Three New Resveratrol Derivatives from the Mangrove Endophytic Fungus Alternaria sp.
Abstract
:1. Introduction
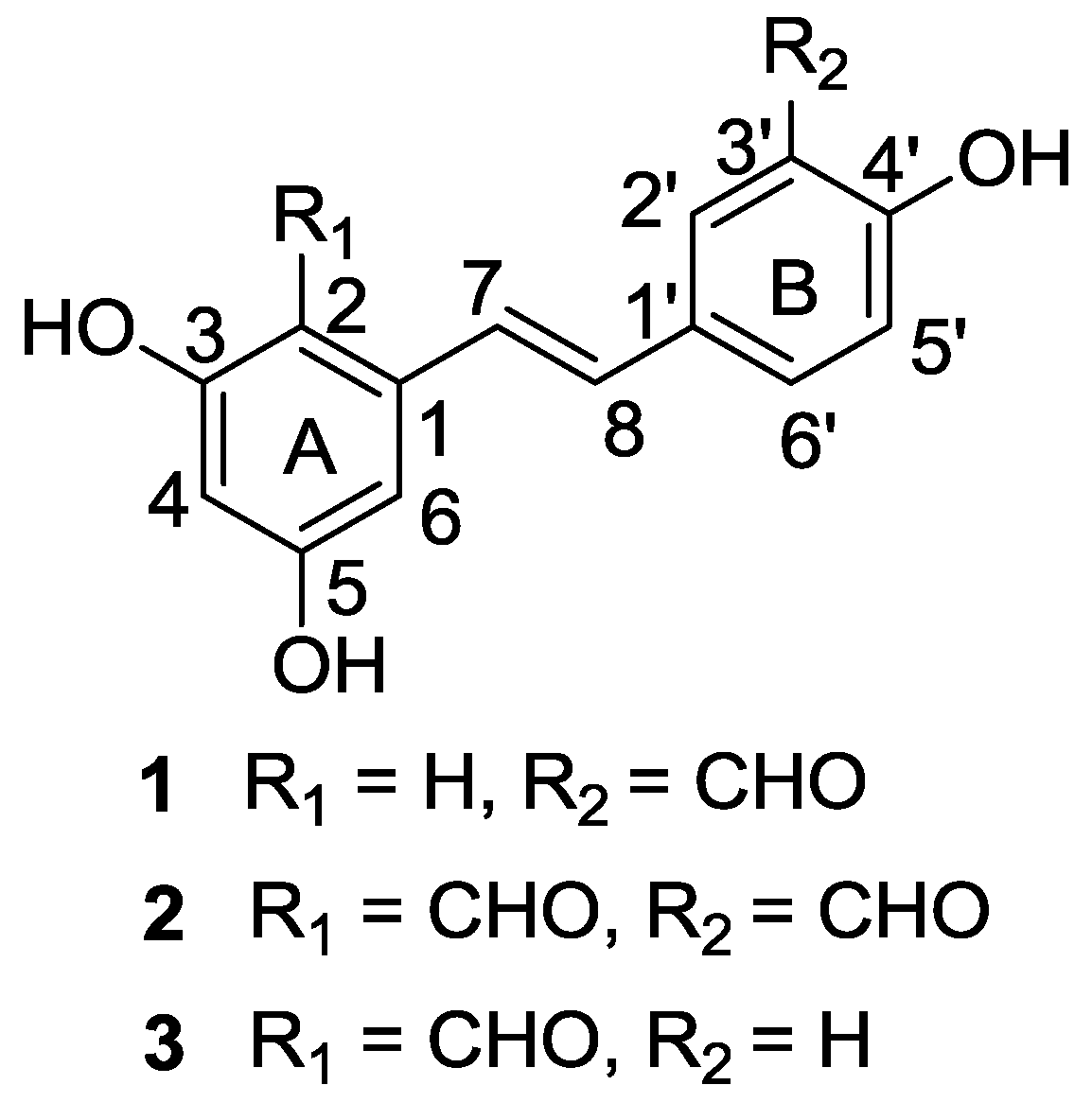
2. Results and Discussion
2.1. Chemical Structure Elucidation
| Position | 1 a | 2 a | 3 a | |||
|---|---|---|---|---|---|---|
| δC, mult. | δH (J in Hz) | δC, mult. | δH (J in Hz) | δC, mult. | δH (J in Hz) | |
| 1 | 139.3, C | - | 144.9, C | - | 145.6,C | - |
| 2 | 105.0, CH | 6.57 (d, 2.4) | 112.0, C | - | 112.0,C | - |
| 3 | 158.8, C | 166.1, C | - | 166.2,C | - | |
| 4 | 102.3, CH | 6.31 (t, 2.4) | 101.8, CH | 6.30 (d, 2.0) | 101.4,CH | 6.27 (d, 1.8) |
| 5 | 158.8, C | - | 165.2, C | - | 165.2,C | - |
| 6 | 105.0, C | 6.57 (d, 2.4) | 106.6, CH | 6.73 (d, 2.0) | 106.3,CH | 6.70 (d, 1.8) |
| 2-CHO | - | - | 193.4, CH | 10.33(s) | 193.4,CH | 10.33(s) |
| 3-OH | - | 8.36(s) | - | 12.55(s) | - | 12.58(s) |
| 5-OH | - | 8.36(s) | - | 11.02(s) | - | 9.74(s) |
| 7 | 128.2, CH | 7.04 (d, 16.2) | 122.3, CH | 7.83 (d, 16.2) | 119.8, CH | 7.67 (d, 16.2) |
| 8 | 126.4, CH | 7.11 (d, 16.2) | 133.0, CH | 7.17 (d, 16.2) | 134.8, CH | 7.08 (d, 15.6) |
| 1′ | 129.9, C | - | 129.3, C | - | 128.6, C | - |
| 2′ | 131.4, CH | 7.95(d, 2.0) | 132.4, CH | 8.08 (d, 1.8) | 128.6, CH | 7.55 (d, 8.4) |
| 3′ | 121.1, CH | - | 121.1, C | - | 115.6, CH | 6.88 (d, 8.4) |
| 4′ | 160.7, C | - | 161.3, C | - | 158.0, C | - |
| 5′ | 117.5, CH | 7.00 (d, 8.4) | 117.6, CH | 7.03 (d, 8.4) | 115.6, CH | 6.88 (d, 8.4) |
| 6′ | 134.6, CH | 7.84 (dd, 2.0, 8.4) | 135.1, CH | 7.97 (dd, 2.1, 8.4) | 158.0, C | 7.55 (d,8.4) |
| 3′-CHO | 197.0, CH | 10.07(s) | 196.8, CH | 10.07(s) | - | - |
| 4′-OH | - | 10.96(s) | - | 9.81(s) | - | 8.69(s) |

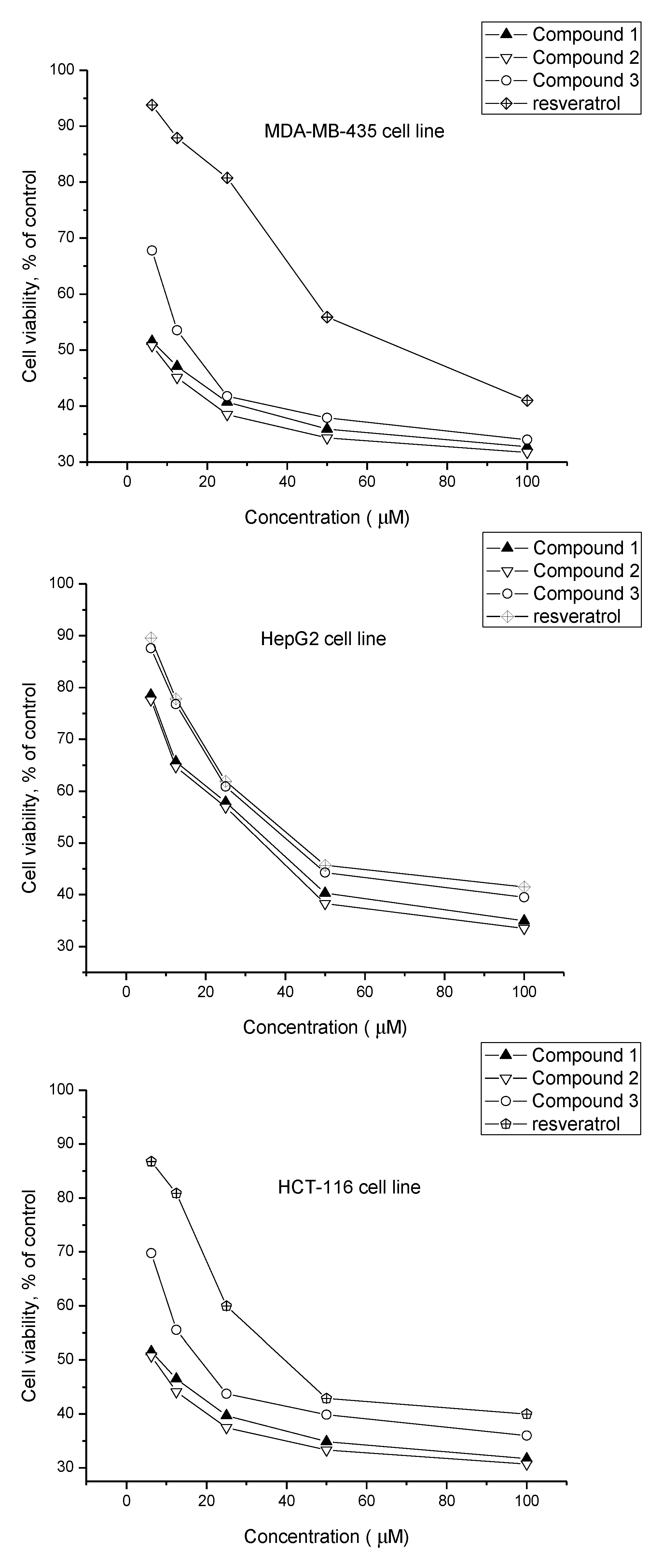
2.2. Biological Activity
| Samples | Cell lines | ||
|---|---|---|---|
| MDA-MB-435 c | HepG2 c | HCT-116 c | |
| 1 | 8.56 ± 0.81 | 35.32 ± 1.67 | 7.82 ± 1.02 |
| 2 | 7.68 ± 0.90 | 32.70 ± 1.50 | 6.93 ± 0.63 |
| 3 | 16.49 ± 0.78 | 41.86 ± 0.57 | 18.63 ± 0.95 |
| Resveratrol b | 68.56 ± 1.36 | 43.52 ± 1.29 | 38.87 ± 1.58 |
| Epirubicin b | 0.56 ± 0.06 | 0.96 ± 0.02 | 0.48 ± 0.03 |
| 1 | 2 | 3 | Resveratrol b | Ascorbic acid b |
|---|---|---|---|---|
| 447.62 ± 5.00 | >900.00 | 572.68 ± 6.41 | 70.22 ± 0.35 | 21.61 ± 0.00 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material and Fermentation
3.3. Extraction and Isolation
3.4. Cytotoxicity Assay
3.5. The DPPH Radical Scavenging Activity Assay
4. Conclusions
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shen, T.; Wang, X.-N.; Lou, H.-X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef]
- Jang, M.S.; Cai, E.N.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; Moon, R.C.; Pezzuto, J.M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Bradamante, S.; Barenghi, L.; Villa, A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004, 22, 169–188. [Google Scholar] [CrossRef]
- Sinha, K.; Chaudhary, G.; Gupta, Y.K. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002, 71, 655–665. [Google Scholar]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; Scherer, B.; Sinclair, D.A. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Valenzano, D.R.; Terzibasi, E.; Genade, T.; Cattaneo, A.; Domenici, L.; Cellerino, A. Resveratrol prolongs life span and retards the onset of age-related markers in a shortlived vertebrate. Curr. Biol. 2006, 16, 296–300. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Shi, J.; Zeng, Q.; Liu, Y.; Pan, Z. Alternaria sp. MG1, a resveratrol-producing fungus: isolation, identification, and optimal cultivation conditions for resveratrol production. Appl. Microbiol. Biotechnol. 2012, 95, 369–379. [Google Scholar] [CrossRef]
- Aly, A.H.; Indriani, I.D.; Edrada-Ebel, R.A.; Wray, V.; Mueller, W.E.G.; Trotzke, F.; Zirrgiebel, U.; Schaechtele, C.; Kubbutat, M.H.G.; Lin, W.; Proksch, P.; Ebel, R. Cytotoxic metabolites from the fungal endophyte Alternaria sp. and their subsequent detection in its host plant Polygonum senegalense. J. Nat. Prod. 2008, 71, 972–980. [Google Scholar] [CrossRef]
- Kjer, J.; Wray, V.; Edrada-Ebel, R.; Ebel, R.; Pretsch, A.; Lin, W.; Proksch, P. Xanalteric acids I and II and related phenolic compounds from an endophytic Alternaria sp. isolated from the mangrove plant Sonneratia alba. J. Nat. Prod. 2009, 72, 2053–2057. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Comp. Rev. Food. Sci. Food Safe 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Rodríguez, J.; Olea-Azar, C.; Cavieres, C.; Norambuena, E.; Delgado-Castro, T.; Soto-Delgado, J.; Araya-Maturana, R. Antioxidant properties and free radical-scavenging reactivity of a family of hydroxynaphthalenones and dihydroxyanthracenones. Bioorg. Med. Chem. 2007, 15, 7058–7065. [Google Scholar] [CrossRef]
- Chong, J.L.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Akamatsu, H.; Taga, M.; Kodama, M.; Johnson, R.; Otani, H.; Kohmoto, K. Molecular karyotypes for Alternaria plant pathogens known to produce host-specific toxins. Curr. Genet. 1999, 35, 647–656. [Google Scholar] [CrossRef]
- Tournas, V.H.; Stack, M.E. Production of alternariol and alternariol methyl ether by Alternaria alternata grown on fruits at various temperatures. J. Food Prot. 2001, 64, 528–532. [Google Scholar]
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Xie, G.E.; Zhu, X.; Li, Q.; Gu, M.H.; He, Z.j.; Wu, J.H.; Li, J.; Lin, Y.C.; Li, M.F.; She, Z.G.; Yuan, J. SZ-685C, a marine anthraquinone, is a potent inducer of apoptosis with anticancer activity by suppression of the Akt/FOXO pathway. Br. J. Pharmacol. 2010, 159, 689–697. [Google Scholar] [CrossRef]
- Bonfante, V.; Bonadonna, G.; Villani, F.; di Fronzo, G.; Martini, A.; Casazza, A.M. Preliminary phase I study of 4′-epi-adriamycin. Cancer Treat. Rep. 1979, 63, 915–918. [Google Scholar]
- Schauer, P.K.; Wittes, R.E.; Gralla, R.J.; Casper, E.S.; Young, C.W. A phase I trial of 4′-epi-adriamycin. Cancer Clin. Trials 1981, 4, 433–437. [Google Scholar]
- Bonfante, V.; Villani, F.; Bonadonna, G. Toxic and therapeutic activity of 4′-epi-doxorubicin. Tumori 1982, 68, 105–111. [Google Scholar]
- Sekhon-Loodu, S.; Warnakulasuriya, S.N.; Rupasinghe, H.P.; Shahidi, F. Antioxidant ability of fractionated apple peel phenolics to inhibit fish oil oxidation. Food Chem. 2013, 140, 189–196. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella auritom. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, J.; Cox, D.G.; Ding, W.; Huang, G.; Lin, Y.; Li, C. Three New Resveratrol Derivatives from the Mangrove Endophytic Fungus Alternaria sp. Mar. Drugs 2014, 12, 2840-2850. https://doi.org/10.3390/md12052840
Wang J, Cox DG, Ding W, Huang G, Lin Y, Li C. Three New Resveratrol Derivatives from the Mangrove Endophytic Fungus Alternaria sp. Marine Drugs. 2014; 12(5):2840-2850. https://doi.org/10.3390/md12052840
Chicago/Turabian StyleWang, Jinhua, Daniel G. Cox, Weijia Ding, Guanghao Huang, Yongcheng Lin, and Chunyuan Li. 2014. "Three New Resveratrol Derivatives from the Mangrove Endophytic Fungus Alternaria sp." Marine Drugs 12, no. 5: 2840-2850. https://doi.org/10.3390/md12052840
APA StyleWang, J., Cox, D. G., Ding, W., Huang, G., Lin, Y., & Li, C. (2014). Three New Resveratrol Derivatives from the Mangrove Endophytic Fungus Alternaria sp. Marine Drugs, 12(5), 2840-2850. https://doi.org/10.3390/md12052840




