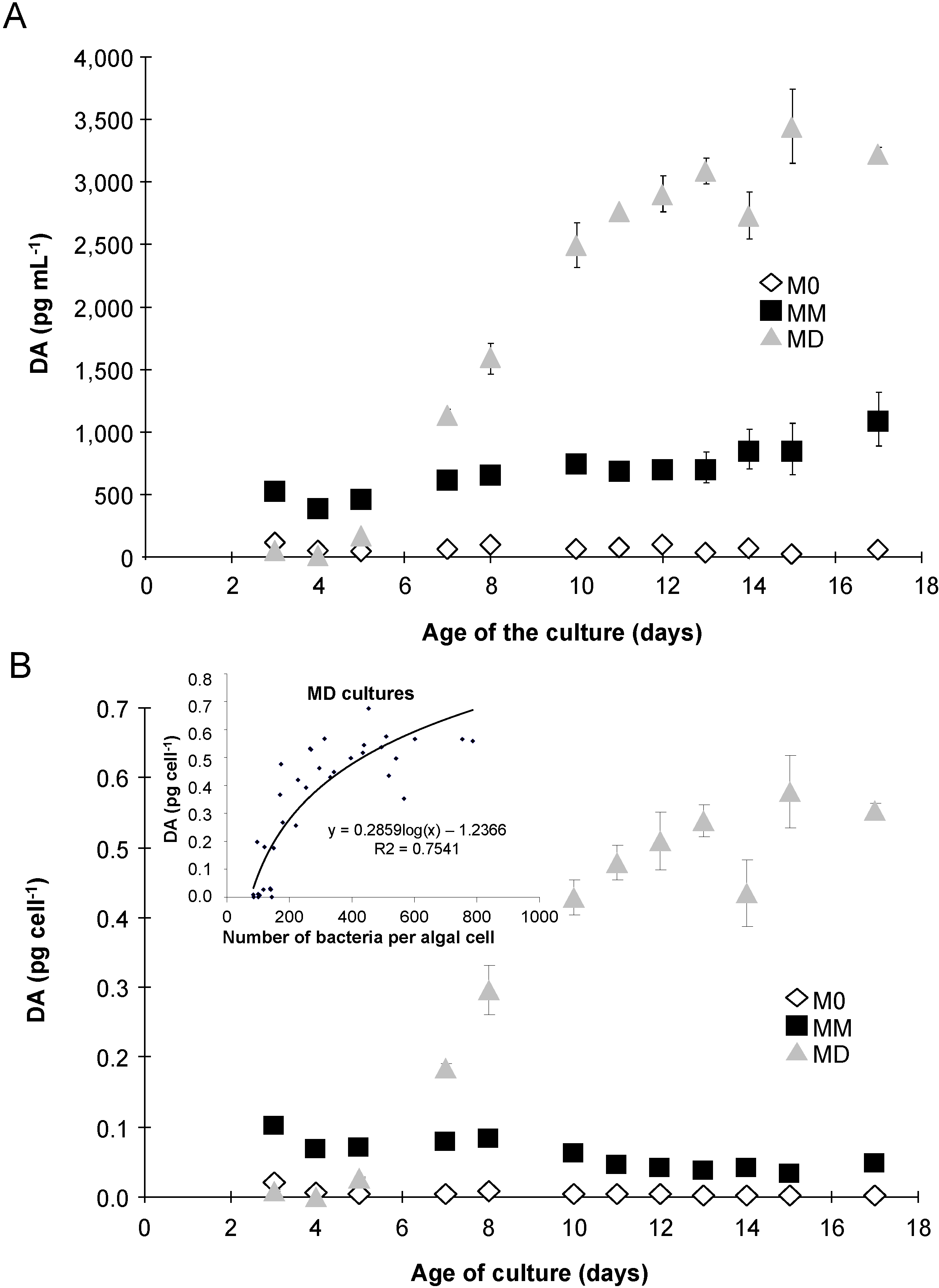
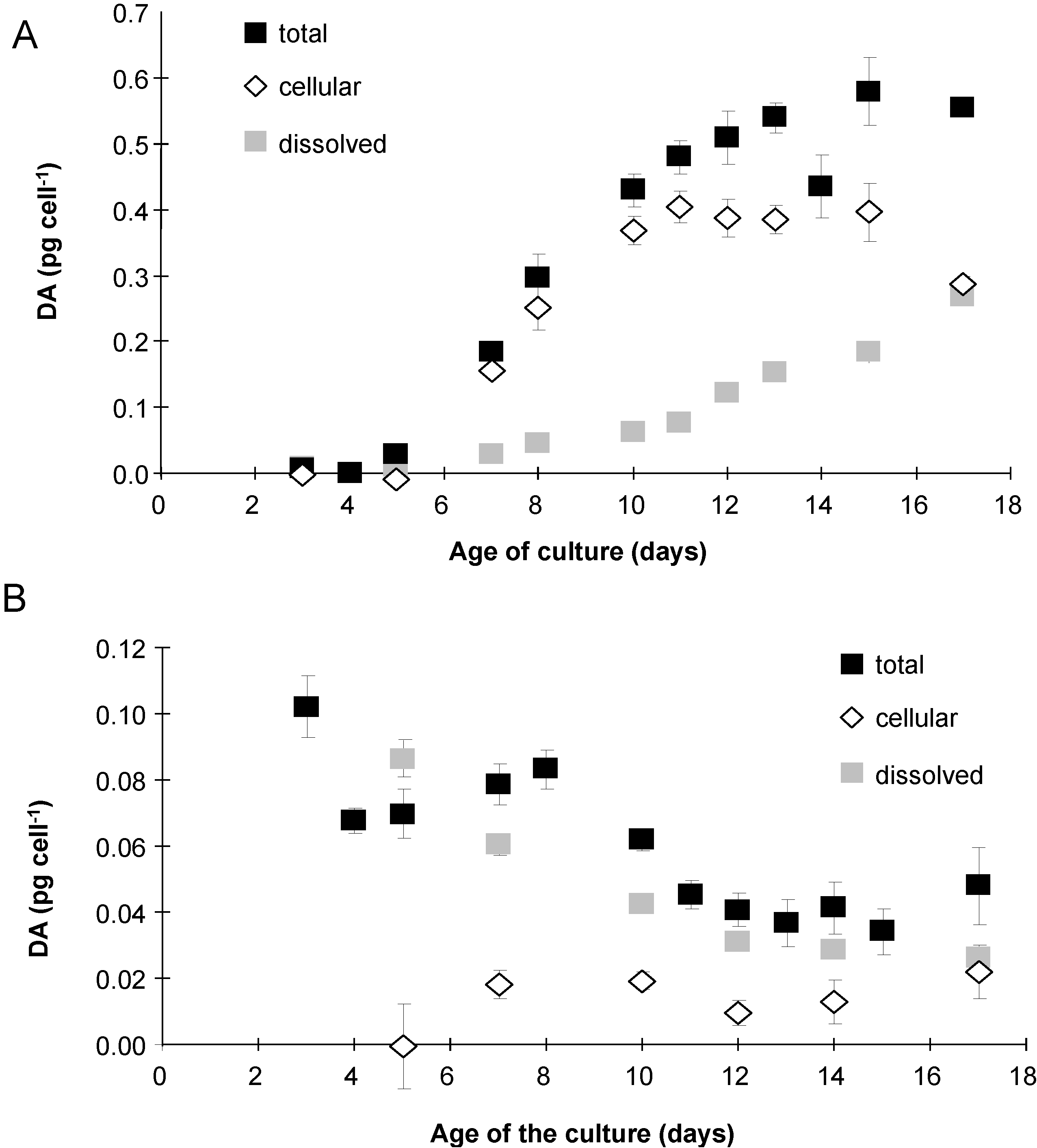
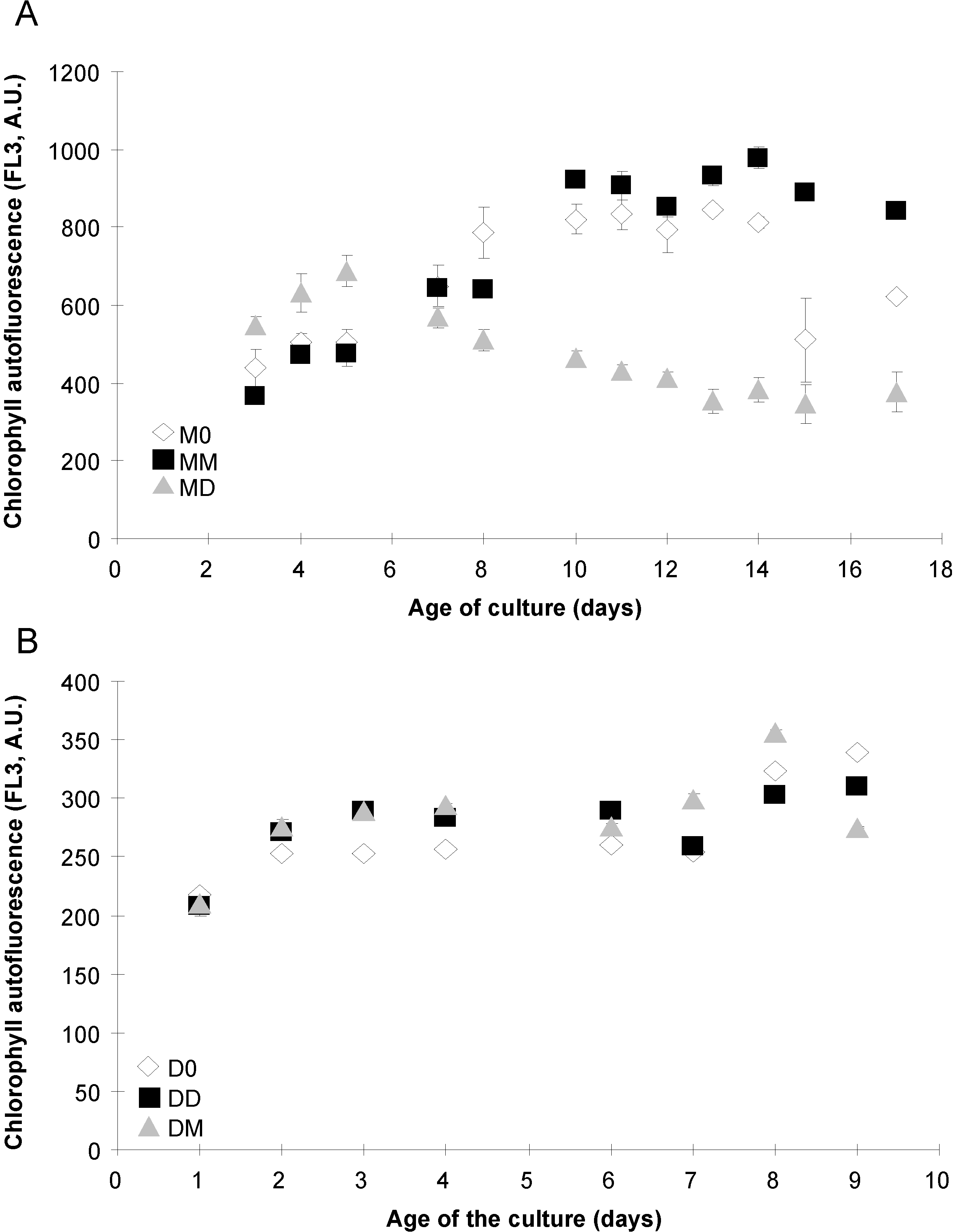
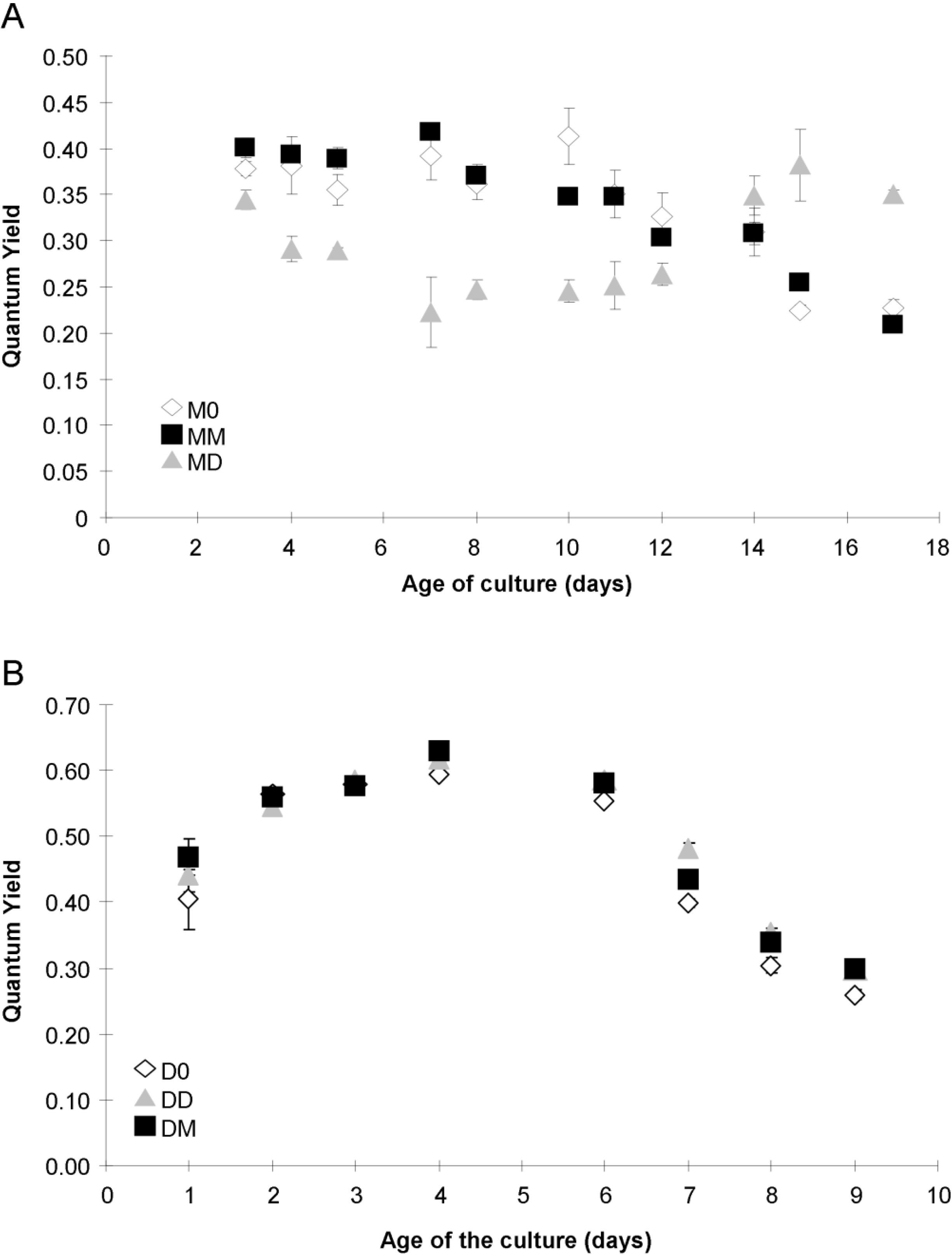
2.5. Esterase Activity and Lipid Content
3. Discussion
4. Materials and Methods
4.1. Culture Conditions
4.2. Removal of Bacteria
4.3. Experiments
| Algal Species Treated with Antibiotics | P. multiseries “M-” | P. delicatissima “D-” | |
|---|---|---|---|
| Bacteria Added | |||
| No bacterial addition “-0” | M0 | D0 | |
| Bacteria from P. multiseries “-M” | MM (P. multiseries control) | DM | |
| Bacteria from P. delicatissima “-D” | MD | DD (P. delicatissima control) | |
4.4. Physiological Measurements
4.5. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bates, S.S.; Bird, C.J.; de Freitas, A.S.W.; Foxall, R.; Gilgan, M.; Hanic, L.A.; Johnson, G.R.; McCulloch, A.W.; Odense, P.; Pocklington, R.; et al. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Can. J. Fish. Aquat. Sci. 1989, 46, 1203–1215. [Google Scholar] [CrossRef]
- Lelong, A.; Hégaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia 2012, 51, 168–216. [Google Scholar]
- Pan, Y.L.; Parsons, M.L.; Busman, M.; Moeller, P.D.R.; Dortch, Q.; Powell, C.L.; Doucette, G.J. Pseudo-nitzschia sp. cf. pseudodelicatissima—A confirmed producer of domoic acid from the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2001, 220, 83–92. [Google Scholar] [CrossRef]
- Thessen, A.E.; Bowers, H.A.; Stoecker, D.K. Intra- and interspecies differences in growth and toxicity of Pseudo-nitzschia while using different nitrogen sources. Harmful Algae 2009, 8, 792–810. [Google Scholar] [CrossRef]
- Wells, M.L.; Trick, C.G.; Cochlan, W.P.; Hughes, M.P.; Trainer, V.L. Domoic acid: The synergy of iron, copper, and the toxicity of diatoms. Limnol. Oceanogr. 2005, 50, 1908–1917. [Google Scholar] [CrossRef]
- Fehling, J.; Davidson, K.; Bates, S.S. Growth dynamics of non-toxic Pseudo-nitzschia delicatissima and toxic P. seriata (Bacillariophyceae) under simulated spring and summer photoperiods. Harmful Algae 2005, 4, 763–769. [Google Scholar] [CrossRef]
- Bates, S.S.; Douglas, D.J.; Doucette, G.J.; Léger, C. Enhancement of domoic acid production by reintroducing bacteria to axenic cultures of the diatom Pseudo-nitzschia multiseries. Nat. Toxins 1995, 3, 428–435. [Google Scholar] [CrossRef]
- Rhodes, L.; Holland, P.A.J.; Selwood, A.; McNabb, P. Mass culture of Pseudo-nitzschia australis for production of a new isomer of domoic acid. In Harmful Algae 2002, Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO; Steidinger, K.A., Landsberg, J.H., Tomas, C.R., Vargo, G.A., Eds.; UNESCO: Paris, France, 2004; pp. 125–127. [Google Scholar]
- Bates, S.S.; Worms, J.J.; Smith, J.C. Effects of ammonium and nitrate on growth and domoic acid production by Nitzschia pungens in batch culture. Can. J. Fish. Aquat. Sci. 1993, 50, 1248–1254. [Google Scholar] [CrossRef]
- Grossart, H.P.; Simon, M. Interactions of planktonic algae and bacteria: Effects on algal growth and organic matter dynamics. Aquat. Microb. Ecol. 2007, 47, 163–176. [Google Scholar] [CrossRef]
- Bruckner, C.; Rehm, C.; Grossart, H.P.; Kroth, P.G. Growth and release of extracellular organic compounds by benthic diatoms depend on interactions with bacteria. Environ. Microb. 2011, 13, 1052–1063. [Google Scholar] [CrossRef]
- Gaerdes, A.; Iversen, M.H.; Grossart, H.P.; Ullrich, M.S. Diatom-associated bacteria are required for aggregation of Thalassiosira weissflogii. ISME J. 2011, 5, 436–445. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takata, Y.; Kodama, M. Direct contact between Pseudo-nitzschia multiseries and bacteria is necessary for the diatom to produce a high level of domoic acid. Fish. Sci. 2009, 75, 771–776. [Google Scholar] [CrossRef]
- Stewart, J.E. Bacterial involvement in determining domoic acid levels in Pseudo-nitzschia multiseries cultures. Aquat. Microb. Ecol. 2008, 50, 135–144. [Google Scholar] [CrossRef]
- Stewart, J.E.; Marks, L.J.; Wood, C.R.; Risser, S.M.; Gray, S. Symbiotic relations between bacteria and the domoic acid producing diatom Pseudo-nitzschia multiseries and the capacity of these bacteria for gluconic acid/gluconolactone formation. Aquat. Microb. Ecol. 1997, 12, 211–221. [Google Scholar] [CrossRef]
- Jochem, F.J. Probing the physiological state of phytoplankton at the single-cell level. Sci. Mar. 2000, 64, 183–195. [Google Scholar] [CrossRef]
- Lelong, A.; Jolley, D.; Hégaret, H.; Soudant, P. The effects of copper toxicity on Pseudo-nitzschia spp. physiology and domoic acid production. Aquat. Toxicol. 2012, 118–119, 37–47. [Google Scholar] [CrossRef]
- Lelong, A.; Bucciarelli, E.; Hégaret, H.; Soudant, P. Iron and copper limitations differently affect growth rates, photosynthetic and physiological parameters of the marine diatom Pseudo-nitzschia delicatissima. Limnol. Oceanogr. 2013, 58, 613–623. [Google Scholar]
- Lelong, A.; Hégaret, H.; Soudant, P. Cell-based measurements to assess physiological status of Pseudo-nitzschia multiseries, a toxic diatom. Res. Microbiol. 2011, 162, 969–981. [Google Scholar] [CrossRef]
- Lelong, A.; Laboratoire des sciences de l’environnement marin (LEMAR), UMR6539, Institut Universitaire Européen de la Mer (IUEM), Plouzané, France. Unpublished work. 2011.
- Douglas, D.J.; Bates, S.S.; Bourque, L.A.; Selvin, R.C. Domoic acid production by axenic and non-axenic cultures of the pennate diatom Nitzschia pungens f. multiseries. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1993; pp. 595–600. [Google Scholar]
- Osada, M.; Stewart, J.E. Gluconic acid/gluconolactone: Physiological influences on domoic acid production by bacteria associated with Pseudo-nitzschia multiseries. Aquat. Microb. Ecol. 1997, 12, 203–209. [Google Scholar] [CrossRef]
- Kaczmarska, I.; Ehrman, J.M.; Bates, S.S.; Green, D.H.; Léger, C.; Harris, J. Diversity and distribution of epibiotic bacteria on Pseudo-nitzschia multiseries (Bacillariophyceae) in culture, and comparison with those on diatoms in native seawater. Harmful Algae 2005, 4, 725–741. [Google Scholar] [CrossRef]
- Sison-Mangus, M.P.; Jiang, S.; Tran, K.N.; Kudela, R.M. Host-specific adaptation governs the interaction of the marine diatom, Pseudo-nitzschia and their microbiota. ISME J. 2014, 8, 63–76. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar]
- Marie, D.; Partensky, F.; Vaulot, D.; Brussaard, C. Enumeration of phytoplankton, bacteria, and viruses in marine samples. Curr. Protoc. Cytom. 2001, 10. [Google Scholar] [CrossRef]
- Jochem, F.J. Dark survival strategies in marine phytoplankton assessed by cytometric measurement of metabolic activity with fluorescein diacetate. Mar. Biol. 1999, 135, 721–728. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and of quenching chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Galbraith, D.W.; Harkins, K.R.; Jefferson, R.A. Flow cytometric characterization of the chlorophyll contents and size distributions of plant protoplasts. Cytometry 1988, 9, 75–83. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lelong, A.; Hégaret, H.; Soudant, P. Link between Domoic Acid Production and Cell Physiology after Exchange of Bacterial Communities between Toxic Pseudo-nitzschia multiseries and Non-Toxic Pseudo-nitzschia delicatissima. Mar. Drugs 2014, 12, 3587-3607. https://doi.org/10.3390/md12063587
Lelong A, Hégaret H, Soudant P. Link between Domoic Acid Production and Cell Physiology after Exchange of Bacterial Communities between Toxic Pseudo-nitzschia multiseries and Non-Toxic Pseudo-nitzschia delicatissima. Marine Drugs. 2014; 12(6):3587-3607. https://doi.org/10.3390/md12063587
Chicago/Turabian StyleLelong, Aurélie, Hélène Hégaret, and Philippe Soudant. 2014. "Link between Domoic Acid Production and Cell Physiology after Exchange of Bacterial Communities between Toxic Pseudo-nitzschia multiseries and Non-Toxic Pseudo-nitzschia delicatissima" Marine Drugs 12, no. 6: 3587-3607. https://doi.org/10.3390/md12063587
APA StyleLelong, A., Hégaret, H., & Soudant, P. (2014). Link between Domoic Acid Production and Cell Physiology after Exchange of Bacterial Communities between Toxic Pseudo-nitzschia multiseries and Non-Toxic Pseudo-nitzschia delicatissima. Marine Drugs, 12(6), 3587-3607. https://doi.org/10.3390/md12063587




